Back to Journals » International Journal of Nanomedicine » Volume 16
Enhancing Cancer Immunotherapy Treatment Goals by Using Nanoparticle Delivery System
Authors Muluh TA , Chen Z, Li Y , Xiong K, Jin J , Fu S , Wu J
Received 17 December 2020
Accepted for publication 14 February 2021
Published 25 March 2021 Volume 2021:16 Pages 2389—2404
DOI https://doi.org/10.2147/IJN.S295300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Tobias Achu Muluh,1,* Zhuo Chen,1,* Yi Li,1,* Kang Xiong,1 Jing Jin,1 ShaoZhi Fu,1– 3 JingBo Wu1– 3
1Department of Oncology, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, Sichuan, People’s Republic of China; 2Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Luzhou, 646000, Sichuan, People’s Republic of China; 3Department of Oncology, Academician (Expert) Workstation of Sichuan Province, Luzhou, Sichuan, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: ShaoZhi Fu; JingBo Wu
Department of Oncology, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, Sichuan, People’s Republic of China
Tel/Fax +86 8303165696
Email [email protected]; [email protected]
Abstract: Recently, there has been an incredible increase in research about the abnormal growth of cells (neoplasm), focusing on the management, treatment and preventing reoccurrence. It has been understood that the natural defense system, composed of a variety of immune defensive cells, does not just limit its function in eliminating neoplastic cells, but also controls the growth and spread of tumor cells of different kinds to other parts of the body. Cancer immunotherapy, is a cancer treatment plan that educates the body’s defensive system to forestall, control, and eliminate tumor cells. The effectiveness of immunotherapy is achieved, to its highest efficacy, by the use of nanoparticles (NPs) for precise and timely delivery of immunotherapies to specific targeted neoplasms, with less or no harm to the healthy cells. Immunotherapies have been affirmed in clinical trials as a cancer regimen for various types of cancers, the side effects resulting from imprecise and non-targeted conveyance is well managed with the use of nanoparticles. Nonetheless, we will concentrate on enhancing cancer immunotherapy approaches by the use of nanoparticles for the productivity of antitumor immunity. Nanoparticles will be presented and utilized as an objective immunotherapy delivery system for high exactness and are thus a promising methodology for cancer treatment.
Keywords: tumor immunotherapy, immune system, nanoparticles, drug delivery, drug release
Introduction
Cancer immunotherapy was founded around the late phase of 1890s by a famous cancer specialist Dr. William B. Coley (1862–1936) who was also a surgeon.1 During a patient’s treatment process, he realized that when some specific bacteria were administered to patients who were suffering from neoplasm, there was a great effect to the growth tissues which could even lead to total disappearance, which serves as a foundation for lots of immunotherapy research nowadays.2 Many researchers have come to demonstrate that when patients are exposed to the bacteria-toxin, there is an activation which boosts the immune system to assault the neoplastic cells which could possibly improve the patient’s situation as well as set them free from malignancy.3 Immunotherapy upgrades the immune defense system’s capacity to perceive, target, and set free from neoplastic cells thereby not modifying or hurting the healthy cells, which makes it a better option for the management and treatment of all kinds of cancer.4 Engaging the immune system is a better approach to battle tumors because of the following realistic views as shown in Table 1.
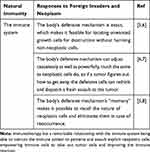 |
Table 1 The Immunity Affiliation with the Immunotherapy |
Immunotherapy has been affirmed clinically as the primary line of treatment and management for most malignancies,9 and may likewise be a powerful therapy for patients with specific tumors that are impervious to treatment.10 Irrespective of the fact that immunotherapy can be given to patients in many ways, conveyance of small-particle medications to their malignant growth target destinations of activity generally presents perhaps the greatest problem in view of the cancer's homogeneous tissue dispersion, renal freedom, and absence of target specificity.11 In order to better manage the above challenges, nanotherapeutics is fast giving hope for the treatment of cancer to both researchers and clinicians.12 Its molecular size measuring approximately 1–100 nm makes it an ideal means by which drug delivery for treating and preventing tumor cells gain more effectiveness and are more precise as well.13
Nanoparticles are typically synthesized from a top-down or bottom-up approach.14 A bottom-up approach relies on nucleating atomic-sized materials into the eventual nanoparticles. While the exact synthesis method depends on the material being generated, some common methods include the Turkevich method (citrate reduction), gas phase synthesis, block copolymer synthesis, and more recently, microbial synthesis. Top-down methods, where a bulk material is physically broken down to make smaller molecules, include milling, laser ablation and spark ablation.14,15
NPs possess an ideal pharmacokinetics with delayed dissemination time, particular endothelial porousness at a few objective tissues, and high explicitness for biological targets are the beautiful nature of nanotherapeutics that drive the scientists to zero in on the utilization of nanoparticles for the medication delivery system.16 The disclosure and utilization of nanoparticles for clinical application has been grasped enthusiastically for the headway of remedial apparatuses which productively prompts the accomplishment of cancer immunotherapy’s objective.17 Nanoparticles are best for focusing on explicit delivery, with a noteworthy lessening in side effects and a decrease in systemic toxicity.18 NPs contain an assortment of molecular bonding sites and shielding the molecules from degradation and controlling their delivery kinetics.19 Nanoparticles assume a significant part in cancer immunotherapy since they have tweaked size, shape, charge and surface properties bringing about better viability.13,20 NP-based immunotherapy drug conveyance systems have considerable abilities for tumor treatment. The critical central purposes of nanoparticles utilized as medication transporters includes high sufficiency, high transporter limit, practicality incorporation of both hydrophilic and hydrophobic substances and probability of variable ways of administration such as oral and inhalant.21 These properties of nanoparticles empowered drug bioavailabilities and efficacy and decrease the unwanted targeting which are significants snags in tumor management.22
Tumor Cells and Body’s Defensive Mechanism Cells
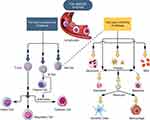
The body’s defensive mechanism relays the functions of the innate and adaptive immunity, which are the focal segments that provide the medium for which microbe diseases are targeted and eliminated,23 the innate immune cells are liable for sure-fire insurance while the adaptive immune cells are answerable for long-haul assurance.24 The cells of the immune system can be seen in Figure 1.
The innate immunity, made up of immune cells ranging from dendritic-cells (DCs), macrophages, and natural-killer-cells (NK-cells), brings about the prior defense system by perceiving rationed pathogen associated molecular patterns (PAMPs) following the mechanism of pattern recognition receptors (PRRs), which are made up of toll-like receptors (TLRs) bound to the membrane.5,25 Antigen presenting cells (APCs) are responsible for capturing pathogens, including macrophages, as well as dendrite-cells, proceeds fractioned into peptide fragments then exposed to the major-histocompatibility-complex (MHC) which are noticeable with the aid of T-cell-receptors (TCR).23,26 In a not shell, NK-cells are capable of eliminating low functional host cells instead of targeting foreign pathogen for minimal presentation of the MHC-l on malignant cells. NK-cells have the capacity to suddenly murder virally contaminated and a wide assortment of tumor cells yet save most healthy cells,27 NK-cells are fit for intervening the executing of tumor cells by a few unmistakable instruments including the emission of an expansive range of cytokines, extravasating just as entering tissue districts, shifting from premalignant to dangerous tissues.2 Also, NK-cells are likewise known to be exceptionally receptive to numerous organic agents, including cytokines, for example, interleukin (IL)-2 or IL-12 and interferons (IFNs), and quickly to expand their cytolytic, secretory, proliferative, and other capacities upon incitement with these agents.
The adaptive defensive immunity continues the innate defensive immunity that has enacted T- and B-cells specific pathogenicity. Just like the defensive ability of the PRRs of the innate immunity,28 T- and B-cells are the immunity foundations of a broad variety ligation of pathogenic-receptors leading to the formation of numerous antigen-receptors.29 The marker present of the molecular surfaces of T-cells can characterize the differentiation into various small entities ranging from cytotoxic-T-lymphocyte (CTL) likewise referred to as CD8+ T-cells, having a function in prompt killing of foreign pathogens or harmed and broken malignant-cells.30,31 To actuate CTLs, it’s fundamental to invigorate T-cells with solid MHC signals or extra signals delivered by ‘‘helper” T-cells (TH) cells. TH-cells (CD4+ T-cells) assume significant parts in adaptive immune guideline though they don’t straightforwardly include microbe phagocytosis or annihilation.32 Additionally, significant adaptive defensive cells such as B-cells are responsible for providing antibodies associated with pathogen inactivation.
“Immunosurveillance” and “Immunoediting” Relation with the Tumor
While a considerable number of our cells grow and divide normally, the process is firmly monitored by lots of factors, including the gene qualities inside the cells, change in shape of cells, change in position, the cell-cell adhesion, the quantity of a particular protein present in a cell and the quantity of enzyme tryptophan pyrrolase in the liver. The effectiveness of tumor immunotherapy is based on the “tumor immunosurveillance”.12 At the point when no more growth is required, cells are advised to quit growing. Tragically, malignant growth cells gain deserts that cause them to disregard these stop signs, and then develop wildly.33 However, notwithstanding the ability of the immune system to react in opposition to most tumor cells, the appearance of tumor signals allow the growing tumor to escape detection or to avoid the immune response.34 The “immunoediting” concept, tries to shed the mechanism of evasion through describing tumor development as a system following three stages which can be seen in Table 2 and Figure 2. During the “immunoediting”, the immune defensive system shapes tumor movement into two contrary directions which secure the host against malignancy and mold tumor immunogenicity through three stages, namely; elimination, equilibrium and escape. In the elimination stage, tumor cells initiate the inborn immune defensive system because of the mechanical interruption of sound tissues. IFN-gamma emissions from macrophages and dendritic cells (DCs) cause direct cell demise and initiate angiostatin chemokine articulation in tumor cells. The antigen-presenting cells (APCs), for example, dendritic cells and macrophages pick up the dying tumor cells along with antigens and are conveyed to depleting lymph nodes to enact CD4+ T-cells and thus CD8+ T-cells.35 Tumor-associated antigen (TAA) actuated T-cells course to the tumor microenvironment and initiate cell demise by delivering cytotoxic proteins including perforin or granzyme, or by the Fas/FasL pathway. The IFN-gamma-initiated proinflammatory tumor microenvironment upgrades apoptosis through a criticism circle of creation of more IFN-gamma and IL-12. Hereditarily changing immune tumor cells that endure the elimination stage then proceeds to the equilibrium phase. Now, tumor cells go into a peaceful stage and there is a harmony between invulnerable assault and malignant growth cell expansion that forestalls tumor outgrowth. As the immune defensive system hinders multiplication of insusceptible delicate variations, tumor cells which stay safe gain changes that shield them from discovery and make an immunosuppressive tumor microenvironment.36 Two notable break components are diminishing TAA introduction and deficiency of MHC1 articulation which ties to T-cell receptors (TCRs) on CD8+ T-cells. Additionally, malignancy cells produce suppressive cytokines, for example, TGF-beta and IL-10 just as inhibitory costimulatory molecules, for example, CTLA-4, PD-1, and PD-L1.35,37 Regulatory T-cells, myeloid-determined dendritic cells, and tumor-related macrophages add to tumor endurance and extension.
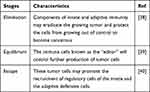 |
Table 2 Tumor Development Stages and Characteristics |
Nanoparticles in Cancer Immunotherapy
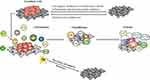
In recent years, remarkable advances and attempts have revealed the ability of nanoparticle production and application for high efficacy and great potency in the field of medicine, especially as a conveyance medium for immunotherapy.41 The promising results are realized because of the physiochemical properties of the nanoparticle consisting of a precise and consistent immunotherapeutic drug conveyance strategy.42 The nanoparticle’s size, nature and the immunotherapeutic conveyance are a few remarkable features that make it a perfect model for enhancing cancer immunotherapy.43 Furthermore, NPs possess a few similarities in feature like malignant cells which lead to an advancement of the permeability and retention effect. NPs normally are composed of metals, organic compounds as well as polymers. They can be manufactured for the conveyance of precise immunotherapy including those of less soluble and absorbability.44 Most commercially manufactured NPs use liabilities that focus on strategies that are entirely localized on the impact of permeability and retention effect (EPR).21 Nevertheless, technology which bring into use the ability of nanoparticle active targeting during which immunotherapy can precisely adhere to the nanoparticles and effectively convey at the malignant cells site where tumor cells are neutralized while sparing healthy cells.45 See Figure 3.
Nanoparticles Enhancing Cancer Immunotherapy
Lots of NPs have been discovered as immunotherapy carriers to convey antitumor immunotherapeutics specifically to tumor cells. These NPs have the capability of offering stability, increasing solubility and causing less toxicity to healthy cells.46 NPs have the potential to supply immunotherapeutics directly at malignancy sites which can be accounted for by the improved duration within the blood stream without altering physiochemical properties of the body.47 The lymphoid node secures the NPs preceding their drug conveyance priority and elimination of the toxic waste products. There is a great decrease in cellular cytotoxicity and reasonable outcome when NPs-immunotherapy are passively used for aiming malignancy.48 Here, we have to understand that there is a variation in patients and several forms of neoplasm exist so how can we better understand the enhancement of immunotherapy considering a single form of neoplasm? These can be achieved based on the morphological structure, dimension, the positive or negative nature of the NPs which are the prior features that play the focal role regarding the duration in circulation, migration into neoplasm sites for tumor targeting and its firmness to immunotherapy preventing untargeted dispatches,49 see Figure 3. Renal clearance and phagocytosis are the two major ways by which NPs are clear from the body based on their sizes respectively. The covalent attachment nature of polyethylene glycation to the NPs can successfully block the liquidation of the NPs and hence enforce their planned drift.50 Irrespective of the fact that these adjustments can effectively block the liquidation of NPs, they as well influence the rate at which immunotherapies are conveyed to the neoplastic tissues.51 Ligation of precise tumor attracted ligands to the NPs is the best way to avoid dispatches of immunotherapy to untargeted and non-precise cells which can be toxic, so the traits of NPs give a quintessential function in toxicity, pharmaco-kinetics, remedy potential, and bio-distribution of immunotherapy against neoplasm.52 With most malignant’s immunotherapy drugs, the introduction of NPs as a means for delivering the immunotherapies offers several workable outcomes over ordinary cure tactics. The healthcare department are plying the utilization of nano-materials such as NPs in various ways including the conveyance of immunotherapy to tumor districts and also for diagnostic purposes.22 The successful transport of the immunotherapeutic drug encompasses the covalent bonding which exists between the immunotherapy and the NPs. Also, the means by which the NPs convey the drugs to the tumor fields.53 In addition, more hope comes as a result of the periodic migration of the immunotherapy within the blood flow to the precise tumor tissues.54 The defensive surveyance molecules can be provoked by NPs which causes phagocytosis of cells and in regards awaken the surveyance response against neoplasm.55 In evaluation, NPs have been evacuated and are successfully used for the conveyance of vaccines clinically, which relay on MHC-l and MHC-ll in dendrite cells.56 Immunotherapeutic vaccines that focus on dendrite-cells in most tumor regiments are classified specifically into different techniques including those with a bacteria-vector and a non-bacteria vector as well as those based on the cellular line for the conveyance system.57 Because the malignant tissues shelter incorporates molecules which can suppress the defensive system component, this bothers the rate at which the defensive system assaults malignant cells to a greater extent.21 NPs demonstrate beyond reasonable doubt to beautify the ability of the molecules that make up the defensive system by means of calling to action the dendrite cells.58 NPs also can engineer DCs through specific attachments which produce the signals that activate the defensive mechanism to play and eliminate malignancy.
Nanoparticles as Immunotherapy Delivery Vehicle
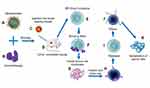
Nanoparticle immunotherapy drug conveyance is an emerging significant department of medicine whereby nanoparticles drug delivery system is helping in the realization of cancer treatment. Nanoscale edifices have at present been created to comprise of two principle segments which include the NPs main component responsible for adhesion and immunotherapeutic drug itself as in Figure 3.59 The immunotherapy in conveyed to neoplasm fields through absorption or surface adhesion. The nano-system immunotherapeutic conveyance strategy is of maximum efficacy to cancer regiments which provide satisfactory results with minimal abnormalities.60 The outstanding performance of the nanoparticles-immunotherapy conveyance system with high preciseness is completely dependent on a few principles that have to be obeyed. Such principles include: the conveyance system should possess the effective and firmly adhesive nature for bonding the immunotherapy61 also, the firmly adhesive complex should be maintained within the circulation and only dispatch at the tumor tissue regions.62 The NP-immunotherapy adhesive molecule should prevent unwanted dispatches of the immunotherapy which can be toxic in the cytoplasm and finally the immunotherapy's precise dispatch should be able to educate the immune defensive system to recognize and eradicate neoplastic cells in the case of reoccurrence.63 The immunotherapy adhesive molecule should be produced from live synthesized molecules which can easily be neutralized by the system and reduce the risk of healthy cell's toxicity.64
Within the previous decade, a range of immunotherapy conveyance systems have been put in play for high precision in clinic and as a therapeutic medium of transport. The NPs that are used to stack immunotherapies possess a surface with bonding sites which are capable of entrapping, engulfing and releasing the immunotherapy, hence preventing degradation and denaturation.65 Therefore, NPs promote increasing and developing interests in the field of cancer immunotherapy treatments.
Transport of Nanoparticle-Immunotherapy Complex to Malignant Cells
When nanoparticles are administrated into the general circulatory system, they will be conveyed to the malignant site by active or passive means. Considering passive conveyance, NPs can make the maximum transport of immunotherapy to the malignant cells because of the understanding of the EPR-impart of malignancy by NPs.22 The nature of the circulatory medium in terms of blood volume and variation in thickness hinders the equilibrium distribution so NPs bonding sites are modified in such a way that they can be easily localized and engulf malignant cells,66 Figure 3F. The malignant cells which have been marked for eradication should have surface properties which can easily be recognized by the NPs engulfing the immunotherapy for precise delivery.67 Paying more attention to malignant cells, the NPs can successfully achieve a substantial delivery of immunotherapy to the malignant cells with a sharp drop in toxicity of non-malignant cells.68 Additionally, findings have likewise demonstrated that various bonding sites (receptors) at the same time can result in nanoparticles showing multivalent qualities and henceforth more grounded cooperation for bonding and conveying of immunotherapy at the tumor cells sites.69 Once conveyed the immunotherapy should be able to completely dissociate from the NPs by erosion as well as degradation or disintegration, Figure 3I. The dispatched immunotherapies struggle for optimal function in eradicating the tumor cells.70 Consequently, there may be a competition among how speedy the immunotherapy gets to the intracellular space, via each energetic (active) delivery system, likewise the talent of the receptor interceded endocytosis, and the way rapidly it will proceed to malignant cells.71 Thus, for an effective outcome to be achieved, the delivery system has to be modified in such a way that the immunotherapeutic carrier gets into the intracellular space before dispatching the immunotherapy.
Specific Delivery of Immunotherapies from the Nanoparticle-Immunotherapy Composite
The outstanding performance of NPs for delivering immunotherapy to precise tumor cells has influenced researchers to work on the mechanism by which the drugs are adhered to the various types of NPs, transported and precisely delivered to targeted tumor cells.69 Several types of NP (Table 3) exist ranging from carbon-based NPs, ceramic NPs, metal NPs, semiconductor NPs, polymeric NPs and lipid-based NPs and have been used for drug delivery. They are classified into different types focusing on the size, morphology, physical and chemical properties of the NPs.72 An outline of NPs for drug conveyancing in malignant tumor cells includes; liposome NPs composing of hydrophilic and hydrophobic which can convey hydrophilic immunotherapies inside the fluid center zone while hydrophobic immunotherapies are inside the hydrophobic district of the bilayer; polymeric NPs are made of polymers and can exemplify hydrophilic and hydrophobic molecules; micelles are made of a hydrophobic monolayer of amphiphilic lipids encasing a hydrophobic center, which can convey hydrophobic anticancer immunotherapies; dendrimers are tediously spread particles comprising of radially symmetric atoms of tree-like arms or branches which can embody hydrophilic and hydrophobic substances; polymersomes are fake vesicles made of a bilayer of engineered amphiphilic block copolymers encasing a hydrophilic center, which convey hydrophilic and hydrophobic immunotherapies; lastly, inorganic NPs are particles shaped by the crystallization of inorganic salts, framing a tridimensional course of action with connected iotas, which can epitomize hydrophilic and hydrophobic and are vesicles made of a hydrophobic bilayer of amphiphilic lipids.53,69,73,74
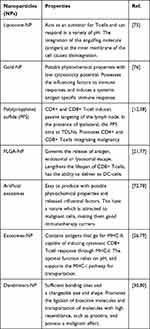 |
Table 3 Most Commonly Used Clinical Nanoparticles |
The Encapsulation Efficiency, Loading Capacity, Stability and Release of Immunotherapy from the Nanoparticle-Immunotherapy Composite
Encapsulation efficiency is the degree of drug that is effectively entangled into the NPs. Encapsulation efficiency (EE%) is determined by (absolute medication added – free non-ensnared drug) divided by the complete medication added.81 Loading capacity is the proportion of drug stacked per unit weight of the NPs, demonstrating the level of mass of the NPs that is due to the embodied drug.82 Loading capacity (LC%) can be determined by the measure of absolute captured drug partitioned by the all-out NP weight. In drug conveyance, yield (given as a percent) is an impression of the measure of drug conveyed per sum epitomized. The incomparable EE%, LC% and stability of NPs engaged them to be used for immunotherapy drug conveyance.83,84
The clearance rate of immunotherapies in the system is solely dependent on the blood circulation and the clearance rate is driven by the renal excretion and the interaction with the reticuloendothelial system of intracellular delivery and release. Once the nanotherapeutics are conveyed to malignant sites, the NPs-immunotherapy-drug composite must dissociate to release the immunotherapy.85 NPs do not only limit the transport of immunotherapy into the cells, they also act as a medium for transporting amino acids and nucleotides into the intercellular space.82 The variation in surface morphology and nature of the NP could help them to actively or passively bind the molecule and transport to the targeted malignant cells. The charge nature of NPs varies which is also an advantage for easy adhesion-dissociation.86 Nevertheless, surface modification of the nanoparticles provides it with stealth-like characteristics, enabling them to have an increased circulation time by limiting the NP’s immunogenicity and inhibiting their recognition and phagocytosis by mononuclear phagocytic cells.87 The used of NPs as a means of delivery comes as a solution for precise delivery which brings about fewer side-effects commonly noticed in chemotherapy. The adhesiveness of immunotherapy to the NPs at a specific recognitive site and dissociation at the malignant sites reduced unwanted targeting and promoted the immunotherapy outcome.88 Thus, smaller nanoparticles are easily cleared through the kidney and larger particles by the reticuloendothelial system. Once the nanoparticles are intracellularly delivered and released, the larger and smaller molecules undergo phagocytosis and endocytosis for their uptake respectively.73,89 The nature and physiochemical traits of the NPs as well as the various integration sites available for bonding contributes greatly to the cellular migration.
Factors Involved in Immunotherapy Release from Nanoparticles
Despite the fact that new approaches for delivering immunotherapy to tumor cells holds a promising future, there are several factors, as elaborated in Table 4, which influence the release of immunotherapy from the NPs into the intracellular space at the precise tumor site.
 |
Table 4 Influencing Factors for Immunotherapy Drug Release |
Important Characteristics and Application of Nanoparticles for Vaccine Delivery
The biological nature of NPs characterize them as good delivery agents of immunotherapy to tumor cells. The overwarming nature and the less or no harmful side-effects to the human system provides additional advantages suitable for vaccine delivery.100 The success of vaccines has been highly improved with the used of a variety of NPs and many more are still in the research process.47 The immunotherapeutic regiment system is made up of the immune response initiators, known as antigen, which can either be in the form of peptide or the nucleotide DNA that codes for the tumor associated antigens.101 The activator, known as the adjuvant, alerts the defensive mechanism if there is an invader or unwanted cell growth and finally the transporter which is highly precise and timely, releases the immunotherapy to evade the invader or neoplasm.102,103 The application of NPs as a transport medium for vaccines is based on their potential such as, preservation of the immunotherapy, the antigen and adjuvants for optimal function in relation to the protection and enabling maximum absorption by the dendrite cells over traditional cancer regiments.104 The NP-delivery system is programmed to influence the defense system positively by activating immunogenic-cells elimination and setting in place the control mechanism that ignites the protective and monitoring system.105 In conclusion, NPs possess flexible properties which can be modified and used based of the requirement of the molecules to be transported.
Improvement of DC-Based Cancer Immunotherapy by NPs
The conveyance immunotherapy by NPs to DCs has been a point of focus for most cancer immunotherapy researchers. The tumor immunity reaction to immunotherapy is solely based on the availability of the drug at the cancer site and it is effective with the use of NPs to present the vaccine for DCs uptake.102,105 When the immunotherapeutic vaccine is conveyed to the dendritic cells in vivo, they are accompanied by antigens/adjuvants which have a similarity to those intracellular cells, thus improving the efficacy as compared to ex vivo with prolonged functional time.106 Furthermore, the physiochemical and biological properties of vaccines focusing on DCs in vivo along with the NPs delivery system and its beautiful nature makes dendritic cell-based immunotherapy a dream come true.
The Challenges and Solution of the NP-Immunotherapy Delivery System
Irrespective of the promising features of the NPs-immunotherapy conveyance system, some demanding situations focusing on in vivo targeting conveyancing of the immunotherapy to malignant cells slows down the full potential of NPs.107 Lots of NPs generally used in the clinic by oncologists are now determined on EPR effect and passive malignant growth aiming for the improvement of immunotherapy efficacy.108 Several side-effects of EPR in xenograft experiments which are more severe than those found in cancer raise a conflict on the usage of EPR and passive targeting.109 The malignant stoma is poorly developed in tumor xenografts hence the limitation in diffusion boundary and patients suffering from malignancy might present low susceptibility to passive tumor concentrated on with the aid of the EPR effect than formerly anticipated in preclinical models. The rate of immunotherapy released, cellular migration and durability do not attend its goals as shown in the xenograft model.110 A good quantity of immunotherapy is expelled without arriving at their targeted point. Therefore, the use of NPs for the conveyance of immunotherapies facilitates the delivery of immunotherapeutic molecules including those insoluble in water and too easily diffused through the cellular membrane for precise delivery.111 Nevertheless, molecules with sustainable sizes ranging from peptides to nucleic acids require a barrier transport mediator for effective uptake and efficacy which is sometimes limited by cellular clearance and by means of phagocytosis.112 When proinflammatory cytokine are conveyed to a portion of APCs, the is a stimulation of a variety of responses which function as organizing and initiating the immune defensive cells to precisely target the malignant cells and evade them out of the body.113 However, the goal of immunotherapy is not limited to malignant cells only but also to activate the defensive cells at the level of the tumor microenvironment (TME). The malignant cells residing at the TME level are reachable with the aid of NPs as the immunotherapy transport medium.114 The phagocytic cells function best for the ability to ingest, and sometimes digest tumor cells. Furthermore, the encapsulation of tumor cells affects TAM functionality, as apoptotic cell uptake promotes macrophage anti-inflammatory functions. Both phagocytosis and efferocytosis affect TAM functionality and how these mechanisms impact on antitumor immunity is being researched.115 A better understanding of NP delivery systems enables researchers to forecast and elaborate on the consequences of cancer immunotherapies on the immune status of the TME. Future cancer immunotherapy treatment can thereby be designed to not only impact directly on tumor cells, but also to favorably modulate TAM phagocytic activity to benefit from the potential of this central immune player to achieve more potent therapeutic efficacy.116
The utilization of numerous drugs in combination has become the essential technique to treat drug resistant tumors. Nonetheless, the organization of combinatorial therapy is restricted by the changing pharmacokinetics of various medications, which brings about conflicting medication take-up and imperfect medication blend at the tumor destinations.117 Customary blend techniques in plan to amplify helpful adequacy dependents on greatest endured portion does not represent the remedial synergism that is delicate to both dosing and booking of different medications. To overcome this, nanoparticle-based blend methodologies against malignant growth drug obstructions including co-encapsulation of drugs with different physicochemical properties, ratiometric control over drug loading, precise delivery, and temporal sequencing on drug release.118 Nanoparticles composed absolutely by three clinically affirmed parts can be utilized for close infrared laser-set off photothermal removal of essential tumors, creating tumor-related antigens, which, within the sight of R837-containing nanoparticles as the adjuvant, can show immunization like capacities.119 NPs can be used for examination or remedial purposes. In examination, they can be used as fluorescent imprints for the disclosure of biomolecules and microorganisms contrast agents in magnetic resonance and other studies. Likewise, NPs can be used for the centered conveyance of drugs, including protein and polynucleotide substances; in photodynamic treatment and warm destruction of tumors, and in prosthetic maintenance.120
Toxicity of Different Types of Nanoparticle Delivery System
The use of NPs in the field of medicine comes with side-effects which can be harmful. The toxicity of the NPs is one of the major challenges. The toxicity of most NPs is based on how they are transported within the fluid circulatory system, the lymphoid flow and the manner by which they are taken into cells and tissues.54 Most of the cells and organs undergo cell and morphology alteration in the presence of the NPs since the NP’s shape, size and surface charger are the principle factors that determine the cellular uptake, disintegration and degradation. If the NPs are not completely degraded, there is a subsequent pile-up at the tissular and organelle level which will eventually become toxic.121 Nevertheless, scientists continue to investigate different techniques of synthesis NPs with low toxicity and less harmful as compared to the tradition tumor immunotherapies. The focus of the routes and ways by which the NPs are managed within the body. The mechanisms by which the human body functions best is well understood and the NPs are produced similarly to those biological molecules within the body which prevent the accumulation since they can easily get degraded.122 So, NPs can be synthesised with minimal toxicity by considering the physiochemical properties and relevant experimental model trials to evaluate and manage the effects on the biological system. Hence, the application of NPs clinically still has a long way to go since lots of researchers are still working on the physiochemical properties of NPs.
Associated Human Health Effects Related to NPs Immunotherapy Delivery System
When the immunotherapy drugs are loaded into the NPs, the carrier-drug composite needs to get into the cell through the membrane by transcytosis or be simply diffused. In summary, the cell membrane needs to be penetrated by the NPs for effective delivery hence causing damage to the membrane; NPs have the ability of altering the process of cell division which is dependent on the constituent and components of the cytoskeleton as well as the transport system affected by the NP delivery system. NPs create an unbalanced energy flow by destroying the mitochondria and altering the cellular metabolism; NPs alter the interface with the formation of lysosomes, thereby hampering autophagy and degradation of macromolecules and triggering cell apoptosis; NPs caused structural changes in membrane proteins thereby disturbing the flow of substances into and out of cells, including intercellular transport and, finally, NPs activate the synthesis of inflammatory mediators by disturbing the normal mechanisms of cell metabolism, tissue and organ metabolism.123–125 The size of NPs permits them to infiltrate through epithelial and endothelial obstructions into the lymph and blood to be conveyed by the circulatory system and lymph stream to various organs and tissues, including the cerebrum, heart, liver, kidneys, spleen, bone marrow and sensory system.126 Nevertheless, the development and application of NPs as immunotherapeutic tools in targeting neoplasm in preclinical has been demonstrated, beyond reasonable doubt, as a strategic approach that holds top notch potential. The in vivo biodistribution, size as well as the pharmacokinetics of the NPs influence the freely lymphatic circulation and also timely and precise delivery to malignant cells.127 Despite the fact that the mechanism ensuring optimal functioning, including dispatches of adjuvants, surface properties and cytotoxicity to healthy cells, are still under study. NPs which might be biocompatible and possess immunotherapeutic adhesive potential needs to be investigated for its ability to stimulate immune defensive cells which includes DCs, T-cells as well as macrophages. The immunological interest and healing efficacy of such NPs and targeting tumor cells with immunotherapy, completely studied would contribute greatly in cancer prevention, vaccines and cancer treatment.128 Combining cancer therapies, such as immuno-chemo therapy as well as other treatment strategies, has proven a favorable synergistic effect on anti-tumor reaction, more efforts are still put in research to further elucidate the connection between NPs enhance immunotherapy and every other remedy routine.129 Also, for NPs enhanced cancer immunotherapy to be effectively translated into the clinic, a variety of types of malignancy need to be evaluated and the potential of NPs and immunotherapy to the tumor type. The defects of other pathways as a results of cancer vaccines have to be causally evaluated.130 Nanoparticle enhancing immunotherapy for the treatment of tumors has an extended way to go for it to be clinically applicable.
Conclusion and Future Perspectives
Enhancing cancer immunotherapy treatment goals by using NP delivery systems is not just a means of treating cancer but also a way by which the immune defensive cells can be educated in such a way that the immune defensive cells possess a memory that can recognize and eradicate malignancy if in any case there is a reoccurrence. The precise and timely delivery of immunotherapy by NPs helps the body to develop a special “immunomemory” which offers protection by recognizing and eliminating reoccurred malignancy or abnormal growth cells for several years after treatment. NPs enhanced cancer immunotherapy has limited toxicity and side-effects unlike chemotherapy that might damage other fast-growing cells, radiotherapy which has a high possibility of damaging other healthy cells within the locality, surgery which removes cells and photothermal therapy (PTT) which is preferred for breast and prostate regimens, because it does not involve the cutting of the skin but has the negativity of killing healthy surrounding cells and failed to prevent a reoccurrence.
Although traditional cancer therapy such as chemotherapy, radiotherapy and surgery are still the most clinically applicable, immunotherapy remains a priority in the days ahead. The advancement of the nanoparticle-based conveyance frameworks as at present utilized may address a monetarily canny and promising alternative treatment method in future. As earlier discussed, we can realize that NP mediated immunotherapy has a great affirmation for neoplasm management. Their significant focal points, for instance, improvement of medication bioavailability and a decrease of the dosing recurrence brings a reason for better administration of the drugs making straightforward means for lasting protection against reoccurrence and effective therapy.131
Another significantly favorable position of the nanoparticles is the reasonableness of the versatile ways of therapy administration such as oral and inhalation. Furthermore, a high strength of the nanoparticles recommends a long timeframe of realistic usability. It tends to the fact that future research will zero in on the improvement of the vectorized conveyance systems combining the focal points of the colloidal transporters, for instance enormous payloads of a medication, with dynamic focusing to the tumor districts.132 Additionally, advancement of imaginative plan advances recommends that nanoparticles can be consolidated into different strong estimation structures which can convey the drugs at the site of activity, protecting their unique properties. These techniques would moreover improve feasibility and practicability of the nanoparticle-based formulations.
Finally, the achievement of this innovation will doubtlessly depend upon the toxicologic issues related with comprehension of the predetermination of nanocarriers and their polymeric constituents in the body, similarly as removal of the peril of the extra regular solvents.
In this regard, the chance of utilizing drug transporters produced using regular polymers such as chitosan or alginate, addresses an appealing viewpoint.
Acknowledgments
The Union Project of Luzhou City and the Southwest Medical University (Nos. 14JC0144, 2018LZXNYD-ZK06) is appreciated for the grants covering this work, the authors wish to thank the members of Department of Oncology and Laboratory of Cancer Research Institute of Southwest Medical University, Luzhou 64600, PRC for stimulating discussions and inspiring research environments for this review.
Author Contributions
All authors contributed to data analysis, drafting or revision of this article, have agreed on the journal to which the article will be submitted, given final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Tsung K, Norton JA. Lessons from Coley’s toxin. Surg Oncol. 2006;15(1):25–28. doi:10.1016/j.suronc.2006.05.002
2. Kramer MG, Masner M, Ferreira FA, Hoffman RM. Bacterial therapy of cancer: promises, limitations, and insights for future directions. Front Microbiol. 2018;9:16. doi:10.3389/fmicb.2018.00016
3. Park JM, Fisher DE. Testimony from the bedside: from Coley’s toxins to targeted immunotherapy. Cancer Cell. 2010;18(1):9–10. doi:10.1016/j.ccr.2010.06.010
4. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi:10.1038/nature10673
5. Le QV, Yang G, Wu Y, Jang HW, Shokouhimehr M, Oh YK. Nanomaterials for modulating innate immune cells in cancer immunotherapy. Asian J Pharm Sci. 2019;14(1):16–29. doi:10.1016/j.ajps.2018.07.003
6. Hussain S. Nanomedicine for treatment of lung cancer. Adv Exp Med Biol. 2016;890:137–147.
7. Zheng DW, Gao F, Cheng Q, et al. A vaccine-based nanosystem for initiating innate immunity and improving tumor immunotherapy. Nat Commun. 2020;11(1):1985. doi:10.1038/s41467-020-15927-0
8. Ribas A. Releasing the brakes on cancer immunotherapy. N Engl J Med. 2015;373(16):1490–1492. doi:10.1056/NEJMp1510079
9. Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62(5):309–335.
10. Lee MH, Liu KH, Thomas JL, Chen JR, Lin HY. Immunotherapy of hepatocellular carcinoma with magnetic PD-1 peptide-imprinted polymer nanocomposite and natural killer cells. Biomolecules. 2019;9(11):651. doi:10.3390/biom9110651
11. Zhang R, Billingsley MM, Mitchell MJ. Biomaterials for vaccine-based cancer immunotherapy. J Control Release. 2018;292:256–276.
12. Zang X, Zhao X, Hu H, Qiao M, Deng Y, Chen D. Nanoparticles for tumor immunotherapy. Eur J Pharm Biopharm. 2017;115:243–256. doi:10.1016/j.ejpb.2017.03.013
13. Wang JJ, Zeng ZW, Xiao RZ, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765–774. doi:10.2147/IJN.S17296
14. Chikan V, McLaurin EJ. Rapid nanoparticle synthesis by magnetic and microwave heating. Nanomaterials (Basel). 2016;6(5):85. doi:10.3390/nano6050085
15. Rane AV, Kanny K, Abitha VK, Thomas S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In: Synthesis of Inorganic Nanomaterials. Woodhead Publishing; 2018:121–139.
16. Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9(1):16–30. doi:10.1021/nn5062029
17. Zhuang J, Holay M, Park JH, Fang RH, Zhang J, Zhang L. Nanoparticle delivery of immunostimulatory agents for cancer immunotherapy. Theranostics. 2019;9(25):7826–7848. doi:10.7150/thno.37216
18. Wang X, Yang L, Chen ZG, Shin DM. Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin. 2008;58(2):97–110. doi:10.3322/CA.2007.0003
19. Taha MS, Cresswell GM, Park J, Lee W, Ratliff TL, Yeo Y. Sustained delivery of carfilzomib by tannic acid-based nanocapsules helps develop antitumor immunity. Nano Lett. 2019;19(11):8333–8341. doi:10.1021/acs.nanolett.9b04147
20. Li J, Lin W, Chen H, Xu Z, Ye Y, Chen M. Dual-target IL-12-containing nanoparticles enhance T cell functions for cancer immunotherapy. Cell Immunol. 2020;349:104042. doi:10.1016/j.cellimm.2020.104042
21. Aikins ME, Xu C, Moon JJ. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc Chem Res. 2020;53(10):2094–2105. doi:10.1021/acs.accounts.0c00456
22. Nasirmoghadas P, Mousakhani A, Behzad F, et al. Nanoparticles in cancer immunotherapies: an innovative strategy. Biotechnol Prog. 2020;e3070. doi:10.1002/btpr.3070
23. De La Rochere P, Guil-Luna S, Decaudin D, Azar G, Sidhu SS, Piaggio E. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018;39(9):748–763. doi:10.1016/j.it.2018.07.001
24. Qu Q, Zhai Z, Xu J, Li S, Chen C, Lu B. IL36 cooperates with anti-CTLA-4 mAbs to facilitate antitumor immune responses. Front Immunol. 2020;11:634. doi:10.3389/fimmu.2020.00634
25. Kepp O, Marabelle A, Zitvogel L, Kroemer G. Oncolysis without viruses - inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020;17(1):49–64. doi:10.1038/s41571-019-0272-7
26. Ichim TE, Zhong Z, Kaushal S, et al. Exosomes as a tumor immune escape mechanism: possible therapeutic implications. J Transl Med. 2008;6:37. doi:10.1186/1479-5876-6-37
27. Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20(5):321–334. doi:10.1038/s41577-019-0269-6
28. Korangath P, Barnett JD, Sharma A, et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer. Sci Adv. 2020;6(13):eaay1601. doi:10.1126/sciadv.aay1601
29. Kaboli PJ, Zhang L, Xiang S, et al. Molecular markers of regulatory T cells in cancer immunotherapy with special focus on Acute Myeloid Leukemia (AML) - a systematic review. Curr Med Chem. 2020;27(28):4673–4698. doi:10.2174/0929867326666191004164041
30. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. doi:10.1038/s41577-019-0210-z
31. Schmid D, Park CG, Hartl CA, et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8(1):1747. doi:10.1038/s41467-017-01830-8
32. Yang WJ, Zhou LJ, Lau J, Hu S, Chen XY. Functional T cell activation by smart nanosystems for effective cancer immunotherapy. Nano Today. 2019;27:28–47. doi:10.1016/j.nantod.2019.05.004
33. Zhu M, Du L, Zhao R, et al. Cell-penetrating nanoparticles activate the inflammasome to enhance antibody production by targeting microtubule-associated protein 1-light chain 3 for degradation. ACS Nano. 2020;14(3):3703–3717. doi:10.1021/acsnano.0c00962
34. Zhang M, Kim JA, Huang AY. Optimizing tumor microenvironment for cancer immunotherapy: beta-glucan-based nanoparticles. Front Immunol. 2018;9:341. doi:10.3389/fimmu.2018.00341
35. Bolli E, O’Rourke JP, Conti L, et al. A virus-like-particle immunotherapy targeting epitope-specific anti-xCT expressed on cancer stem cell inhibits the progression of metastatic cancer in vivo. Oncoimmunology. 2018;7(3):e1408746. doi:10.1080/2162402X.2017.1408746
36. Chiavenna SM, Jaworski JP, Vendrell A. State of the art in anti-cancer mAbs. J Biomed Sci. 2017;24(1):15. doi:10.1186/s12929-016-0311-y
37. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. doi:10.1186/s12943-015-0321-5
38. Yoon HY, Selvan ST, Yang Y, et al. Engineering nanoparticle strategies for effective cancer immunotherapy. Biomaterials. 2018;178:597–607. doi:10.1016/j.biomaterials.2018.03.036
39. Yang F, Shi K, Jia YP, Hao Y, Peng JR, Qian ZY. Advanced biomaterials for cancer immunotherapy. Acta Pharmacol Sin. 2020;41(7):911–927.
40. Wang L, Ma Q, Yao R, Liu J. Current status and development of anti-PD-1/PD-L1 immunotherapy for lung cancer. Int Immunopharmacol. 2020;79:106088. doi:10.1016/j.intimp.2019.106088
41. Zhang E, Xing R, Liu S, Qin Y, Li K, Li P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr Polym. 2019;222:115004. doi:10.1016/j.carbpol.2019.115004
42. Yan S, Zhao P, Yu T, Gu N. Current applications and future prospects of nanotechnology in cancer immunotherapy. Cancer Biol Med. 2019;16(3):486–497. doi:10.20892/j.issn.2095-3941.2018.0493
43. Velpurisiva P, Gad A, Piel B, Jadia R, Rai P. Nanoparticle design strategies for effective cancer immunotherapy. J Biomed (Syd). 2017;2(2):64–77. doi:10.7150/jbm.18877
44. Vigneron N. Human tumor antigens and cancer immunotherapy. Biomed Res Int. 2015;2015:948501. doi:10.1155/2015/948501
45. van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–1017. doi:10.1038/s41565-019-0567-y
46. Tzeng SY, Patel KK, Wilson DR, Meyer RA, Rhodes KR, Green JJ. In situ genetic engineering of tumors for long-lasting and systemic immunotherapy. Proc Natl Acad Sci U S A. 2020;117(8):4043–4052. doi:10.1073/pnas.1916039117
47. Urbanavicius D, Alvarez T, Such GK, Johnston APR, Mintern JD. The potential of nanoparticle vaccines as a treatment for cancer. Mol Immunol. 2018;98:2–7. doi:10.1016/j.molimm.2017.12.022
48. Surendran SP, Moon MJ, Park R, Jeong YY. Bioactive nanoparticles for cancer immunotherapy. Int J Mol Sci. 2018;19(12):3877. doi:10.3390/ijms19123877
49. Qian HQ, Liu BR, Jiang XQ. Application of nanomaterials in cancer immunotherapy. Mater Today Chem. 2018;7:53–64. doi:10.1016/j.mtchem.2018.01.001
50. Park W, Heo YJ, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:24. doi:10.1186/s40824-018-0133-y
51. Bergman PJ. Cancer Immunotherapies. Vet Clin North Am Small Anim Pract. 2019;49(5):881–902. doi:10.1016/j.cvsm.2019.04.010
52. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
53. Caster JM, Callaghan C, Seyedin SN, Henderson K, Sun B, Wang AZ. Optimizing advances in nanoparticle delivery for cancer immunotherapy. Adv Drug Deliv Rev. 2019;144:3–15. doi:10.1016/j.addr.2019.07.009
54. Buss CG, Bhatia SN. Nanoparticle delivery of immunostimulatory oligonucleotides enhances response to checkpoint inhibitor therapeutics. Proc Natl Acad Sci U S A. 2020;117(24):13428–13436. doi:10.1073/pnas.2001569117
55. Banstola A, Jeong JH, Yook S. Immunoadjuvants for cancer immunotherapy: a review of recent developments. Acta Biomater. 2020;114:16–30. doi:10.1016/j.actbio.2020.07.063
56. Bai Y, Wang Y, Zhang X, et al. Potential applications of nanoparticles for tumor microenvironment remodeling to ameliorate cancer immunotherapy. Int J Pharm. 2019;570:118636. doi:10.1016/j.ijpharm.2019.118636
57. Benson Z, Manjili SH, Habibi M, et al. Conditioning neoadjuvant therapies for improved immunotherapy of cancer. Biochem Pharmacol. 2017;145:12–17. doi:10.1016/j.bcp.2017.08.007
58. Leventhal DS, Sokolovska A, Li N, et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat Commun. 2020;11(1):2739. doi:10.1038/s41467-020-16602-0
59. de Andrade LF, Apolinário AC, Rangel-Yagui CO, Stephano MA, Tavares LC. Chitosan nanoparticles for the delivery of a new compound active against multidrug-resistant Staphylococcus aureus. J Drug Deliv Sci Technol. 2020;55.
60. Cheng CT, Castro G, Liu CH, Lau P. Advanced nanotechnology: an arsenal to enhance immunotherapy in fighting cancer. Clin Chim Acta. 2019;492:12–19. doi:10.1016/j.cca.2019.01.027
61. Fan W, Yung B, Huang P, Chen X. Nanotechnology for multimodal synergistic cancer therapy. Chem Rev. 2017;117(22):13566–13638. doi:10.1021/acs.chemrev.7b00258
62. Gao S, Yang D, Fang Y, et al. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics. 2019;9(1):126–151. doi:10.7150/thno.29431
63. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196.
64. Sheng WY, Huang L. Cancer immunotherapy and nanomedicine. Pharm Res. 2011;28(2):200–214. doi:10.1007/s11095-010-0258-8
65. Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine. 2018;14(2):237–246. doi:10.1016/j.nano.2017.10.013
66. Mu Q, Wang H, Zhang M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin Drug Deliv. 2017;14(1):123–136. doi:10.1080/17425247.2016.1208650
67. Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17(4):251–266. doi:10.1038/s41571-019-0308-z
68. Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18(7):553–566. doi:10.1038/s41573-019-0025-4
69. Gupta J, Safdari HA, Hoque M. Nanoparticle mediated cancer immunotherapy. Semin Cancer Biol. 2020;69:307–324. doi:10.1016/j.semcancer.2020.03.015
70. Guevara ML, Persano F, Persano S. Nano-immunotherapy: overcoming tumour immune evasion. Semin Cancer Biol. 2019;69:238–248. doi:10.1016/j.semcancer.2019.11.010
71. Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer. 2019;19(10):587–602. doi:10.1038/s41568-019-0186-9
72. Huang P, Wang X, Liang X, et al. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 2019;85:1–26. doi:10.1016/j.actbio.2018.12.028
73. Hess KL, Medintz IL, Jewell CM. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today. 2019;27:73–98. doi:10.1016/j.nantod.2019.04.005
74. Hashemi V, Farhadi S, Ghasemi Chaleshtari M, et al. Nanomedicine for improvement of dendritic cell-based cancer immunotherapy. Int Immunopharmacol. 2020;83:106446. doi:10.1016/j.intimp.2020.106446
75. Asadi N, Davaran S, Panahi Y, et al. Application of nanostructured drug delivery systems in immunotherapy of cancer: a review. Artif Cells Nanomed Biotechnol. 2017;45(1):18–23. doi:10.1080/21691401.2016.1178136
76. Bae J, Parayath N, Ma W, Amiji M, Munshi N, Anderson KC. BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8(+) cytotoxic T lymphocytes against multiple myeloma: clinical applications. Leukemia. 2020;34(1):210–223. doi:10.1038/s41375-019-0540-7
77. Li X, Wang X, Ito A, Tsuji NM. A nanoscale metal organic frameworks-based vaccine synergises with PD-1 blockade to potentiate anti-tumour immunity. Nat Commun. 2020;11(1):3858. doi:10.1038/s41467-020-17637-z
78. Jo SD, Nam GH, Kwak G, Yang Y, Kwon IC. Harnessing designed nanoparticles: current strategies and future perspectives in cancer immunotherapy. Nano Today. 2017;17:23–37. doi:10.1016/j.nantod.2017.10.008
79. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi:10.1186/s12916-016-0623-5
80. Marwah H, Garg T, Goyal AK, Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016;23(2):564–578. doi:10.3109/10717544.2014.935532
81. Wilkosz N, Lazarski G, Kovacik L, et al. Molecular insight into drug-loading capacity of PEG-PLGA nanoparticles for itraconazole. J Phys Chem B. 2018;122(28):7080–7090. doi:10.1021/acs.jpcb.8b03742
82. Koch PD, Rodell CB, Kohler RH, Pittet MJ, Weissleder R. Myeloid cell-targeted nanocarriers efficiently inhibit cellular inhibitor of apoptosis for cancer immunotherapy. Cell Chem Biol. 2020;27(1):94–104 e105. doi:10.1016/j.chembiol.2019.12.007
83. Kang M, Hong J, Jung M, et al. T-cell-mimicking nanoparticles for cancer immunotherapy. Adv Mater. 2020;32(39):e2003368. doi:10.1002/adma.202003368
84. Rosenblatt KM, Bunjes H. Evaluation of the drug loading capacity of different lipid nanoparticle dispersions by passive drug loading. Eur J Pharm Biopharm. 2017;117:49–59. doi:10.1016/j.ejpb.2017.03.010
85. Vijayan V, Mohapatra A, Uthaman S, Park IK. Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics. 2019;11(10):534. doi:10.3390/pharmaceutics11100534
86. Nam J, Son S, Park KS, Zou WP, Shea LD, Moon JJ. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater. 2019;4(6):398–414. doi:10.1038/s41578-019-0108-1
87. Cho NH, Cheong TC, Min JH, et al. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6(10):675–682. doi:10.1038/nnano.2011.149
88. Scheetz L, Park KS, Li Q, et al. Engineering patient-specific cancer immunotherapies. Nat Biomed Eng. 2019;3(10):768–782. doi:10.1038/s41551-019-0436-x
89. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. doi:10.3322/caac.21596
90. Munakata L, Tanimoto Y, Osa A, et al. Lipid nanoparticles of Type-A CpG D35 suppress tumor growth by changing tumor immune-microenvironment and activate CD8 T cells in mice. J Control Release. 2019;313:106–119. doi:10.1016/j.jconrel.2019.09.011
91. Qian X, Shi Z, Qi H, et al. A novel Granzyme B nanoparticle delivery system simulates immune cell functions for suppression of solid tumors. Theranostics. 2019;9(25):7616–7627. doi:10.7150/thno.35900
92. Abd-Allah H, Abdel-Aziz RTA, Nasr M. Chitosan nanoparticles making their way to clinical practice: a feasibility study on their topical use for acne treatment. Int J Biol Macromol. 2020;156:262–270. doi:10.1016/j.ijbiomac.2020.04.040
93. Ding RL, Xie F, Hu Y, et al. Preparation of endostatin-loaded chitosan nanoparticles and evaluation of the antitumor effect of such nanoparticles on the Lewis lung cancer model. Drug Deliv. 2017;24(1):300–308. doi:10.1080/10717544.2016.1247927
94. Yan Y, Ding H. pH-responsive nanoparticles for cancer immunotherapy: a brief review. Nanomaterials (Basel). 2020;10(8):1613. doi:10.3390/nano10081613
95. Dey A, Manna S, Kumar S, Chattopadhyay S, Saha B, Roy S. Immunostimulatory effect of chitosan conjugated green copper oxide nanoparticles in tumor immunotherapy. Cytokine. 2020;127:154958. doi:10.1016/j.cyto.2019.154958
96. Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61(2):91–112. doi:10.3322/caac.20102
97. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi:10.1038/ncomms13193
98. Xu J, Xu L, Wang C, et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11(5):4463–4474. doi:10.1021/acsnano.7b00715
99. Ni K, Luo T, Nash GT, Lin W. Nanoscale metal-organic frameworks for cancer immunotherapy. Acc Chem Res. 2020;53(9):1739–1748. doi:10.1021/acs.accounts.0c00313
100. Park YM, Lee SJ, Kim YS, et al. Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw. 2013;13(5):177–183. doi:10.4110/in.2013.13.5.177
101. Fusciello M, Fontana F, Tahtinen S, et al. Artificially cloaked viral nanovaccine for cancer immunotherapy. Nat Commun. 2019;10(1):5747. doi:10.1038/s41467-019-13744-8
102. O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–167.
103. Sengupta S. Cancer nanomedicine: lessons for immuno-oncology. Trends Cancer. 2017;3(8):551–560. doi:10.1016/j.trecan.2017.06.006
104. Kim JY, Kim YJ, Kim JS, et al. Adjuvant effect of a natural TLR4 ligand on dendritic cell-based cancer immunotherapy. Cancer Lett. 2011;313(2):226–234. doi:10.1016/j.canlet.2011.09.009
105. Shi GN, Zhang CN, Xu R, et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials. 2017;113:191–202. doi:10.1016/j.biomaterials.2016.10.047
106. Lizotte PH, Wen AM, Sheen MR, et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016;11(3):295–303. doi:10.1038/nnano.2015.292
107. Saleh T, Shojaosadati SA. Multifunctional nanoparticles for cancer immunotherapy. Hum Vaccin Immunother. 2016;12(7):1863–1875. doi:10.1080/21645515.2016.1147635
108. Moyer TJ, Kato Y, Abraham W, et al. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat Med. 2020;26(3):430–440. doi:10.1038/s41591-020-0753-3
109. Min Y, Roche KC, Tian S, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12(9):877–882. doi:10.1038/nnano.2017.113
110. Gao J, Wang WQ, Pei Q, Lord MS, Yu HJ. Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol Sin. 2020;41(7):986–994. doi:10.1038/s41401-020-0400-z
111. Fontana F, Liu D, Hirvonen J, Santos HA. Delivery of therapeutics with nanoparticles: what’s new in cancer immunotherapy? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1):e1421. doi:10.1002/wnan.1421
112. Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection–possibilities for cancer treatment. Anticancer Drugs. 2016;27(4):269–277. doi:10.1097/CAD.0000000000000337
113. Perry JL, Tian S, Sengottuvel N, et al. Pulmonary delivery of nanoparticle-bound toll-like receptor 9 agonist for the treatment of metastatic lung cancer. ACS Nano. 2020;14(6):7200–7215. doi:10.1021/acsnano.0c02207
114. Ou W, Thapa RK, Jiang L, et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J Control Release. 2018;281:84–96. doi:10.1016/j.jconrel.2018.05.018
115. Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904.
116. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi:10.1038/nm.3909
117. Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1104–1111. doi:10.1016/j.bcp.2012.01.008
118. Xie Z, Fan T, An J, et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem Soc Rev. 2020;49(22):8065–8087. doi:10.1039/D0CS00215A
119. Tao W, Ji X, Zhu X, et al. Two-dimensional antimonene-based photonic nanomedicine for cancer theranostics. Adv Mater. 2018;30(38):e1802061. doi:10.1002/adma.201802061
120. Luo M, Fan T, Zhou Y, Zhang H, Mei L. 2D black phosphorus–based biomedical applications. Adv Funct Mater. 2019;29(13):1808306. doi:10.1002/adfm.201808306
121. Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res Lett. 2018;13(1):44. doi:10.1186/s11671-018-2457-x
122. Xie Z, Peng M, Lu R, et al. Black phosphorus-based photothermal therapy with aCD47-mediated immune checkpoint blockade for enhanced cancer immunotherapy. Light Sci Appl. 2020;9:161. doi:10.1038/s41377-020-00388-3
123. Qiu M, Singh A, Wang D, et al. Biocompatible and biodegradable inorganic nanostructures for nanomedicine: silicon and black phosphorus. Nano Today. 2019;25:135–155. doi:10.1016/j.nantod.2019.02.012
124. Tao W, Ji X, Xu X, et al. Antimonene quantum dots: synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew Chem Int Ed Engl. 2017;56(39):11896–11900. doi:10.1002/anie.201703657
125. Unsoy G, Gunduz U. Smart drug delivery systems in cancer therapy. Curr Drug Targets. 2018;19(3):202–212. doi:10.2174/1389450117666160401124624
126. Tang Z, Kong N, Ouyang J, et al. Phosphorus science-oriented design and synthesis of multifunctional nanomaterials for biomedical applications. Matter. 2020;2(2):297–322. doi:10.1016/j.matt.2019.12.007
127. Naskar S, Sharma S, Kuotsu K. Chitosan-based nanoparticles: an overview of biomedical applications and its preparation. J Drug Deliv Sci Technol. 2019;49:66–81.
128. Orange M, Reuter U, Hobohm U. Coley’s lessons remembered: augmenting mistletoe therapy. Integr Cancer Ther. 2016;15(4):502–511. doi:10.1177/1534735416649916
129. Wang C, Beiss V, Steinmetz NF, Simon AE. Cowpea mosaic virus nanoparticles and empty virus-like particles show distinct but overlapping immunostimulatory properties. J Virol. 2019;93(21). doi:10.1128/JVI.00129-19
130. Patyar S, Joshi R, Byrav DS, Prakash A, Medhi B, Das BK. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17(1):21. doi:10.1186/1423-0127-17-21
131. Carlson RD, Flickinger JC, Snook AE. Talkin’ toxins: from Coley’s to modern cancer immunotherapy. Toxins (Basel). 2020;12(4):241. doi:10.3390/toxins12040241
132. DeBerardinis RJ, Phimister EG. Tumor microenvironment, metabolism, and immunotherapy. N Engl J Med. 2020;382(9):869–871. doi:10.1056/NEJMcibr1914890
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.