Back to Journals » Clinical Interventions in Aging » Volume 18
Enhanced Recovery After Surgery (ERAS) Program for Anterior Cervical Discectomy and Fusion (ACDF) in Patients Over 60 Years Old
Authors Wang P, Kong C, Teng Z, Zhang S, Cui P, Wang S, Zhao G, Lu S
Received 22 May 2023
Accepted for publication 22 September 2023
Published 27 September 2023 Volume 2023:18 Pages 1619—1627
DOI https://doi.org/10.2147/CIA.S422418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Peng Wang,1– 3,* Chao Kong,1– 3,* Ze Teng,4 Sitao Zhang,1– 3 Peng Cui,1– 3 Shuaikang Wang,1– 3 Guoguang Zhao,2,3 Shibao Lu1– 3
1Department of Orthopedics, Xuanwu Hospital of Capital Medical University, Beijing, 100053, People’s Republic of China; 2National Clinical Research Center for Geriatric Diseases, Beijing, 100053, People’s Republic of China; 3Beijing Municipal Geriatric Medical Research Center, Beijing, 100053, People’s Republic of China; 4Department of Radiology, Cancer Hospital Chinese Academy of Medical Sciences, Beijing, 100021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shibao Lu, Department of Orthopedics, Xuanwu Hospital of Capital Medical University, 45 Changchun Street, Xicheng, Beijing, 100053, People’s Republic of China, Email [email protected] Guoguang Zhao, National Clinical Research Center for Geriatric Diseases, 45 Changchun Street, Xicheng, Beijing, 100053, People’s Republic of China, Email [email protected]
Background: Enhanced recovery after surgery (ERAS) is currently widely used in many surgical specialties, but there is still a lack of concern about the cervical ERAS program for old patients (> 60 years old). We aimed to determine whether our ERAS program significantly improved satisfaction and outcomes in old patients (> 60 years old) with anterior cervical discectomy and fusion (ACDF).
Methods: This is a retrospective cohort study. The study enrolled patients if they were over the age of 60 years old underwent ACDF from July 2019 and June 2021 (ERAS group) and from January 2018 and June 2019 (non-ERAS group). Data including demographic, comorbidity, and surgical information were collected. We also evaluated ERAS process compliance, primary outcome, surgical complication, and length of stay (LOS).
Results: There were 135 patients in the ERAS group, and 122 patients in the non-ERAS group were included. A comparison of the demographic data revealed that there were no statistically significant intergroup differences observed between the group. Overall, ERAS pathway compliance was 91.9%. There were no significant differences in the fusion levels, operative time, intraoperative blood loss, postoperative VAS score, and complications between the ERAS and non-ERAS groups. In addition, there was no significant difference in readmission and mortality at 30-day follow-up between the two groups. However, we observed a statistically significant decrease in the LOS in the ERAS group (8.68± 2.34 of ERAS group versus 10.43± 4.05 in non-ERAS group, p=0.013).
Conclusion: This report describes the first ERAS protocol used in old patients with ACDF. Our ERAS program is safe and associated with incremental benefits with respect to LOS in old patients with ACDF.
Keywords: enhanced recovery after surgery, old, anterior cervical discectomy and fusion
Background
Since life expectancy and medical technological advancements have increased, China has become one of the countries with the largest elderly population and the fastest aging population in the world.1 In China there are 260 million people >60 years old, and the prevalence of elderly patients with degenerative diseases of the cervical spine is also increasing, diseases that seriously disturb an individual’s living condition. The desire of elderly patients to improve their quality of life is becoming stronger, and the demand for elective surgery by elderly patients is increasing. Although the elderly patient cohort is the fastest expanding segment of the population undergoing surgery, there is a paucity of clinical research specific to elderly patients undergoing anterior cervical discectomy and fusion (ACDF).2,3 The ailments of elderly patients are often accompanied by comorbidities, malnutrition, polypharmacy, functional decline, and psychological alterations, and the specific social needs of patients may coexist and overlap, which creates a condition of limited adaptation to surgical stress that consistently affects surgical outcome.4 To improve the surgical safety of elderly patients, it is necessary to decrease surgical stress and improve the ability of the patient to resist surgical stress.
Enhanced recovery after surgery (ERAS) was first proposed by Henrik Kehlet in 1997.5 ERAS is a series of perioperative optimization measures established using evidence-based medical data to reduce perioperative physical and psychological traumatic stress, which lead to a reduction in the occurrence of complications and a faster recovery after surgery. Currently, ERAS is widely used in many surgical specialties.6–8 However, although some studies have determined the implementation of ERAS for several cervical surgeries,3,9,10 there is still a lack of studies concerning the cervical ERAS program for elderly patients (>60 years old).
ACDF is one of the basic surgical procedures in spinal surgery and is commonly performed for the treatment of cervical degenerative diseases. Although the incision for ACDF is small, the incision is deep and the adjacent tissue structure is complicated. Therefore, there are certain surgical risks and complications of ACDF. A series of preoperative, intraoperative, and postoperative measures of ERAS could theoretically lead to a reduction in the occurrence of ACDF surgery-related complications. However, to the best of our knowledge, the actual benefits and safety of ERAS in elderly patients undergoing ACDF surgery remain unknown. The aim of the present study was to measure the effectiveness and safety of an ERAS program in elderly patients undergoing ACDF surgery.
Methods
Study Design and Data Collection
The present study is a retrospective analysis of data collected from elderly patients (>60 years old) who underwent ACDF surgery between July 2019 and June 2021 (ERAS group) and an age- and sex-matched non-ERAS group who underwent ACDF surgery between January 2018 and June 2019. Both groups were treated by the same surgical team. The retrospective non-ERAS group included patients who had been treated according to traditional perioperative protocols. All patients were diagnosed by two spinal specialists based on clinical symptoms and image inspection. Surgery was indicated when patients had typical symptoms of myelopathy or radiculopathy who did not respond to conservative treatments for these symptoms. Inclusion criteria for enrollment included patients with cervical spondylosis, spondylotic myelopathy, and radiculopathy with either hard (osteophytic) or soft disc prolapse, and those who failed conservative treatment. Individuals who had an infectious disease, trauma, cauda equina injury, or neoplasm were excluded from the study, as well as those undergoing a revision of a previous surgical fusion. All surgeons used an operating microscope to perform discectomies and removal of possible anterior compressions. The posterior longitudinal ligament was routinely excised at the intervertebral space. All ACDF surgeries applied the concept of precision and minimal invasiveness throughout the entire operation. A precise positioning and trans-interstitial approach was used to reduce tissue damage, reduce bleeding, and avoid nerve damage. In order to avoid the occurrence of postoperative delirium, our main measures include maintaining the patient’s blood pressure at plus or minus 10% of baseline during anesthesia, reducing the use of midazolam and using dexmedetomidine, and routinely inhaling oxygen for 6 hours after surgery in both ERAS and non-ERAS group. Discharge criteria were: mobilization without help, adequate pain control (VAS < 3), normal body temperature, no wound infection, and no severe complications.
The demographic information, comorbidities, and operative and anesthetic details of patients are presented in Table 1. The primary outcome data that were analyzed included treatment complications, length of stay (LOS), postoperative pain status, and 30-day readmission rate. All data were collected by manually reviewing the electronic medical record.
 |
Table 1 Patient Demographics |
ERAS Program
The ERAS program was proposed and planned in 2017. The proposed ERAS program for elderly patients undergoing ACDF surgery was written in combination with other ERAS projects and with the clinical experience of many experts. The core expert group consisted of anesthesiologists, spinal surgeons, nutritionists, physical therapists, physicians, geriatricians, and nurses. With the approval of the Ethical Committee for Human Subjects of the Xuanwu Hospital of Capital Medical University (permit date, 2018.4.3; approval no. 2018086), the ERAS program for patients undergoing ACDF surgery was implemented from July 2019. We previously described the development and implementation of an evidence-based ERAS pathway for short-level lumbar fusion.11 Using this pathway as a template, the ERAS program was adjusted to address the distinct perioperative needs of patients undergoing ACDF surgery. The ERAS interventions used in the present study were divided into preoperative, intraoperative, and postoperative steps.
Preoperative steps: (1) Patient education and counseling. A nurse explained the pre- and postoperative stages of the ERAS procedure, the discharge criteria, and the main scenarios that can occur early after discharge. (2) No prolonged fasting. Eating was allowed until 6 hours before surgery. (3) Fluid and carbohydrate loading. A carbohydrate drink was allowed up to 2 hours before surgery. (4) Preemptive analgesia: 75 mg pregabalin given orally the day before surgery.
Intraoperative steps: (1) Antibiotic prophylaxis within 1 hour of incision. (2) Tranexamic acid within 30 min of incision. (3) Local anesthesia was used before incision and after suturing (4) Maintenance of normothermia, which involved keeping the core body temperature at 36–37°C.
Postoperative steps: (1) Early oral feeding. This involved drinking water early after recovery from anesthesia, early feeding starting 6 hours and protein powder supplement during the liquid phase. (2) Early ambulation. This involved postoperative ambulation after 24 hours. (3) Early removal of bladder catheter after 24 hours. (4) Multimodal analgesia: visual analog scales <4, no analgesia, or a minimal oral dose of nonopioid [acetaminophen, non-steroidal anti-inflammatory drug (NSAID), or gabapentin]; visual analog scales 4–6, oral or intravenous acetaminophen, NSAID, or gabapentin; visual analog scales ≥7, opioid.
Control Group
To analyze the impact of ERAS on patient outcomes, we assessed a control cohort of adults older than 60 years who underwent ACDF surgery, performed by the same surgical group during the same period.
In the non-ERAS group, preoperative fasting is for at least 8 h and oral feeding on postoperative 1 day. Perioperative analgesia was prescribed according to the experience of the attending physicians and patient controlled analgesia (containing sufentanil and other agents in 100 mL saline). ERAS group’s intraoperative steps were rarely used. Postoperative rehabilitation exercise was staying in bed before surgical drainage pulled out. Urinary catheter removal was based on attending physicians' preference.
Statistical Analysis
All statistical analyses were performed using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA). Patient demographics, comorbidities data, markers of baseline health and clinical outcomes were compared between the ERAS and the non-ERAS groups using a Student’s t-test and χ2 test. For continuous variables, groups were compared using a Student’s t-test if the data were normally distributed or using the Mann–Whitney U-test if they were not. If variances from the ERAS and non-ERAS groups were found to be unequal using the F-test, the Satterthwaite two-sample t-test was used. Otherwise, the two-sample t-test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Demographics
There were 135 patients in the ERAS group (51 men and 84 women; mean age, 67.28±5.84 years old; mean BMI, 25.13±3.08) and 122 patients in the non-ERAS group (43 men and 79 women; mean age, 66.03±5.71 years old; mean BMI, 24.66±3.52). The baseline demographic characteristics and operative details of these two groups are compared in Table 1. Compared with the non-ERAS group, the ERAS group had a significantly higher incidence of hypertension and heart disease. No significant differences were observed in the mean preoperative visual analog scale (VAS) scores or the neck disability index (NDI) score. There were no significant differences between both groups in the preoperative total protein and albumin levels. Furthermore, no significant differences in the American Society of Anesthesiologists (ASA) grades were observed between the two groups.
Compliance with the ERAS Program
The ERAS program used in the present study included 11 intervention elements, and the overall program compliance was 91.9% (Table 2). All preoperative and intraoperative ERAS elements and postoperative multimodal analgesia were used in 100% of cases. The elements with relatively low compliance were early oral feeding (63.7%) and early ambulation (64.4%).
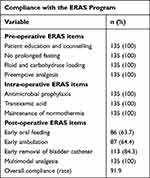 |
Table 2 ERAS Pathway Compliance |
Outcomes
The main clinical outcomes are shown in Table 3. There were no significant differences for the fusion levels, intraoperative blood loss, and postoperative VAS scores between the ERAS and the non-ERAS groups. There were also no significant differences in readmission and mortality at the 30-day follow-up between the ERAS and non-ERAS groups. However, a statistically significant decrease in the LOS in the ERAS group was observed (ERAS group, 8.68±2.34 versus non-ERAS group, 10.43±4.05; p=0.013). The ERAS group had a lower incidence of complications than the non-ERAS group, but these differences were not statistically significant (p=0.494; Table 4). The major and minor complications are detailed in Table 4, and no statistically significant intergroup differences were observed. However, the rates of nausea/emesis were significantly decreased in the ERAS group compared with the non-ERAS group (p=0.048; Table 4).
 |
Table 3 Postoperative Outcomes |
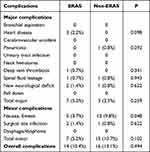 |
Table 4 Over Complication Data |
Discussion
The introduction of ERAS at our institution for patients undergoing ACDF surgery resulted in a significant decrease in the LOS without causing an increase in postoperative complications and VAS score. The average LOS was 8 days after ACDF surgery in elderly patients, which was a decrease in almost 2 days compared with the conventional protocol. According to the results of the present study, it seems applicable to implement the described ERAS protocol in elderly patients undergoing ACDF surgery, which may also help to reduce costs.
Common diseases, such as cervical disc herniation, cervical spondylosis, and posterior longitudinal ligament ossification, can cause damage to the cervical spinal cord, nerve root compression, and irritation trigger corresponding clinical symptoms, which results in the requirement for ACDF surgery. The main symptoms patients with spinal cord and nerve root damage experience are upper limb numbness, pain, fatigue, and muscle atrophy. In severe cases, these symptoms can manifest as unstable walking or even paralysis. The purpose of ACDF surgery is to relieve the compression of the spinal cord and nerve roots, rebuild the stability of the cervical spine surgery segment, promote the recovery of the patient’s nerve function, and improve the quality of life.
Age-related reduction in physiological reserves and functional capacity are inevitable and affect all organ systems, and this leads to a typical presentation of many comorbidities in elderly patients.12 Therefore, surgeons are reluctant to perform spinal surgery on elderly patients due to the high risk of perioperative complications.13 In addition to normal indications for surgery, the surgeon must balance the risks of adverse events with the expected benefits of surgery according to nutritional state, inflammatory activity, and anticipated host response. The concept of ERAS was first proposed >20 years ago and is currently extended to spinal surgery. According to the principles of classic ERAS, the main function of ERAS is to reduce stress, which is significant for elderly patients. Although several studies have been published on ERAS for ACDF surgery,9,10,14,15 there have been limited studies that focus on patients >60 years old. Compared with other ERAS for ACDF in young patient studies, the pillars of our ERAS protocol are similar: 1) high patient satisfaction at the center of his or her management, 2) a combination of interventions to nutritional support, reducing the pain and early ambulation.
In the present study, the average LOS in the ERAS group was 8.68±2.34 days, which was shorter than that in the non-ERAS group (10.43±4.05 days) but longer than that in other studies,9,16 this suggests that ERAS relatively aggressive approach is relatively safe in elderly patients. There are numerous factors that affect LOS, including preoperative comorbidities and postoperative complications. The incidence rate of complications in the ERAS group of the present study was similar to that reported in the literature.9,16 However, compared with other studies, the patients included in the present study were older and may have more comorbidities before surgery, which required more comprehensive preoperative examination and more targeted preoperative treatment. Furthermore, unlike in Western countries, postoperative rehabilitation exercises are conducted in wards in China rather than patients being referred to rehabilitation units. This is due to financial limitations and a shortage of rehabilitation medical resources in public hospitals. As a result, hospitalization for even several days is still the convention in most Chinese hospitals for better recovery.
Nutritional optimization is an essential component of perioperative treatment as malnutrition is a high-risk factor of major postoperative complications. Malnutrition thus increases the LOS and the burden on health-care systems.12 The European Society for Clinical Nutrition and Metabolism 2021 guidelines highlighted the significance of nutrition in ERAS.17 The success of surgery depends not only exclusively on technical surgical skills but also on nutritional management,18 which is due to the metabolic imbalance response caused by the surgery itself. For spinal surgeons, nutritional management is an inter-professional challenge. Preoperative carbohydrate fluids given up to 2 hours prior to surgery in contrast to the traditional midnight preoperative fast reduces not only surgical stress but also the incidence of postoperative insulin resistance. Clinical observational studies have demonstrated that perioperative hyperglycemia is associated with adverse outcomes in diabetic and non-diabetic patients.19 In addition, shortening the fasting time before surgery has been proven to enhance patient comfort prior to surgery, and it has been theorized that reducing patient catabolism has a positive impact on perioperative muscle preservation.20 Protein catabolism is a considerable feature at the postoperative phase, and shorting both the fasting time and nutritional therapy as soon as possible may provide the energy for optimal healing and counteract muscle catabolism and reduce postoperative wound infection.21 A previous study has shown that BMI <24 kg.m−2 is the cutoff for all-cause mortality in older patients.18 However, obscured by obesity reduced muscle mass and malnutrition may be ignored in surgical patients, some studies have shown high rates of malnutrition in older patients.22,23 Therefore, at our institution protein powder is routinely supplemented during the liquid phase after surgery, as suggested by Chan.12 For elderly patients undergoing ACDF surgery, the nutritional program at our institution includes prevention and reduction of catabolism before and after surgery. In this study, there was no significant difference in the incidence of postoperative infection between the two groups, which may be related to the absence of preoperative hyponutrition in both groups. Based on our clinical experience, avoidance of unnecessarily prolonged pre- and postoperative fasting and the supplementation of protein powder during the liquid phase after surgery is safe and reduces the feeling of hunger and anxiety in patients.
Multimodal, non-opioid-based analgesia has become the cornerstone of the ERAS program at our institution for effective analgesia after ACDF surgery. Well-managed pain, the so-called “fifth vital sign”, is widely recognized as an important metric for the success of surgery and recovery, and even as a surrogate for patient satisfaction.24 Many factors contribute to postoperative pain after spinal surgery and, in addition to nociceptive pain from surgical incision, these patients experience musculoskeletal pain from surgical traction and manipulation of bone, muscle, ligaments, and joints.25 Although opioids remain an effective therapy for pain control, substantial evidence has shown the wide range of adverse effects of opioid use, including nausea, ileus, respiratory depression, hyperalgesia, and delirium associated with opioid analgesia.26 Multimodal analgesia involves the use of multiple mechanisms of pain control that act synergistically to improve the analgesic effect and reduce the doses of opioids, thereby reduce the risk of side-effects from opioids27 and subsequently reduce the LOS and hospital cost. The ERAS program outlined in the present study included preoperative oral non-opioid medications to reduce the need for opioids postoperatively, and the results demonstrated that there was no significant difference between the two groups regarding the postoperative VAS score.
Early mobilization was another important aspect of the ERAS program outlined in the present study. Although early mobilization was first proposed by Emil Ries in 189928, it is only within the last 20 years that early mobilization has become accepted among surgeons. This early mobilization has led to significant progress in postoperative care with the development of ERAS. The harm of traditional prolonged bed rest in postoperative care is well-known, and it should therefore not be advocated. Despite the known benefits of early mobility, the answers to several questions regarding this intervention remain unknown. For example: 1) How soon after uncomplicated ACDF surgery should >60-year-old patients get out of bed and ambulate 2) Do patients >60 years old underwent ACDF surgery treated with early mobilization safe? Compared with a previous ERAS study of patients undergoing ACDF surgery,9,10 in the present study, patients who were >60 years old, who had a worse physical function and given the complexity of this population, were ambulated, the catheter was removed within 24 hours, and in-bed active/passive limb movement was conducted within 4 hours after surgery. The results of the present study demonstrated that this improvement is safe and effective in improving the efficacy of elderly patients undergoing ACDF surgery.
The relatively low compliance factors of early oral nutrition and early ambulation in our study because of the following facts: 1) Some elderly patients were slower to recover from anesthesia, resulting in low compliance with early oral feeding. 2) Some elderly patients are affected by the traditional idea of long stay in bed after surgery or feared of falling down, resisted early ambulation, resulting in reduced compliance with early ambulation. Given this low compliance factors of early oral nutrition (63.7%) and early ambulation (64.4%), we should strengthen these two parts of preoperative education and have a duty nurse at the bedside who is responsible for assessing the risk of swallow in elderly patients after they recovered from anesthesia and for guided early ambulation on the first day after surgery.
However, the present study does have several limitations. This study has a retrospective design and has a small sample size. The observation time was limited to the hospitalization period, and given the lack of long-term follow-up data, definitive conclusions may not be drawn from these results. In addition, the ERAS and non-ERAS groups were assessed at different times, which may have introduced analytical bias into the study. Moreover, we lack of frailty assessment. Frailty is an age-related, progressive decline in multiple physiological reserves that results in adverse postoperative outcomes, and frailty assessment is more convincing. Furthermore, multicenter studies with a larger cohort and long-term follow-up are required to confirm the safety and efficacy of the presented ERAS program in elderly patients after ACDF surgery.
Conclusions
In summary, in the present study, the implementation of the outlined ERAS protocol for ACDF surgery was associated with a significant decrease in the LOS without sacrificing quality of care in terms of clinical outcomes. While still in its infancy, modified approaches to the outlined ERAS program will likely improve patient satisfaction and outcomes.
Abbreviations
ERAS, enhanced recovery after surgery; LOS, length of stay; BMI, body mass index; ASA, American Society of Anesthesiologists; VAS, Visual Analogue Score.
Data Sharing Statement
The data set used during the current study is available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was reviewed and approved by the institutional review board following the declaration of Helsinki principles in Xuanwu Hospital Capital Medical University (permit data 2018.4.3; no. 2018086). Informed consent was provided by all participating individuals.
Acknowledgments
We thank the staff at the Department of Orthopedics, Xuanwu Hospital Capital Medical University, and all the patients who participated in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Beijing Municipal Medical Science Institute-Public Welfare Development Reform Pilot Project: (Capital Medical Research No. 2019-2).
Disclosure
The authors declare no financial and non-financial competing interests in this work.
References
1. Zhang C, Feng J, Wang S, et al. Incidence of and trends in Hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med. 2020;17(8):e1003180. doi:10.1371/journal.pmed.1003180
2. Peden CJ, Grocott MP. National research strategies: what outcomes are important in peri-operative elderly care? Anaesthesia. 2014;69(Suppl 1):61–69. doi:10.1111/anae.12491
3. Soffin EM, Wetmore DS, Barber LA, et al. An enhanced recovery after surgery pathway: association with rapid discharge and minimal complications after anterior cervical spine surgery. Neurosurg Focus. 2019;46(4):E9. doi:10.3171/2019.1.FOCUS18643
4. Bettelli G. Preoperative evaluation in geriatric surgery: comorbidity, functional status and pharmacological history. Minerva Anestesiol. 2011;77(6):637–646.
5. Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617. doi:10.1093/bja/78.5.606
6. Meillat H, Brun C, Zemmour C, et al. Laparoscopy is not enough: full ERAS compliance is the key to improvement of short-term outcomes after colectomy for cancer. Surg Endosc. 2020;34(5):2067–2075. doi:10.1007/s00464-019-06987-5
7. Scheib SA, Thomassee M, Kenner JL. Enhanced recovery after surgery in gynecology: a review of the literature. J Minim Invasive Gynecol. 2019;26(2):327–343. doi:10.1016/j.jmig.2018.12.010
8. Una Orejon R, Mateo Torres E, Huercio Martinez I, et al. Application of ERAS (Enhanced Recovery After Surgery) and laparoscopic surgery in the management of patients with bladder cancer. Arch Esp Urol. 2018;71(2):178–186.
9. Debono B, Sabatier P, Boniface G, et al. Implementation of enhanced recovery after surgery (ERAS) protocol for anterior cervical discectomy and fusion: a propensity score-matched analysis. Eur Spine J. 2021;30(2):560–567. doi:10.1007/s00586-020-06445-0
10. Debono B, Corniola MV, Pietton R, Sabatier P, Hamel O, Tessitore E. Benefits of Enhanced Recovery After Surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurg Focus. 2019;46(4):E6. doi:10.3171/2019.1.FOCUS18669
11. Wang P, Wang Q, Kong C, et al. Enhanced recovery after surgery (ERAS) program for elderly patients with short-level lumbar fusion. J Orthop Surg Res. 2020;15(1):299. doi:10.1186/s13018-020-01814-3
12. Chan SP, Ip KY, Irwin MG. Peri-operative optimisation of elderly and frail patients: a narrative review. Anaesthesia. 2019;74(Suppl 1):80–89. doi:10.1111/anae.14512
13. Watanabe T, Kanayama M, Takahata M, et al. Perioperative complications of spine surgery in patients 80 years of age or older: a multicenter prospective cohort study. J Neurosurg Spine;2019. 1–9. doi:10.3171/2019.9.SPINE19754
14. Arshi A, Wang C, Park HY, et al. Ambulatory anterior cervical discectomy and fusion is associated with a higher risk of revision surgery and perioperative complications: an analysis of a large nationwide database. Spine J. 2018;18(7):1180–1187. doi:10.1016/j.spinee.2017.11.012
15. Fu MC, Gruskay JA, Samuel AM, et al. Outpatient anterior cervical discectomy and fusion is associated with fewer short-term complications in one- and two-level cases: a propensity-adjusted analysis. Spine. 2017;42(14):1044–1049. doi:10.1097/BRS.0000000000001988
16. Leng X, Zhang Y, Wang G, et al. An enhanced recovery after surgery pathway: LOS reduction, rapid discharge and minimal complications after anterior cervical spine surgery. BMC Musculoskelet Disord. 2022;23(1):252. doi:10.1186/s12891-022-05185-0
17. Weimann A, Braga M, Carli F, et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. 2021;40(7):4745–4761. doi:10.1016/j.clnu.2021.03.031
18. Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623–650. doi:10.1016/j.clnu.2017.02.013
19. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8–14. doi:10.1097/SLA.0b013e31827b6bbc
20. Pogatschnik C, Steiger E. Review of preoperative carbohydrate loading. Nutr Clin Pract. 2015;30(5):660–664. doi:10.1177/0884533615594013
21. Aahlin EK, Trano G, Johns N, et al. Risk factors, complications and survival after upper abdominal surgery: a prospective cohort study. BMC Surg. 2015;15(1):83. doi:10.1186/s12893-015-0069-2
22. Stratton RJ, Hackston A, Longmore D, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr. 2004;92(5):799–808. doi:10.1079/BJN20041258
23. Corish CA, Bardon LA. Malnutrition in older adults: screening and determinants. Proc Nutr Soc. 2019;78(3):372–379. doi:10.1017/S0029665118002628
24. Beverly A, Kaye AD, Ljungqvist O, Urman RD. Essential elements of multimodal analgesia in Enhanced Recovery After Surgery (ERAS) guidelines. Anesthesiol Clin. 2017;35(2):e115–e143. doi:10.1016/j.anclin.2017.01.018
25. Sharma S, Balireddy RK, Vorenkamp KE, Durieux ME. Beyond opioid patient-controlled analgesia: a systematic review of analgesia after major spine surgery. Reg Anesth Pain Med. 2012;37(1):79–98. doi:10.1097/AAP.0b013e3182340869
26. Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol. 2007;21(1):65–83. doi:10.1016/j.bpa.2006.12.004
27. Beverly A, Kaye AD, Urman RD. SCAMPs for multimodal post-operative analgesia: a concept to standardize and individualize care. Curr Pain Headache Rep. 2017;21(1):5. doi:10.1007/s11916-017-0603-2
28. Ries E. Some radical changes in the after-treatment of celiotomy cases. JAMA. 1899;XXXIII(8):454–456. doi:10.1001/jama.1899.92450600020001g
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
