Back to Journals » International Journal of Nanomedicine » Volume 13
Enhancing anticancer effects, decreasing risks and solving practical problems facing 3-bromopyruvate in clinical oncology: 10 years of research experience
Authors El Sayed SM
Received 9 April 2018
Accepted for publication 8 June 2018
Published 15 August 2018 Volume 2018:13 Pages 4699—4709
DOI https://doi.org/10.2147/IJN.S170564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Video abstract presented by Salah Mohamed El Sayed.
Views: 942
Salah Mohamed El Sayed1,2
1Department of Clinical Biochemistry and Molecular Medicine, Taibah College of Medicine, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia; 2Department of Medical Biochemistry, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt
Abstract: 3-Bromopyruvate (3BP) is a promising powerful general anticancer agent. Unfortunately, 3BP release faces many practical and biochemical problems in clinical human oncology, for example, 3BP induces burning venous sensation (during intravenous infusion) and rapid inactivation by thiol groups of glutathione and proteins. 3BP exhibits resistance in glutathione-rich tumors without being able to exert selective targeting. 3BP does not cross the blood–brain barrier and cannot treat nervous system tumors. Importantly, 3BP cannot persist in tumor tissues due to the phenomenon of enhanced permeability and retention effect. Here, the author presents the practical solutions for clinical problems facing 3BP use in clinical oncology, based on over 10 years of experience in 3BP research. Crude (unformulated 3BP that is purchased from chemical companies without being formulated in liposomes or other nanocarriers) should not be administered in clinical oncology. Instead, 3BP is better formulated with liposomes, polyethylene glycol (PEG), PEGylated liposomes (stealth liposomes) or perillyl alcohol that are used currently with many chemotherapeutics for treating clinical tumors in cancer patients. Formulating 3BP with targeted liposomes, for example, with folate, transferrin or other ligands, improves tumor targeting. Formulating 3BP with liposomes, PEG, stealth liposomes or perillyl alcohol may improve its pharmacokinetics, hide it from thiols in the circulation, protect it from serum proteins and enzymes, prevent burning sensation, prolong 3BP’s longevity and facilitate crossing the BBB. Formulating 3BP with stealth liposomes protects 3BP from the reticuloendothelial cells. Liposomal 3BP formulations may retain 3BP better inside the relatively large tumor capillary pores (abolish enhanced permeability and retention effect) sparing normal tissues, facilitate new delivery routes for 3BP (eg, topical and intranasal 3BP administration using perillyl alcohol) and improve cancer cytotoxicity. Formulating 3BP may be promising in overcoming many obstacles in clinical oncology.
Keywords: 3-bromopyruvate, 3BP release, PEG formulation, practical problems, liposomes and targeting cancer
Introduction
3-Bromopyruvate (3BP) is a promising general anticancer agent that kills almost all types of cancer cells1 through targeting many critical points in cancer biology.2 3BP proved effective in treating different human cancers.1 3BP is a marvelous killer of cancer cells in vitro in cell culture (two-dimensional [2D] model) at low micromolar concentrations that usually do not harm normal cells.3 Moreover, 3BP is an antimetabolite owing to its structural similarity to acetate (precursor of lipogenesis), pyruvate (precursor of Krebs cycle) and lactate (Warburg effect), as shown in Figure 1A, which confers numerous criteria to cancer biology, chemoresistance and the unique biological features of tumors distinguishing them from normal cells.3,5 Cancer cells exhibit characteristic metabolic and biological features that distinguish them from normal cells (Table 1). Cancer cells are characterized by hyperglycolysis, overexpression of glucose transporters and monocarboxylate transporters, overexpression of hexokinase II3–7 and decreased antioxidant power that may help designing effective targeted cancer therapy.
  | Table 1 Key metabolic and biological differences between normal cells and cancer cells |
Antiangiogenic effects of 3BP5 and inhibition of adenosine triphosphate-binding cassettes6 cooperate to terminate tumor chemoresistance and radioresistance. The most exciting report about 3BP was the safe eradication of all experimental hepatoma tumors in rats, while non-treated tumor-bearing rats suffered badly.7 Unfortunately, the clinical practice of using 3BP in treating human patients2 does not go side by side with these amazing results gained when treating 2D cultured cancer cells or experimental xenograft tumors in small animals, for example, rats and mice,5 in vitro 3D spheroids, clonogenic assays or anchorage-independent growth assays using crude (unformulated) 3BP powder.4–5 Lack of promising anticancer effects of 3BP in clinical oncology may possibly be due to accelerated glutathione (GSH)-induced 3BP metabolism, attachment to serum proteins and binding to thiol (SH)-rich molecules, eg, GSH, that may neutralize the whole delivered 3BP dose.1 Importantly, the small molecular size of 3BP (Figure 1A), in addition to the possible presence of some biological differences between human tumors and experimental xenograft tumors may not allow 3BP to remain well inside the tumor tissues. Based on over 10 years of researcher’s experience with 3BP chemistry, pharmacology and cancer research in 2D models, animals and human studies, the author tries to give some practical solutions to many clinical problems that may hinder achieving an utmost benefit of 3BP in clinical oncology.
Practical problems and obstacles faced when using 3BP in clinical oncology
Many major obstacles are faced with the clinical use of crude (unformulated) 3BP as a practical chemotherapeutic agent in modern oncology in human patients (Table 2). These obstacles include: 3BP-induced burning sensation at veins (during 3BP intravenous infusion),2 rapid attachment of 3BP to GSH and serum proteins at the thiol groups,1 lack of early response to 3BP in some cases,2 resistance of some cancer cells rich in GSH to 3BP8 and lack of 3BP-induced targeting the tumor tissues. Moreover, excess tumor lactate (Warburg effect) may antagonize 3BP (due to structural antagonism), in addition to the probable inability of 3BP to diffuse massively through tumor tissues. Moreover, 3BP cannot persist inside the tumors (possibly due to the phenomenon of enhanced permeability and retention [EPR] effect).9 Importantly, unformulated 3BP is not suitable for treating nervous system tumors as 3BP does not cross the blood–brain barrier (BBB).10 Unfortunately, the small molecular size of chemotherapeutic drugs (eg, 3BP) may impair 3BP retention in tumor tissues.9 However, 3BP toxicity is easier to control (compared to many other chemotherapeutics) using thiols, for example, N-acetyl L-cysteine or GSH.1 Finally, combinations of 3BP with other anticancer agents need to be optimized as the author discusses here.
Solving the problem: “lack of early response to 3BP in many cases”
Unfortunately, the strong amazing cytotoxic effects encountered on using 3BP in 2D models of cell culture are not the same results obtained on treating many human tumors. This urged the author to search for the causes and possible treatment lines (Table 2). The distance from venous administration (infusion) site of 3BP till the target tumor sites to be reached is relatively long. 3BP in serum comes in contact with serum proteins where thiol groups may attach with 3BP and abolish its anticancer effects.1 Metabolism of 3BP through conjugation to GSH followed by 3BP inactivation was recently reported by the author, which was supported by dozens of published reports.1 For treating this problem, coating 3BP in liposomes or in polyethylene glycol (PEG) produces PEGylated 3BP (PEG-3BP; Figure 1B) that may act as a barrier to prevent direct 3BP attachment to serum proteins (Table 3).
In the majority of cases, 3BP is expected to give satisfactory cytotoxic oxidative effects at the cellular level as many cancer cells are sensitive to 3BP-induced oxidant effects.11 However, some tumor types and cancer cells rich in GSH may exhibit resistance to 3BP.8 In addition, 3BP may not do well in the presence of high GSH content in some cancer cells. To solve this problem clinically, patients should take safe, approved GSH-depleting agents, for example, paracetamol2 or ethacrynic acid (diuretic), prior to taking 3BP. Alternatively, PEG-3BP may prevent a large fraction of 3BP from being consumed in conjugation with thiol groups (in proteins and GSH), and this consequently prolongs the longevity of 3BP.
Solving the problem: “EPR effect”
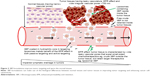
At the tumor itself, 3BP may not be able to go through the tumor tissue and cells possibly due to the heterogeneous nature of tumor biology, for example, high vascular porosity, wide tumor vascular gaps and leakiness that collectively expel chemotherapeutics outside the tumors, that is, the EPR effect (Figure 2).9
Being a small molecule, 3BP may not only enter into the tumor tissues easily, but also gets out easily due to enhanced permeability. Retention of 3BP inside the tumor tissue may not be achieved to get the desired cytotoxic effects (Table 2). The author suggests that this issue can be simply solved by coating 3BP in liposomes or PEGylated liposomes (PEG-3BP) to increase the size and or molecular weight of the delivered 3BP to the tumor site, in order to allow for better retention of 3BP inside the tumors (Figure 1B). Stealth liposomal drug formulations were reported to be helpful to augment targeting and cytotoxicity of currently used chemotherapeutics, for example, doxorubicin.12 The author recommends stealth liposomes to be applied to 3BP through enclosing 3BP in liposomes coated with PEG (Figure 1B).
Some people may argue that 3BP is better delivered through direct intratumoral injection. This is not enough as cancer is a systemic disease rather than a localized tumor mass. Systemic 3BP delivery is vital for cancer therapy where intratumoral 3BP injection is never a substitute for that. Moreover, EPR effect may limit the therapeutic benefits of intratumoral 3BP injection.
Solving the problem: “3BP may not be powerful against some human tumors and 3D models”
The author’s experience (in Japan) using unformulated 3BP as an anticancer treatment in 3D culture models (spheroid growth, anchorage-independent growth assay and clonogenic assay) supported the strong cytotoxic effects of 3BP in preventing the growth of spheroids and colonies, provided that 3BP is added early after culturing the cancer cells (before colony formation starts).4,5 However, the author noticed that if 3D models were allowed to grow in 3BP-free conditions (for a few days or weeks) till they reach a well-formed 3D configuration, daily treatment using crude 3BP was not effective (unpublished data) and could not be compared to adding 3BP to 2D cell culture models or adding 3BP immediately after culturing cancer cells to delay the growth of cancer cell colonies. Despite daily continuous addition of 3BP in the culture medium around 3D spheroids and colonies, growth of spheroids and colonies continued to progress slowly and no massive damage to cancer cells was noted compared to the rapid morphological damage noted in cultured 2D glioma cells. The author may conclude that the 3D configuration in tumor spheroids and colonies may not allow crude 3BP to reach the cells successfully wholly, as occurring in cultured 2D cells (that were cultured in 3BP-containing cell culture medium). Unfortunately, treating well-formed 3D spheroids and colonies using crude unformulated 3BP may predict a similar outcome to clinical human tumors.
Based on this, majority of the practical problems faced when using 3BP in clinical oncology are related to 3BP delivery, distribution and targeting, that is, improving 3BP delivery may preserve 3BP’s molecular structure, while improving 3BP’s distribution and targeting may improve the therapeutic outcomes (Figure 2). According to the author, all these problems may be solved upon formulating 3BP with liposomes or other suitable nanocarriers (Table 2).
Liposomes are lipid bilayered nanospheres used as drug delivery systems (Figure 1B). Liposomes are attractive drug carriers due to their efficiency, biocompatibility, low toxicity, non-immunogenicity, enhanced solubility of the carried chemotherapeutic agents and their ability to encapsulate a large number of drugs.13 Liposomes can encapsulate hydrophilic drugs (as 3BP) and hydrophobic drugs, improve the pharmacokinetic and pharmacodynamic profiles of the therapeutic payload, and promote controlled and sustained release of drugs.12,13 Advantages of liposomal drug formulations may also be applied to 3BP and include preferential drug accumulation, higher drug concentration at the tumor sites, enhanced 3BP retention effect, better intracellular uptake and stability, and improved drug biodistribution and pharmacokinetics (Table 2). In addition to increasing the therapeutic efficacy and lowering the toxicity,14 stability of 3BP and its ability to cross the cell membrane may dramatically improve upon using liposomal 3BP formulations. Recently, liposomal 3BP formulations did well in treating tumor spheroids.14
Importantly, cationic liposomes may not be preferable for formulating crude 3BP as the positive surface charge of cationic liposomes causes nonspecific interactions with anionic blood components, resulting in possible rapid 3BP clearance from the circulation by the reticuloendothelial system.15 Moreover, inclusion of fusogenic lipids in liposomal structure (Table 3) helps in releasing the liposomal cargo (eg, 3BP) at the acidic pH in tumors.16 Glick et al reported that the first-order decay rate of 3BP at physiological temperature and pH has a half-life of only 77 minutes, and that decreasing buffer pH decreases the decay rate.17 Application of these findings depends on the safety issue and biodistribution of 3BP. The relatively short half-life of 3BP confers treatment safety, where the drug is catabolized early without being retained in body tissues. Also, the relatively longer half-life of 3BP at acidic pH is quite suitable for a longer effect and retention at the acidic tumor microenvironment, provided that 3BP is adequately delivered inside the tumor tissues. Interestingly, imaging agents allow visualization of distribution of nanoparticles (eg, liposomes).18
Solving the problem: “3BP-induced burning venous sensation and phlebitis”
3BP proved effective and safe in many in vivo animal studies. Human studies regarding using 3BP for treating malignancies are very few. A prior patient consent, patient orientation and education about 3BP anticancer effects and possible side effects, in addition to ethical committee approval, are quite mandatory. Using same ethical guidelines, administration of 3BP to healthy volunteers under strict medical supervision and support is strongly required to encourage 3BP use in clinical practice. This also seals the gap regarding the biological differences and response to treatment between the animal studies and patients’ treatment.
The author and co-researchers followed the strict previously mentioned guidelines (patient’s consent, ethical committee approval, patient orientation and medical supervision) to report the single published case study using unformulated 3BP to treat a patient having metastatic melanoma.2 3BP-induced burning sensation at the veins during intravenous infusion was reported to be quite intolerable to the extent that forced reducing the infusion rate or changing the infusion site to another vein.2 This is expected to be more problematic if 3BP is tried to be given through direct intravenous bolus injection. Considering that 3BP is an alkylating agent1,2,7,14 and an acid in solution, burning sensation may be due to 3BP-induced alkylation or 3BP-induced strong acidity. Slow intravenous 3BP infusion was accompanied with mild burning sensation that decreased by slowing the rate of 3BP infusion.2 This concludes that 3BP is quite difficult to be administered through intravenous injection. There may be arguments that 3BP was safely given to animals intravenously. However, assuming that the calculated dose of 3BP is 1 mg/kg/mL, the given volume of solvent (eg, saline) containing 3BP to animals (<2 kg body weight) will be <2 mL. This is quite smaller compared to the volume of solvent (sterile saline or water for injection) that should be given to humans weighing >70 kg body weight (>70 mL). For that, direct intravenous injection of more concentrated 3BP (eg, 70 mg dissolved in 3 mL saline or sterile water) seems to be quite intolerable due to the expected burning sensation. Phlebitis may be encountered with prolonged and/or increased dose of 3BP administration (via intravenous infusion). As a healthy human volunteer who was administered 3BP infusion (with other volunteers from different countries), the author experienced intolerable burning sensation at the vein. Dose administered was 1 mg/kg dissolved in isotonic saline. Usually, veins receiving 3BP infusion become unsuitable for subsequent 3BP administration. A similar picture was reported previously in a melanoma patient.2
Some research ideas may suggest neutralizing 3BP with sodium bicarbonate to get a suitable pH (pH 7.4) to prevent any 3BP-induced acidity or burning sensation. According to the author, a possible chemical reaction (neutralization) between the acidic bromopyruvic acid (3BP) and sodium bicarbonate may occur with possible disruption of the molecular structure of 3BP. In earlier studies, Ko et al dissolved unformulated crude 3BP in diluted PBS (1X). The solutions of 3BP (Sigma-Aldrich Co., St Louis, MO, USA) were prepared in PBS adjusted to pH 7.0–7.5, wherein the 3BP solutions were sterilized using Millexâ GV (EMD Millipore, NY, USA) 0.22 mm filter unit and used immediately. Freshly prepared solutions were used in all the reported studies.7 According to the author, 3BP should never be subjected to alkalies during dissolution in order to preserve its chemical structure stability for better anticancer effects.
A possible practical solution (given by the author) that preserves 3BP chemical structure and treats this problem is formulating 3BP with liposomes or enclosing 3BP in PEG (Table 2). This may prevent the alkylation effects (burning sensation) exerted by 3BP. This may also confer longevity to 3BP by masking it from thiol groups in serum proteins, tissue proteins and GSH. Moreover, PEGylation masks drugs (eg, 3BP; Figure 1A) from being taken up by the reticuloendothelial system.19 PEG molecules confer a protective hydrophilic layer on liposomal surface that prevents their aggregation and interaction with the blood components.20 Drugs (eg, 3BP) PEGylation may prevent opsonization of chemotherapeutics21 by shielding 3BP surface charge, enhancing the repulsive interaction between polymer-coated liposomes (containing 3BP) and blood components, increasing surface hydrophilicity and forming a polymeric layer over the liposomal surface (Figure 1B).
Solving the problem: “It is not possible to give an intravenous bolus injection of 3BP”
Coating 3BP in liposomes (3BP liposomes) or PEG (PEG-3BP) or both (PEGylated 3BP liposomes; Figure 1B) may keep 3BP away from unnecessary exposure to serum proteins, serum thiols (eg, GSH), serum enzymes and other molecules that may neutralize 3BP, consume a significant fraction of the delivered 3BP dose or reduce its potency. Moreover, conjugating 3BP to natural terpenes, for example, perillyl alcohol, grants the benefit of preserving a large fraction of 3BP from being consumed in chemical attachments to thiol groups of GSH and serum proteins (Tables 2 and 3). Collectively, such 3BP formulation may be promising in facilitating giving a safe non-painful 3BP bolus intravenous injection.
Solving the problem: “3BP does not cross the BBB”
Crossing the BBB is a prerequisite for effective chemotherapy of nervous system tumors. Unfortunately, 3BP does not cross the BBB.10 However, 3BP proved effective in killing glioma cells and aggressive glioblastoma cells in vitro and also in xenograft animal models (ectopic animal models) where no BBB exists.4,5 3BP was effective in treating gliomas4,5 (Table 4). To date, no study or report proved the effectiveness of crude 3BP in treating experimental gliomas implanted in the brain (orthotopic tumor model). As 3BP does not cross the BBB, the author suggests a possible solution by enclosing 3BP in liposomes (can cross the BBB).
  | Table 4 Optimal delivery of 3BP to special sites and chosen types of tumors |
Solving the problem: “lack of selective targeting of 3BP to tumor tissues”
Administering crude 3BP (without liposomal formulations) through intravenous infusion deprives 3BP of selective tumor targeting (Table 3) as 3BP may be distributed to all tissues (apart from central nervous system). However, liposomal 3BP formulations may settle better in the pores and abnormal vasculature of the tumor tissues. Liposomal 3BP particles, PEGylated 3BP or PEGylated liposomal 3BP particles (stealth liposomes; Figure 1B) may not be retained well at the normal tissue sites (do not exhibit EPR effect) compared to a better retention at the tumor tissues (Figure 2). Liposomal drug delivery is approved for human use in many current chemotherapeutic medications (Tables 2 and 3).
Actively targeted liposomes may minimize 3BP off-target effects (Table 3). Actively targeted liposomal delivery systems are prepared by conjugating targeting ligands (eg, folate and transferrin), peptides and monoclonal antibodies to the liposomal surface.22 Folate and transferrin receptors are overexpressed in many cancer cells and have been used to make liposomes more specific to tumor tissues21 (Table 3).
Solving the problem: “precautions regarding 3BP combinations with supportive treatments”
3BP is well-known to exert potent oxidative stress-induced cytotoxicity in different tumor cells, which needs strict precautions. No antioxidant medications should be allowed to be taken with 3BP to avoid abolishing its cytotoxic effects. Antioxidant vitamins (E, A and C) are better not to be co-administered with 3BP, in order to allow for better antitumor effects. These can be given a few hours after 3BP delivery to rescue the normal cells from 3BP-induced oxidative damage. Proteins and peptides (eg, albumin infusion) should never be co-administered with 3BP or given immediately before or after 3BP treatment. Thiol groups of cysteine amino acids (in proteins or GSH) attach permanently to 3BP, inducing its inactivation.1 In patients having hypoproteinemia or hypoalbuminemia necessitating albumin infusion, protein infusion should be given about 8 hours of 3BP administration. Formulating 3BP in liposomal carriers, PEG or PEGylated (stealth) liposomes may preserve a relatively large fraction of administered 3BP from being consumed in unnecessary attachments to serum proteins (Table 2).
Solving the problem: “3BP intoxication and special delivery conditions to certain tumors”
Many previous reports confirmed the author’s conclusion that 3BP metabolism occurs through permanent attachment to thiol groups of GSH, N-acetyl L-cysteine (NAC) and proteins. If a large dose of 3BP was administered to patients and/or 3BP-induced toxicity appeared, GSH should be administered immediately.1 However, some tumors may have a special nature that may warrant using special delivery methods of 3BP for better penetration of tumor cells (Table 4).
Solving the problem: “lack of effective topical 3BP cream and ointment”
Cancer patients may need topical anticancer agents in case of malignant wounds, inoperable fungating breast cancers, superficial malignant skin ulcers, ulcerating superficial tumors, some gastrointestinal tract tumors (rectal cancer) and others. Applying locally dissolved 3BP in watery solutions is quite impractical due to the rapid escape of 3BP solutions away from the tumor cells. Lack of adequate contact time between 3BP and the tumor tissue, in addition to the possibility of unwanted binding of locally administered 3BP to tissue proteins in the tumor tissue, may hinder or prevent 3BP-induced anticancer effects. Hence, the need for topical long-lasting contact of 3BP to local sites tissue arises (Table 4). Manufacturing topical creams or ointments containing liposomal 3BP formulations is promising to potentiate the cytotoxic effects exerted by systemic administration of 3BP. Combination of 3BP and citric acid in topical creams or ointments may be promising in potentiating such cytotoxicity.
Nagoba et al suggested that mixing 3 g of citric acid with 100 g of white soft paraffin (100%) pure petroleum jelly was effective as a local chemotherapy.23 Same thing may be done with 3BP (as a crude powder or liposomal 3BP), taking into account the author’s experience that both citric acid powder and crude 3BP powder have the same degree of solubility in water. Based on this similarity between 3BP and citric acid, mixing 3 g of crude 3BP (or liposomal 3BP) with 100 g of white soft paraffin (100%) pure petroleum jelly in a mortar (in a complete aseptic sterile atmosphere) may be promising to get a local 3BP formulation (Table 4).
3BP combinations with other chemotherapeutics in treatment protocols
3BP may be used as a monotherapy or as a part of a combination therapy in oncology protocols. When combining 3BP with other chemotherapeutics, it is better to revise the literature to select the best reported drugs.1 Citrate was reported to intensify 3BP cytotoxic effects against cancer cells.4 However, citrate can be given orally, but not intravenously. Citric acid (better than sodium citrate) is a natural acid present in oranges and fruits. Its anticancer effects were confirmed in previous reports possibly due to citrate-induced inhibition of the glycolytic enzyme phosphofructokinase.4 Adding oral citric acid to intravenous 3BP formulations may be promising in treating gastrointestinal cancer, in which 3BP causes systemic cytotoxicity to the tumor cells where citric acid causes local cytotoxicity.
Conclusion
Formulating 3BP may be promising in overcoming many obstacles in clinical oncology.
Acknowledgment
The author is grateful to professor Kiyoshi Fukui (the prominent well-known scientist in D-amino acid oxidase research), Division of Enzyme Pathophysiology, The Institute for Enzyme Research, The University of Tokushima, Japan. Professor Kiyoshi Fukui was kind and patient in teaching the author how to write a scientific paper and how to do scientific research in his highly prestigious laboratory with the help of his excellent and co-operative Japanese and international staff members. Under kind patronage and support by Professor Kiyoshi Fukui, three papers in 3-bromopyruvate research were published. The author expresses that he can never reward the favor of professor Kiyoshi Fukui for his patience, support and endless help. The author is grateful to The University of Tokushima, the Japanese people and Japan for the scientific make-up that enabled him to write this article. The author is also grateful to Dr Stephen Strum (the highly knowledgeable board certified American oncologist), Dr Orn Adalsteinsson and Dr Jagadish for their fruitful scientific discussions that benefited a lot in 3BP research. The author is also so grateful to Taibah University, Saudi Arabia for allowing a helpful environment to conduct this study. The author is very grateful to Obaidi, Mr Raed Ali Al-Raddadi, Mr Sultan Al-Hussini, Mr Mohamed Abdelsamad and Mr Wael Barakat from the administrative department, College of Medicine, Taibah University for their technical help and support to this work.
The author declares that there is no financial or non-financial competing interests with any other partner. There is no financial benefit. The article is fully supported by the author.
Disclosure
The author reports no conflicts of interest in this work.
References
El Sayed SM, Baghdadi H, Zolaly M, Almaramhy HH, Ayat M, Donki JG. The promising anticancer drug 3-bromopyruvate is metabolized through glutathione conjugation which affects chemoresistance and clinical practice: An evidence-based view. Med Hypotheses. 2017;100:67–77. | ||
El Sayed SM, Mohamed WG, Seddik MA, et al. Safety and outcome of treatment of metastatic melanoma using 3-bromopyruvate: a concise literature review and case study. Chin J Cancer. 2014;33(7):356–364. | ||
Lis P, Dyląg M, Niedźwiecka K, et al. The HK2 dependent “Warburg effect” and mitochondrial oxidative phosphorylation in cancer: targets for effective therapy with 3-bromopyruvate. Molecules. 2016;21(12):E1730. | ||
El Sayed SM, El-Magd RM, Shishido Y, et al. 3-Bromopyruvate antagonizes effects of lactate and pyruvate, synergizes with citrate and exerts novel anti-glioma effects. J Bioenerg Biomembr. 2012;44(1):61–79. | ||
El Sayed SM, El-Magd RM, Shishido Y, et al. D-Amino acid oxidase-induced oxidative stress, 3-bromopyruvate and citrate inhibit angiogenesis, exhibiting potent anticancer effects. J Bioenerg Biomembr. 2012;44(5):513–523. | ||
Wu L, Xu J, Yuan W, et al. The reversal effects of 3-bromopyruvate on multidrug resistance in vitro and in vivo derived from human breast MCF-7/ADR cells. PLoS One. 2014;9(11):e112132. | ||
Ko YH, Smith BL, Wang Y, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324(1):269–275. | ||
Qin JZ, Xin H, Nickoloff BJ. 3-Bromopyruvate induces necrotic cell death in sensitive melanoma cell lines. Biochem Biophys Res Commun. 2010;396(2):495–500. | ||
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. | ||
Wicks RT, Azadi J, Mangraviti A, et al. Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro Oncol. 2015;17(1):70–80. | ||
El Sayed SM, Mahmoud AA, El Sawy SA, et al. Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect). Med Hypotheses. 2013;81(5):866–870. | ||
Ghannam MM, El Gebaly R, Fadel M. Targeting doxorubicin encapsulated in stealth liposomes to solid tumors by non thermal diode laser. Lipids Health Dis. 2016;15:68. | ||
Voinea M, Simionescu M. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J Cell Mol Med. 2002;6(4):465–474. | ||
Gandham SK, Talekar M, Singh A, Amiji MM. Inhibition of hexokinase-2 with targeted liposomal 3-bromopyruvate in an ovarian tumor spheroid model of aerobic glycolysis. Int J Nanomedicine. 2015;10:4405–4423. | ||
Zhao W, Zhuang S, Qi XR, Xr Q. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int J Nanomedicine. 2011;6:3087–3098. | ||
Guo X, Szoka FC. Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG – diortho ester – lipid conjugate. Bioconjug Chem. 2001;12(2):291–300. | ||
Glick M, Biddle P, Jantzi J, Weaver S, Schirch D. The antitumor agent 3-bromopyruvate has a short half-life at physiological conditions. Biochem Biophys Res Commun. 2014;452(1):170–173. | ||
Fülöp A, Sammour DA, Erich K, et al. Molecular imaging of brain localization of liposomes in mice using MALDI mass spectrometry. Sci Rep. 2016;6:33791. | ||
Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010;197(197):3–53. | ||
Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62(2):90–99. | ||
Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPPS J. 2007;9(2):E128–E147. | ||
Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–1626. | ||
Nagoba BS, Punpale A, Poddar A, Suryawanshi NM, Swami GA, Selkar SP. Citric acid treatment of chronic nonhealing ulcerated tophaceous gout with bursitis. Int J Low Extrem Wounds. 2013;12(4):276–278. | ||
Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26(1):57–64. | ||
Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. | ||
Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–187. | ||
Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14(2):303–315. | ||
Liechty WB, Peppas NA. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm. 2012;80(2):241–246. | ||
Kularatne SA, Low PS. Targeting of nanoparticles: folate receptor. Methods Mol Biol. 2010;624:249–265. | ||
Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373(1–2):116–123. | ||
Zhai G, Wu J, Yu B, Guo C, Yang X, Lee RJ. A transferrin receptor-targeted liposomal formulation for docetaxel. J Nanosci Nanotechnol. 2010;10(8):5129–5136. | ||
Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. | ||
Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J Control Release. 2012;160(2):264–273. | ||
Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6(8):865–878. | ||
Paszko E, Senge MO. Immunoliposomes. Curr Med Chem. 2012;19(31):5239–5277. | ||
van Woensel M, Wauthoz N, Rosière R, et al. Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers. 2013;5(3):1020–1048. | ||
Chen TC, da Fonseca CO, Schönthal AH. Perillyl alcohol and its drug-conjugated derivatives as potential novel methods of treating brain metastases. Int J Mol Sci. 2016;17(9):1463. | ||
Zhang Q, Pan J, North PE, et al. Aerosolized 3-bromopyruvate inhibits lung tumorigenesis without causing liver toxicity. Cancer Prev Res. 2012;5(5):717–725. | ||
Chen TC, Yu J, Nouri Nigjeh E, et al. A perillyl alcohol-conjugated analog of 3-bromopyruvate without cellular uptake dependency on monocarboxylate transporter 1 and with activity in 3-BP-resistant tumor cells. Cancer Lett. 2017;400(17):161–174S0304-3835. | ||
Meure LA, Knott R, Foster NR, Dehghani F. The depressurization of an expanded solution into aqueous media for the bulk production of liposomes. Langmuir. 2009;25(1):326–337. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.