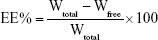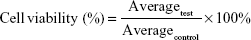Back to Journals » Drug Design, Development and Therapy » Volume 11
Enzymatic synthesis and in vitro evaluation of folate-functionalized liposomes
Authors Guo B, Xu D, Liu X, Yi J
Received 20 January 2017
Accepted for publication 10 May 2017
Published 20 June 2017 Volume 2017:11 Pages 1839—1847
DOI https://doi.org/10.2147/DDDT.S132841
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Bohong Guo, Danqiao Xu, Xiaohong Liu, Jun Yi
Department of Pharmaceutics, Guangdong Pharmaceutical University, Guangzhou, China
Abstract: In this study, folate–poly(ethylene glycol)3400–cholesterol conjugates (FA–PEG–Chol) were enzymatically synthesized in one step and incorporated into liposomes to prepare folate (FA)-functionalized liposomes for targeted drug delivery. The FA-functionalized liposomes loaded with betulinic acid (BA) (FA-L-BA) were prepared by thin lipid film method. The FA-L-BA was characterized by their morphology, particle size, zeta potential, encapsulation efficiency (EE), stability, cell cytotoxicity and cellular uptake. The average size of FA-L-BA was 222±8 nm. The spherical particles exhibited a negative electrical charge of -20.12±1.45 mV and high EE of 91.61%±1.16%. The liposomes were taken up selectively by HepG2 cells. FA-L-BA showed enhanced cytotoxicity (50% inhibitory concentration [IC50] =63.07±2.22 µg/mL) compared to nontargeted control normal liposomes loaded with BA (L-BA; IC50 =93.14±2.19 µg/mL) in HepG2 cells in vitro. In addition, FA-functionalized liposomes loaded with Ir-1 (FA-L-Ir-1) showed significantly higher cellular uptake in HepG2 cells compared to nontargeted control normal liposomes loaded with Ir-1 (L-Ir-1). This novel approach for the liposomes surface modified with FA by a one-step enzymatic amidation was expected to provide potential application as a drug carrier for active targeted delivery to tumor cells.
Keywords: enzymatic synthesis, liposomes, folate, surface modification, betulinic acid
Introduction
It is well known that folate (FA) as a high-affinity ligand is recognized by FA receptor (FR; Kd ~10−10 M), which is overexpressed at the surface of many types of cancer cells.1 The receptor-mediated uptake of FA has been proposed as a potentially useful target in cancer treatment and as a route to promote the entry of attached macromolecules or liposomes into cells.2–4 Synthetic polymers conjugated to FA as a signal mediator for such biological recognition have been developed in many drug-targeting carrier studies.5 Guo et al6 reported a lipophilic conjugate of FA (FA–poly(ethylene glycol)3350–cholesterol, FA–PEG–Chol), which was synthesized and evaluated for receptor-mediated targeting of liposomes to tumor cells. However, these chemical modifications require a high reaction temperature, high pressure and complicated and controlled multiple-step reactions and are difficult to purify. On the other hand, enzymatic synthesis is mild and a small amount of by-products are produced due to the substrate specificity of the enzyme. Furthermore, lipase possesses enhanced stability, easy separation from the reaction mixture and reusability.7 Previous reports have demonstrated the enzymatic synthesis of liposomal ligand,8–11 which has various advantages, such as high initial rate, high substrate conversion, high regioselectivity, environmental protection and a reduction in energy consumption. Biocatalytic processes have become a useful and green alternative in stereoselective synthesis due to their multiple advantages.12–14
In the current study, we reported the synthesis of a lipophilic FA derivative, FA–PEG–Chol, which was based on amide linkages. FA conjugation was synthesized by a nonaqueous enzymatic reaction in one step. The FA group was located at the outer end of PEG, away from the bilayer. We chose a PEG length (PEG, Mr 3400; designated as PEG3400) for the FA–PEG–Chol conjugates to reduce steric interference with receptor binding. Compared to what previously reported,6 the procedure of enzymatic synthesis was much simpler. The novel enzymatic reaction systems could be used for highly efficient and regioselective preparation of amidations.
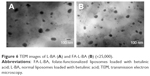
Betulinic acid (BA), a lupane-type pentacyclic triterpenoid saponin from tree bark, could induce the apoptosis of tumor cells without toxicity to normal cells in vitro and in vivo.15 BA has potent anticancer activity on many kinds of tumor cells, including colon cancer, skin cancer, prostate cancer, lung cancer, liver cancer and breast cancer cell lines.16–19 However, because of its weak hydrosolubility (0.02 μg/mL) and high lipophilicity, BA has been difficult to study the efficacy in vivo and a pharmaceutical formulation has not yet been available.20,21 Therefore, the encapsulation of BA into liposomes would be an appropriate method to avoid these drawbacks. In this study, BA was encapsulated in FA-functionalized liposomes with egg phosphatidylcholine (EPC), Chol and FA–PEG–Chol. Characterization of the liposomes, including transmission electron microscopy (TEM) morphology, size, zeta potential, entrapment efficiency, and stability, was carried out. In vitro cell cytotoxicity and cellular uptake on cancer HepG2 and A549 cells were evaluated.
Materials and methods
Materials
BA was purchased from Nanjing Zelang Medical Technology Co., Ltd. (98%; Nanjing, China). EPC and distearoylphosphatidylethandamine (DSPE)–PEG2000 were purchased from Lipoid Co. (Ludwigshafen, Germany). Chol was purchased from Shanghai Advanced Vehicle Technology Ltd., Co. (Shanghai, China). Sephadex G-50 was purchased from Pharmacia Biotech (Uppsala, Sweden). Novozym 435 from Candida Antarctica lipase B was purchased from Novozymes Co. (Bagsvard, Denmark). FA and NH2–PEG3400–NH2 were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Cholesteryl chloroformate was purchased from Shanghai Aladdin Bio-Chem Technology (Shanghai, China). [Ir(ppy)2(BTCP)]PF6 (Ir-1) was gifted by professor Yunjun Liu from the School of Pharmacy, Guangdong Pharmaceutical University, and used as fluorescence probe.22 Methanol and acetonitrile (high-performance liquid chromatography [HPLC] grade reagent) were obtained from Dikma Technologies Inc. (Beijing, China). Cancer cell lines of HepG2 (human hepatocellular carcinoma) and A549 (human lung carcinoma) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Dimethyl sulfoxide (DMSO), RPMI-1640, 3-4,5 dimethylthiazol-2,5 diphenyl tetrazolium bromide (MTT) and fetal bovine serum (FBS) were purchased from Sigma-Aldrich Co. All the reagents used were of analytical grade. Ultrapure MilliQ water was used throughout the experiments.
1H nuclear magnetic resonance (NMR) spectra were performed on a 500 MHz proton NMR spectrometer (Bruker Corporation, Billerica, MA, USA). Fourier transform infrared (FTIR) spectra were carried out on a Nicolet 5DX. Electrospray ionization-mass spectrometry (ESI-MS) was carried out on a Waters Xevo TQD LC-MS. The size and zeta potential of the particles were determined using a Zeta Sizer 3000 (Malvern Instruments, Malvern, UK). The morphology of the particles was determined using a JEM-1400 TEM from Hitachi Ltd. (Tokyo, Japan).
Methods
Synthesis of FA–PEG–Chol
The mixtures of FA (0.0112 g, 50 μmol), NH2–PEG3400–NH2 (0.085 g, 25 μmol) and cholesteryl chloroformate (0.0110 g, 50 μmol) were dissolved in DMSO (10 mL), and the mixtures were oscillated (250 rpm) at 40°C for 10 min. Then, Novozym 435 (500 U) was added into the abovementioned solution, and the mixtures were oscillated (250 rpm) at 40°C for 24 h. After oscillation, the samples were filtered to remove enzyme. The mixtures were dialyzed against deionized water using a spectrum dialysis membrane with a molecular weight cutoff (MWCO) of 2,000 Da to remove unreacted FA and cholesteryl chloroformate and low molecular weight by-products. The product was then purified by size exclusion chromatography using Sephadex LH-20 that was swollen in and eluted with CHCl3:MeOH (2:1). The product, FA–PEG–Chol, was then dried by lyophilization, which yielded a yellow powder product. The identity of the product was confirmed by thin-layer chromatography (TLC), FTIR, MS and 1H NMR in d-DMSO.
Liposome preparation
BA liposomes were prepared using the thin lipid film method.23 Briefly, an appropriate amount of EPC, Chol, DSPE–PEG2000 and BA (EPC:Chol:DSPE–PEG2000=77:52:4.4, molar ratio) were dissolved in a mixture of CHCl3:MeOH (3:1) under magnetic stirring. The solvents were removed under pressure (40°C), resulting in a thin lipid film formation. This film was then hydrated with 10 mL phosphate-buffered solution (PBS; pH =7.4), obtaining multilamellar liposomes. The liposomal suspension was then sonicated (Scientz-IID Ultrasonic Cell Disruptor; Ningbo Scientz Biotechnology Co., Ltd, Ningbo, China) at 200 W and 40 Hz for 120 s to form small unilamellar liposomes. The final injection of liposomes was filtered through polycarbonate filter with 0.45 and 0.22 μm pores (Nucleopore; Tubingen, Germany). The final unilamellar liposomal vesicles were filled into cillin bottles (1 mL each) together with a cryoprotectant of mannitol and were freeze-dried in a freeze drier (LGJ-18A; Four-Ring Science Instrument Plant Beijing Co., Ltd, Beijing, China). Normal liposomes loaded with BA (L-BA) were thus obtained.
The ratio of the EPC/Chol was the same as described earlier. FA–PEG–Chol (the same molar ratio as DSPE–PEG2000) was dissolved in a mixture of CHCl3:MeOH (3:1), then added up into lipid mixtures. The solution was prepared according to the abovementioned procedure, and the FA-functionalized liposomes loaded with BA (FA-L-BA) were obtained.
Fluorescent liposomes were prepared using the above described procedures. The lipid compositions of FA-functionalized liposomes loaded with Ir-1 (FA-L-Ir-1) and nonfunctionalized liposomes loaded with Ir-1 (L-Ir-1) were the same as the liposomes containing BA.
Morphological study by TEM
The morphology of liposomes was studied under the TEM (JEM-1400; Hitachi Ltd.).
Vesicle size and zeta potential analysis
The determination of the particle size and zeta potential of the L-BA and FA-L-BA was performed by dynamic light scattering (DLS), using a Zeta Sizer 3000. The formulations were appropriately diluted with PBS (pH =7.4) for the purpose of avoiding the multi-scattering phenomena. All the measures were determined at least three times at room temperature.
Determination of encapsulation efficiency (EE)
The sample determination was performed by using an HPLC system (10 AVP, Shimadzu, Kyoto, Japan). The sample separation was carried out at 30°C using a reverse phase C18 column (Thermo Fisher Scientific, Waltham, MA, USA; 5 μm, 4.6×250 mm) protected with an Easy Guard Column (10×4.0 mm). The mobile phase consisted of acetonitrile and water (adjusted to pH 3 with acetic acid). The ratio of acetonitrile:H2O (v/v) was adjusted to 80:20. The detection wavelength was 210 nm, and a flow rate of 1.0 mL/min was employed. A sample volume of 20 μL was injected. Each determination was carried out in triplicate.
The EE was calculated by the percentage of drug encapsulated into liposomes (Wtotal–Wfree) relative to the total amount of drug (Wtotal) in liposome suspension.
To determine the Wtotal in liposome suspension, 0.2 mL BA liposome was disrupted by the addition of 10 mL methanol to form a clear solution. The concentration of drug was measured by using HPLC analysis.
The encapsulated fraction and nonencapsulated fraction were separated using Sephadex G-50 column filtration. A volume of 0.2 mL liposome suspension was separated on the column (1.3×25 cm) and eluted with water at a flow rate of 1.0 mL/min. The eluting solution was collected in 20 test tubes that were changed every 2 min. Each tube was diluted to an appropriate concentration; then, all samples and standards were measured at 210 nm. The elution curve was then generated based on the drug concentration and individual dilution factors. The resulted water fraction containing the liposomes free of nonentrapped drug was collected, and the Wfree was determined. The EE was calculated according to the following equation:
|
Stability of the liposomes
The physical stability was assessed by comparing the changes in mean diameters, zeta potential, and EE of the liposomes. Aliquots (5 mL) of FA-L-BA in PBS (pH 7.4) were added to glass vials and stored in refrigerator (4°C) at different periods of time.24
Cell culture
In this study, HepG2 and A549 cells were used to evaluate the targeting effect of the FA-functionalized liposomes, as the two cell lines were reported to be of FR overexpression (positive cell model, FR[+]) and lack FR expression (negative cell model, FR[−]), respectively.25 Cells were grown in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10% FBS and antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) and incubated at 37°C in a humidified atmosphere of 5% CO2. BA was dissolved in DMSO to make a stock solution at a concentration of 11.2–90 μg/mL and then stored at 4°C. L-BA and FA-L-BA lyophilized powders were dissolved in PBS and sonicated for 20 min became a stock solution.
In vitro cytotoxicity study
The cytotoxic activity of free drug (BA) and formulations (FA-L-BA and L-BA) was determined in HepG2 and A549 cells using an MTT assay.26 Breifly, cells were inoculated into 96-well microassay culture plates (8×103 cells/well) and grown overnight at 37°C in a 5% CO2 incubator. Then, the cells were treated with free drug and formulations to achieve final equivalent BA concentrations ranging from 11.2 to 90 μg/mL. Control wells were prepared by the addition of culture medium (100 μL). After 48 h, MTT solution (20 μL, 5 mg/mL) was added to each well. After 4 h, 200 μL DMSO was added to solubilize the MTT formazan and the product was quantified spectrophotometrically by measuring the absorbance at 490 nm in a microplate spectrophotometer (Thermo Scientific Multiskan FC; Thermo Fisher Scientific). Each experiment was repeated at least three times to obtain the mean values, and the data were fitted the next equation to calculate the percentage of cell viability. The concentration that inhibits 50% of the cellular growth (IC50) was calculated from the cell viability data as the drug concentration in which the cell growth was inhibited by 50%.
|
Cellular uptake
To evaluate the FA-functionalized liposomes that could be guided to tumor cell via FR-mediated delivery, we used Ir-1 as the fluorescence probe and loaded it in the liposomes. Briefly, A549 and HepG2 cells were placed in 12-well microassay culture plates (15×104 cells/well) and grown at 37°C in a 5% CO2 incubator until 60% confluence. Then, the cells were treated with L-Ir-1 and FA-L-Ir-1 (equivalent Ir-1 concentration of 6.26 μmol/mL) for 1 h. In another group, cells were preincubated with free FA (1 mM) for 2 h to block the binding of FA to target the receptors on tumor cells before their co-incubation with FA-L-Ir-1. Then, the plates were incubated in a 5% CO2 incubator at 37°C for 1 h. Upon completion of the incubation, the wells were washed two times with PBS and the cells were visualized by fluorescent microscopy (PerkinElmer Inc., Waltham, MA, USA).
Statistical analysis
All the experiments were performed in triplicate, and the results were given as mean ± standard deviation (SD). One-way ANOVA was used to analyze the differences between two treatment groups. Differences were considered to be significant at P<0.05 and extremely significant at P<0.01.
Results and discussion
Synthesis and characterization of FA–PEG–Chol
FA–PEG–Chol conjugates were synthesized by the reaction of FA, NH2-PEG3400-NH2 and cholesteryl chloroformate in the presence of Novozym 435 after oscillation at 40°C for 24 h. The synthetic route for FA–PEG–Chol is shown in Figure 1. FA–PEG–Chol was characterized by ES-MS, FTIR and 1H NMR as shown in Figures 2–4; TLC: Rf =0.60 (CH2Cl2:methanol =4:1). The peak of the m/z was 4,175 Da, which was consistent with the theoretical target value (4,175 Da); IR v/cm−1: 3,341 (–NH–), 2,885 (–CH3–), 1,963 (–CH–), 1,716 (C=O), 1,607 (N–H). 1H NMR (500 MHz, DMSO-d6) analysis showed principal peaks (in ppm) related to the FA moiety (8.58 [d], 7.19 [s], 7.03 [s], 6.64 [d], 4.47 [d], 4.03 [s]), the PEG moiety (3.32–3.65 [m]), and the Chol moiety (5.29 [d], 4.59 [d], 2.28–0.65 [m]).
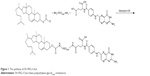  | Figure 1 The synthesis of FA–PEG–Chol. |
  | Figure 2 The 1H NMR spectra of FA–PEG–Chol. |
  | Figure 3 The FTIR spectra of FA–PEG–Chol. |
  | Figure 4 The MS of FA–PEG–Chol. |
In this study, we have developed a practical and efficient methodology for the construction of FA–PEG–Chol by a one-step synthetic procedure. In the structure of FA–PEG–Chol, the Chol group could insert the lipid layer and the FA group is located at the outer end of PEG, away from the bilayer. The schematic structure of FA-functionalized liposomes is shown in Figure 5. With the Novozyme 435 as efficient biocatalyst, the amidation of FA, PEG-derivatized and cholesteryl chloroformate proceeded smoothly to give the targeting molecule (FA-PEG-Chol) in high yields (up to 57% yield).
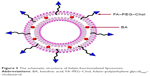  | Figure 5 The schematic structure of folate-functionalized liposomes. |
Characterization of liposomes
The morphology of the liposomes was observed in a TEM image as shown in Figure 6. It was evident that the liposomes were well dispersed with a regular spherical shape. No significant changes were observed in any of the characteristics following the addition of FA to the liposomes. FA-L-BA and L-BA presented similar size distributions with a mean diameter of around 220 nm. As shown in Figure 7 from the DLS, the average particle size of the prepared FA-L-BA was 222±8 nm and the polydispersity index (PI) was 0.18±0.01. The zeta potentials of FA-L-BA and L-BA were −20.12±1.45 mV and −21.67±1.73 mV, respectively. The negative zeta potentials may be due to the presence of the negatively charged lipid DSPE–PEG2000 in the formulation. It appeared that FA–PEG–Chol would not interfere with BA entrapment.
  | Figure 7 Particle-size distribution of FA-L-BA. |
Determination of EE
The retention time of BA in HPLC was approximately 16.7±0.2 min, with Relative Standard Deviations (RSDs) for both interday and intraday values of <1.5% at all concentrations. The total recovery for this method was 99.01%±1.19% (mean ± SD), and the calibration curve was linear for the entire calibration range of 49.8–149.4 μg/mL (r=0.9998).
The liposome elution curve is shown in Figure 8. The free fraction and encapsulated fraction were completely separated. The EE was the proportion of encapsulated fraction in total preparation (encapsulated fraction + free fraction). The EE of FA-L-BA and L-BA was 91.61%±1.16% and 90.24%± 1.03%, respectively (P>0.05). No relevant differences were observed in terms of encapsulation parameters between the two BA liposomes. This fact was according to previous reports,8,27 which explored the receptor-targeted delivery system, suggesting that whether the ligand was modified on the surface of liposomes, the EE was not changed.
  | Figure 8 Elution curve of BA liposomes. |
Storage stability
The stability of FA-L-BA was investigated by measuring the particle size, zeta potential and EE at predetermined time intervals as described previously. As shown in Table 1, after 3 months, no significant increase in BA leakage was observed when liposomes were stored at 4°C. The particle size and zeta potential had smaller changes during the 3-month storing period, compared to that of the freshly made.
Cytotoxic activity evaluation in vitro
In vitro cytotoxic activity was determined by MTT assay. As shown in Figure 9, FA-L-BA inhibited cell survival more forcefully than the same concentration of L-BA in HepG2 cells, while the cell survival did not have obvious difference in A549 cells. FR-targeted liposomes showed enhanced cytotoxicity against FR (+) but not FR (−) cancer cells. As shown in Table 2, the IC50 values of the FA-L-BA and L-BA were 63.07±2.22 μg/mL and 93.14±2.19 μg/mL in HepG2 cells (P<0.05), respectively. In another cell line, A549, the IC50 values of the FA-L-BA and L-BA were 96.30±0.29 μg/mL and 96.95±1.07 μg/mL (P>0.05), respectively. This may be attributed to the effect of the FR-targeted endocytosis.
Cellular uptake
In this study, the fluorescent microscope analysis was employed to study the cellular uptake properties of the conventional and FR-targeted liposomes with or without FA in HepG2 and A549 cells. As shown in Figure 10A–C, the fluorescence intensity was increased in HepG2 cells incubated with FA-L-Ir-1 for 1 h in comparison to L-Ir-1 and FA-L-Ir-1 liposomes plus preincubated 1 mM free FA. However, in A549 cells, as show in figure 10D–F, there was no significant difference in the extent of cellular uptake incubated with L-Ir-1 or FA-L-Ir-1 liposomes with or without FA in the culture medium. This result can be explicated that excess free FA could bind to the FR on the cell surface competitively, which would inhibit the effect of FA-functionalized liposomes to target cancer cells. These results are in line with the report for a FA–pluronic F127 magnetic nanoparticle clusters in an earlier study.28 In addition, it suggested that liposomes modified with FA–PEG–Chol might be endocytosed via the FR.29
Conclusion
In this study, a new synthetic strategy has allowed the synthesis of FA–PEG–Chol via a biocatalytic route in one step. In the synthesis route, the lipase B from Candida antarctica (Novozym 435) was used to selectively amidate the primary amino function introduced into FA and cholesteryl chloroformate.
An enzymatic approach was preferred to a chemical one which may require more drastic conditions and lead to the generation of side products. The liposomes were prepared by thin film method. The FA-L-BA had a good distribution in size, and an EE higher than 90%. FA-L-BA showed much greater cytotoxicity compared to nontargeted control L-BA in HepG2 cells. However, for A549 cells (FR [-]) incubated in FA-L-BA, the cell viability remained nearly unchanged to compare with that in L-BA at various BA concentrations. Nonaqueous enzymatic catalysis of liposomal ligand offered an easy approach for surface FA modification, and the FA-functionalized liposomes are promising drug carriers for active targeted delivery to tumor cells.
Acknowledgment
This work was financially supported by the National Nature Science Foundation of China (No 81403111).
Disclosure
The authors report no conflicts of interest in this work.
References
Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 2000;6(5):1949–1957. | ||
Dong S, Cho HJ, Lee YW, Roman M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules. 2014;15(5):1560–1567. | ||
Datz S, Argyo C, Gattner M, et al. Genetically designed biomolecular capping system for mesoporous silica nanoparticles enables receptor-mediated cell uptake and controlled drug release. Nanoscale. 2016;8(15):8101–8110. | ||
Tong L, Chen W, Wu J, Li H. Folic acid-coupled nano-paclitaxel liposome reverses drug resistance in SKOV3/TAX ovarian cancer cells. Anticancer Drugs. 2014;25(3):244–254. | ||
Pan XQ, Wang H, Lee RJ. Antitumor activity of folate receptor-targeted liposomal doxorubicin in a KB oral carcinoma murine xenograft model. Pharm Res. 2003;20(3):417–422. | ||
Guo WJ, Lee T, Sudimack J, et al. Receptor-specific delivery of liposomes via folate-peg-chol. J Liposome Res. 2000;10(2–3):179–195. | ||
Kim H, Park C. Enzymatic synthesis of phenethyl ester from phenethyl alcohol with acyl donors. Enzyme Microb Technol. 2017;100:37–44. | ||
Guo BH, Cheng Y, Lin LP, Lin DH, Wu W. Preparation and characterization of galactose-modified liposomes by a nonaqueous enzymatic reaction. J Liposome Res. 2011;21(3):255–260. | ||
Letrou-Bonneval E, Chevre R, Lambert O, et al. Galactosylated multimodular lipoplexes for specific gene transfer into primary hepatocytes. J Gene Med. 2008;10(11):1198–1209. | ||
Nie H, Cheng Y, Zheng PJ, et al. Lipase-catalyzed synthesis of novel galactosylated cholesterol. Chinese Chem Lett. 2015;26:543–546. | ||
Wu W, Cheng Y, Guo BH, Wu Q. Pharmacokinetics of liver-targeted docetaxel liposomes modified with 6-O-acyl-D-galactose esters in rabbits. Biomed Rep. 2014;2(4):545–548. | ||
Huisman GW, Collier SJ. On the development of new biocatalytic processes for practical pharmaceutical synthesis. Curr Opin Chem Biol. 2013;17(2):284–292. | ||
Lopez-Iglesias M, Gotor-Fernandez V. Recent advances in biocatalytic promiscuity: hydrolase-catalyzed reactions for nonconventional transformations. Chem Rec. 2015;15(4):743–759. | ||
Wohlgemuth R, Plazl I, Žnidaršič-Plazl P, Gernaey KV, Woodley JM. Microscale technology and biocatalytic processes: opportunities and challenges for synthesis. Trends Biotechnol. 2015;33(5):302–314. | ||
Zhang X, Hu J, Chen Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol Med Rep. 2016;14(5):4489–4495. | ||
Bai L, Zhang H, Liu Q, et al. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016;7(6):2870–2877. | ||
Hsu TI, Wang MC, Chen SY, et al. Betulinic acid decreases specificity protein 1 (Sp1) level via increasing the sumoylation of sp1 to inhibit lung cancer growth. Mol Pharmacol. 2012;82(6):1115–1128. | ||
Park SY, Kim HJ, Kim KR, et al. Betulinic acid, a bioactive pentacyclic triterpenoid, inhibits skeletal-related events induced by breast cancer bone metastases and treatment. Toxicol Appl Pharmacol. 2014;275(2):152–162. | ||
Potze L, di Franco S, Kessler JH, Stassi G, Medema JP. Betulinic acid kills colon cancer stem cells. Curr Stem Cell Res Ther. 2016;11(5):427–433. | ||
Jager S, Winkler K, Pfiiller U, et al. Solubility studies of oleanolic acid and betulinic acid in aqueous solutions and plant extracts of Vscum album L. Planta Med. 2007;73(2):157–162. | ||
Mullauer FB, van Bloois L, Daalhuisen JB, et al. Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anticancer Drugs. 2011;22(3):223–233. | ||
Zhang C, Lai SH, Zeng CC, et al. The induction of apoptosis in SGC-7901 cells through the ROS-mediated mitochondrial dysfunction pathway by a Ir(III) complex. J Biol Inorg Chem. 2016;21(8):1047–1060. | ||
Cavalcanti IM, Mendonça EA, Lira MC, et al. The encapsulation of β-lapachone in 2-hydroxypropyl-β-cyclodextrin inclusion complex into liposomes: a hysicochemical evaluation and molecular modeling approach. Eur J Pharm Sci. 2011;44(3):332–340. | ||
Crosasso P, Ceruti M, Brusa P, Arpicco S, Dosio F, Cattel L. Preparation, characterization, and properties of sterically stabilized paclitaxel-containing liposomes. J Control Release. 2000;63(1–2):19–30. | ||
Liu Q, Xu S, Niu C, et al. Distinguish cancer cells based on targeting turn-on fluorescence imaging by folate functionalized green emitting carbon dots. Biosens Bioelectron. 2015;64:119–125. | ||
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. | ||
Ye P, Zhang W, Yang T, et al. Folate receptor-targeted liposomes enhanced the antitumor potency of imatinib through the combination of active targeting and molecular targeting. Int J Nanomedicine. 2014;9:2167–2178. | ||
Lin JJ, Chen JS, Huang SJ, et al. Folic acid-Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials. 2009;30(28):5114–5124. | ||
Kamen BA, Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci U S A. 1986;83:5983–5987. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.