Back to Journals » OncoTargets and Therapy » Volume 11
Enhanced anticancer effects of low-dose curcumin with non-invasive pulsed electric field on PANC-1 cells
Authors Lu CH, Lin SH, Hsieh CH, Chen WT, Chao CY
Received 22 February 2018
Accepted for publication 4 June 2018
Published 10 August 2018 Volume 2018:11 Pages 4723—4732
DOI https://doi.org/10.2147/OTT.S166264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Chueh-Hsuan Lu,1,2 Shu-Hui Lin,2 Chih-Hsiung Hsieh,1,2 Wei-Ting Chen,1,2 Chih-Yu Chao1–3
1Department of Physics, Lab for Medical Physics and Biomedical Engineering, National Taiwan University, Taipei, Taiwan, Republic of China; 2Biomedical and Molecular Imaging Center, National Taiwan University College of Medicine, Taipei, Taiwan, Republic of China; 3Institute of Applied Physics, National Taiwan University, Taipei, Taiwan, Republic of China
Background: Pulsed electric field (PEF) has been considered as a cell permeability enhancing agent for cancer treatment. Nevertheless, application of PEF for conventional electrochemotherapy is usually at high intensity, and contact or even invasive electrodes are typically used, which may cause unwanted side effects. In this study, a non-invasive way of applying low intensity, non-contact PEF was adopted to study its combination effect with herb, curcumin, against pancreatic cancer cells and the mechanism involved.
Methods: The pancreatic cancer PANC-1 cells were treated with curcumin and PEF alone or in combination, and MTT assay was used to determine the viability of PANC-1 cells. Apoptosis and uptake of curcumin were analyzed by microscopy and flow cytometry. Western blot was further performed to evaluate the expression of apoptotic proteins.
Results: Our results demonstrated that PEF synergized with curcumin to inhibit the proliferation of PANC-1 cells in a field strength- and dose-dependent manner and caused apoptotic death of PANC-1 cells. The apoptotic induction of combination treatment was characterized by an increase in Bax/Bcl-2 ratio, and cleavage of caspase-8, -9, and -3. Moreover, the increase of curcumin uptake via electro-endocytosis was clearly observed in the cells following the exposure of PEF.
Conclusion: We show for the first time that a non-contact approach using low intensity electric field in a pulsed waveform could enhance the anticancer effect of low-dose curcumin on PANC-1 cells through triggering both extrinsic and intrinsic pathways. The findings highlight the potential of this alternative treatment, non-invasive electric field and curcumin, to increase therapeutic efficacy with minimum cytotoxicity and side effects, which may provide a new aspect of cancer treatment in combination of PEF and other anticancer agents.
Keywords: pulsed electric field, curcumin, combination treatment, synergistic effect, pancreatic cancer
Introduction
Pancreatic cancer is one of the most common tumors and the fourth leading cause of cancer-related deaths in men and women.1 It is usually diagnosed at the unresectable stage, and the majority of cases with advanced pancreatic cancer respond poorly to chemotherapy or radiotherapy. Despite improved therapeutic methods, the prognosis of pancreatic cancer still remains poor with a 5-year survival rate of only 2%–27%.2,3 Moreover, the incidence and death rates of pancreatic cancer have been increasing compared to those of most other cancers over the past few years.1 Therefore, there is a continuing need to develop novel agents or alternative strategies to treat pancreatic cancer.
In recent years, natural products have attracted growing scientific attention because of their low toxicity and therapeutic potential against various cancer types.4 Curcumin, a natural phenolic compound isolated from the rhizome of the herb Curcuma longa, has been widely used as a food and in traditional medicine for thousands of years. It has been shown to exhibit diverse pharmacologic properties such as antimicrobial, antioxidant, and anti-inflammatory activities5 as well as anticancer activity against various cancer cells.6–9 Curcumin can inhibit cell proliferation and activate a multi-signal transduction pathway related to apoptosis, through the regulation of Bcl-2 family proteins, the release of cytochrome c, and the activation of caspases.10–12 Most of the studies that have demonstrated the anticancer effects of curcumin used concentrations ranging from 10 to 50 μM.6,7,13–17 However, it is important to note that a concentration that is cytotoxic to cancer cells could also be toxic to normal cells. For example, Balasubramanian and Eckert showed that curcumin (10–20 μM) induced the apoptosis in normal human keratinocytes.18 Human retina endothelial cells and T cells have also been shown to undergo apoptosis when they were exposed to curcumin at concentrations of 10 and 25 μM, respectively.19,20 Thus, treatment of cancer cells with lower concentration of curcumin would be a favorable alternative. Unfortunately, there is a major obstacle that curcumin has poor bioavailability due to its water insolubility and instability.21
Pulsed electric field (PEF) has long been investigated as a technique for cancer treatment, known as electrochemotherapy. The basis of electrochemotherapy is the combination of impermeant or poorly permeant anticancer agents and reversible membrane electroporation induced by short, high intensity PEF above hundreds of volts per centimeter to 1 kV/cm.22 However, such a strong electric field may also cause undesirable side effects, which are mainly pain sensation and muscle contraction. Moreover, the high local current density could lead to edema and even local burns in some cases.23 Some approaches have been proposed to reduce the required voltage for drug incorporation into cells. For example, Fulimoto et al have shown the enhancement of the therapeutic effects of drugs by using low intensity with longer duration.24 Shankayi et al have developed the low intensity and higher repetition frequency electrochemotherapy.25 In spite of that, in these studies, the PEF was delivered by the invasive insertion of the electrodes. Furthermore, the decreasing viability of cells has been reported as a cytotoxic effect of electrolysis, which was the expected result of an electric current passing through the samples due to direct contact of electrodes.26
There have been recent studies of PEF using indirect contact of electrodes demonstrating the calculation of electric field effect and the induction of biological effects.27–29 Nevertheless, the electric fields of conducting these experiments are at high intensities (>1,000 V/cm) and near the verge of electric breakdown, which can be a danger of causing electric current to flow through the body. This paper presents the first demonstration of a combination of low-dose curcumin and non-invasive low intensity PEF with contactless electrodes in the cancer treatment of PANC-1 cells. Our results demonstrated that PEF synergized with curcumin to inhibit cancer cell proliferation, accompanied by an increase in Bax/Bcl-2 ratio, and cleavage of caspase-8, -9, and -3. The increased uptake of curcumin also unambiguously confirmed the enhanced cytotoxic effects of curcumin in PANC-1 cells under exposure to PEF. These results first indicate that non-invasive PEF could enable low-dose curcumin to achieve efficient therapeutic effects in the anticancer treatment of PANC-1 cells, which may provide a new aspect of cancer treatment in combination of PEF and other anticancer agents.
Materials and methods
Cell culture
Human pancreatic cancer cell line PANC-1 and human embryonic kidney (HEK293) cells were obtained from the Bioresource Collection and Research Center of the Food Industry Research and Development Institute (Hsinchu, Taiwan, Republic of China). Cells were plated in 75 cm3 cell culture flasks and grown in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (HyClone, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone) and 1% penicillin-streptomycin (Gibco Life Technologies, Thermo Fisher Scientific) in a humidified 5% CO2 incubator at 37°C.
PEF application
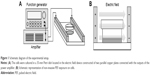
The externally applied PEF of various intensities (10, 30, 60, 90, and 120 V/cm) at 2 Hz with pulse width 2 ms was generated by a function generator (Agilent 33,220A; Agilent Technology, Palo Alto, CA, USA), which was connected to the input of a power amplifier (Trek PZD700; Trek Inc., Medina, NY, USA). The electric field device was constructed of two parallel copper plates separated by 40 mm and was connected with the output of the power amplifier (Figure 1A). The electric field was produced between two parallel plates, and the intensity was determined and equivalent to the applied voltage divided by the distance between two plates. The cells were cultured in a 35-mm Petri dish and continuously exposed to PEF for 24–72 hours. According to the dielectric properties, the field can penetrate the plastic of Petri dish, and thus, cells were under exposure to the fields (Figure 1B). Curcumin (Sigma-Aldrich, St Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) as a 10 mg/mL stock solution and stored at −20°C. Cells were divided into four groups with different treatments: 1) a Control group (Ctrl) was without any treatment; 2) a Curcumin group (Cur) was treated with 2 μg/mL curcumin; 3) a PEF group with a series of 2 Hz, 60 V/cm PEF (2 ms duration); and 4) a Combination group (Cur+ PEF) with 2 μg/mL curcumin and a series of 2 Hz, 60 V/cm PEF (2 ms duration).
MTT assay
Cell viability was accessed by 3-(4,5-dimethylthiazol-2-yl)–2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich) assay. Cells were seeded at 6×104 cells per 35 mm Petri dish and incubated overnight. After 48 hours treatment as described above, the cells were incubated in DMEM containing 0.5 mg/mL MTT for 4 hours at 37°C. Then, the medium was removed, and DMSO was added to dissolve the formazan crystals. The supernatant from each sample was transferred into a 96-well plate, and the absorbance was read at 570 nm using Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific).
Hoechst 33342 staining
Hoechst 33342 (Thermo Fisher Scientific) staining was used to detect morphological characteristics of the nucleus. PANC-1 cells were cultured on glass coverslips in 35 mm Petri dishes. Following incubation with 48 hours treatment, cells were washed with phosphate buffered saline (PBS) (Hyclone) and fixed with 4% paraformaldehyde (PFA) (Sigma-Aldrich) for 10 minutes at room temperature. After washing with PBS, cells were stained with Hoechst 33342 for 10 minutes in the dark and then washed again with PBS. The cells were mounted using Fluoroshield mounting medium (Abcam, Cambridge, UK). The photograph of stained cells was taken under a fluorescent microscope (Axio Imager A1, ZEISS) at 40× magnification.
Cleaved caspase-3 immunofluorescence staining
For immunofluorescence staining, fixed cells were then washed with PBS, permeabilized with 0.1% Triton X-100 (BioShop Canada Inc, Burlington, Ontario, Canada) in PBS for 15 minutes. After that, cells were washed with PBS and blocked with 1% bovine serum albumin (BSA) (Bioshop Canada Inc) in PBS for 30 minutes at 37°C, followed by incubation with diluted primary antibodies against cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. Next, cells were washed with PBS and incubated with Alexa647-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 1 hour at 37°C in the dark. After a further wash with PBS, the cells were mounted using Fluoroshield mounting medium with DAPI (Abcam). The photograph was taken under a fluorescent microscope at 20× magnification.
Flow cytometric analysis of apoptosis
Apoptotic cells were examined by the Annexin V-FITC/PI detection kit (BD Biosciences, San Jose, CA, USA). Cells were harvested with trypsin-EDTA (Gibco) and collected after 48 hours treatment. Then, the cells were washed with cold PBS and resuspended in binding buffer containing Annexin V-FITC and PI. The cell suspensions were incubated for 15 minutes at room temperature in the dark and analyzed by FACSCantoTM II system (BD Biosciences).
Western blot analysis
Cells were collected and washed with cold PBS, and then lysed on ice for 30 minutes in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1.0% Triton X-100, 0.1% SDS, 1 mM EDTA, 1% phosphate, and protease inhibitor cocktail) (Millipore, Billerica, MA, USA). Equivalent amounts of protein were resolved by 10% SDS-PAGE and electrotransferred onto polyvinylidene fluoride membrane (PVDF) (Millipore) in transfer buffer (10 mM CAPS, pH 11.0, 10% methanol) (BioShop Canada Inc). The membranes were blocked with 5% nonfat dry milk/Tris-buffered saline, 0.1% Tween 20 (TBST; blocking buffer) for 1 hour at room temperature and then incubated overnight at 4°C with diluted primary antibodies in blocking buffer. The specific primary antibodies against Bcl-2, cleaved caspase-8, cleaved caspase-9, cleaved caspase-3 (Cell Signaling Technology), Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (GeneTex, Irvine, CA, USA) were used. After washing with TBST, the membranes were incubated with HRP-conjugated anti-goat (GeneTex) or anti-rabbit (Jackson Immunoresearch) secondary antibody. Chemiluminescence was detected using WesternBright ECL Western blotting reagent (Advansta Inc., Menlo Park, CA, USA). The intensities of bands were quantified by ImageJ software (NIH).
Cellular uptake of curcumin
After 48 hours treatment, cells were washed with PBS and fixed with 4% PFA for 10 minutes at room temperature. Finally, the cells were mounted using Fluoroshield mounting medium with DAPI for fluorescent imaging at 100× magnification. The absorption of curcumin was also quantified by flow cytometry. Cells were collected and washed with cold PBS. Afterward, curcumin was excited at 420 nm wavelength and detected at 550 nm by flow cytometry. Results were presented as percentage increase of the mean fluorescence intensity of the treated samples, compared to untreated controls.
Statistical analysis
The results were presented as mean ± SD. Statistical analysis using one-way analysis of variance (ANOVA) was performed with SigmaPlot software. The results were considered to be statistically significant when values of p were less than 0.05. Each experiment was done in triplicate.
Results
PEF enhances curcumin-induced inhibition of PANC-1 cell growth
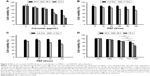
The inhibitory effect of PEF and curcumin alone or in combination on the growth of PANC-1 cells was examined by the MTT assay. In the combination treatment, the cells were continuously exposed to PEF for 24, 48, and 72 hours following the administration of curcumin. As shown in Figure 2A, treatment with curcumin induced a dose- and time-dependent decrease in the viability of PANC-1 cells. However, curcumin at the concentration of 5 and 10 μg/mL sharply decreased cell viability after 48 and 72 hours. Thus, we chose the concentration of 2 μg/mL for the following experiments. We performed the investigation of various PEF intensities ranging from 10 to 120 V/cm to examine its effect on curcumin activity in PANC-1 and non-cancer HEK293 cells. As shown in Figure 2B, our results showed that the efficacy of curcumin in PANC-1 cells was enhanced under the exposure of PEF in an intensity-dependent manner, except at the lower intensity of 10 and 30 V/cm. Although the combination of curcumin with PEF at either 90 or 120 V/cm cooperatively reduced the viability of PANC-1 cells, it also caused a decrease in the cell viability of non-cancer HEK293 cells (Figure 2C). Notably, curcumin in combination with 60 V/cm PEF showed the capability of inducing cytotoxicity in PANC-1 cells, but was found non-harmful toward non-cancer HEK293 cells. This indicates that non-cancer HEK293 cells treated with the co-treatment of curcumin and PEF shows less sensitivity as compared with pancreatic cancer PANC-1 cells. Based on these results, we studied the effect of curcumin in combination with 60 V/cm PEF on PANC-1 cells for the subsequent experiments. As shown in Figure 2D, the viability of PANC-1 cells in the curcumin group can be further reduced in a time-dependent manner when combined with 60 V/cm PEF. On the other hand, cell proliferation of the PEF group did not differ from that of the control group, indicating that PEF alone did not cause much damage to the cells. These results showed that the combination treatment significantly inhibited the cell viability of PANC-1 cells and that the PEF synergistically enhanced the antiproliferative effects of curcumin in PANC-1 cells.
PEF enhances curcumin-induced apoptosis in PANC-1 cells
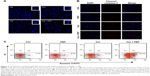
To confirm whether the reduction in cell viability was associated with induction of apoptosis, Hoechst 33342 staining and immunofluorescent detection were initially performed. As shown in Figure 3A, following treatment with PEF and curcumin alone or in combination for 48 hours, the morphological alterations, such as condensation of chromatin and nuclear fragmentation, were clearly observed. In addition, immunofluorescent staining also demonstrated the changes in expression of cleaved caspase-3 (Figure 3B). Both of these results indicate that the cells underwent apoptotic processes. In the further experiment, cells were analyzed by flow cytometry using Annexin V-FITC/PI staining to quantify the extent of apoptosis. Apoptotic rates were 7.6%±1.7%, 8.9%±2.3%, 15.5%±2.3%, and 31.6%±3.5% for control, PEF, curcumin, and curcumin combined with PEF, respectively (Figure 3C). These results suggest that combination treatment has a more prominent effect in inducing apoptosis than their respective individual treatments.
Induction of apoptosis through alterations of Bcl-2 family proteins and caspases activation
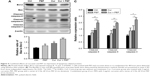
To further investigate the molecular mechanism of apoptosis induced by combination treatment with PEF and curcumin in PANC-1 cells, the expression levels of Bcl-2, Bax, and caspase-8, -9, and -3 were examined after 48 hours treatment. The Western blot analysis in Figure 4A shows that PEF co-treated with curcumin decreased Bcl-2 and increased Bax protein levels, which in turn led to a significant increase in the Bax to Bcl-2 ratio (Figure 4B). In addition, the expression levels of cleaved caspase-8, -9, and -3 were upregulated significantly after combination treatment (Figure 4C). These results suggest that upregulation of Bax and cleaved caspase-8, -9, and -3 expression, and downregulation of Bcl-2 expression could mediate apoptosis of PANC-1 cells induced by combination treatment with PEF and curcumin.
Cellular uptake of curcumin
Based on the electrically enhanced effects on antiproliferation and pro-apoptosis mentioned above, the effect of PEF on cellular uptake of curcumin was studied. Here, fluorescence microscopy was used to visualize cellular uptake of curcumin, which is known to exhibit green fluorescence.30 Figure 5A showed differential interference contrast (DIC) images, fluorescence images, and their merged images of cells after 48 hours treatment. There were no green fluorescence signals in control and PEF groups, while cells treated with curcumin showed clear signals of fluorescence. Particularly, fluorescence in cells treated with PEF and curcumin in combination was more evident. Furthermore, the uptake of curcumin into the PANC-1 cells was quantified by flow cytometry. The relative changes in intracellular curcumin content were detected as shown in Figure 5B. We found that the absorption of curcumin was further enhanced in combination treatment compared with curcumin alone. These results suggest the PEF enhancement of cellular uptake for curcumin is a potential mechanism for the increased anticancer ability.
Discussion
Curcumin exerts anticancer effects in various cancer cells at concentrations generally higher than 10 μM, and such concentrations can cause cytotoxic effect to normal cells. Moreover, it is well known that curcumin suffers from low bioavailability because of its poor absorption. Previous reports have shown that the effectiveness of curcumin could be increased in human breast cancer MCF-7 cells and human leukemia HL-10 cells when electroporation was applied.31,32 However, such intense PEF with invasive electrodes have been reported to cause unwanted side effects. Therefore, research for finding adequate approaches that enable low concentration of curcumin to achieve maximum effect with minimum side effect is of vital importance. In this paper, low intensity PEF was administered non-invasively to investigate its synergistic effect on low-dose curcumin in PANC-1 cells. The concept of such a non-invasive strategy is somewhat similar to that of tumor treating fields (TTF), which is a few volts/centimeter field using attached electrodes.33 However, the method we adopted in this study was not only different in terms of waveform and field strength but also a non-invasive treatment in a non-contact manner. There has been an in vivo rat study using contactless electrodes to investigate the biological effects under the exposure of high voltage (15–20 kV) alternating current electric field,34 in which this concept was also proposed to apply to humans.35 Nevertheless, in our study, we conducted a non-contact experiment using low voltage in a pulsed waveform and a herb, curcumin, to treat cancer for the first time.
Consistent with other reports,36 we observed the time- and dose-dependent cytotoxic effect of curcumin on PANC-1 cells, and we chose curcumin at a low concentration of 2 μg/mL (5.4 μM) to examine the combination effects of PEF and curcumin. Our results showed that the exposure of PEF alone at various intensities did not have a significant change in the viability of PANC-1 and non-cancer HEK293 cells. Nevertheless, the anticancer activity of curcumin in PANC-1 cells can be enhanced under the exposure of PEF, especially at the field strength above 30 V/cm, and the PEF at intensity of 90 and 120 V/cm combined with curcumin was also found toxic to non-cancer HEK293 cells. It is worth mentioning that a reduction in the viability of PANC-1 cells in response to the combination treatment of curcumin and 60 V/cm PEF was not observed in non-cancer HEK293 cells, indicating that the 60 V/cm PEF has selectivity for PANC-1 cells. Besides, the survival rate from the time-course MTT assay demonstrated that the 60 V/cm PEF combined with curcumin exerted synergistic antiproliferative effect on PANC-1 cells in a time-dependent manner (Figure 2D). There was particularly a significant enhancement in the efficacy of curcumin under the continuous application of PEF for 72 hours. Many reports have shown that curcumin alone can be effective against pancreatic cancer. However, it is notable that the concentration of curcumin we used in this study is lower than those used in other research studies.17,37,38 Furthermore, the enhanced cytotoxic efficacy of curcumin by non-invasive PEF demonstrated comparable result to the application of standard PEF on conventional drugs or curcumin.31,39,40 Most importantly, the approach performed in this study is a non-invasive, gentle, and long-term continuous treatment using contactless electrodes. In view of the modifiability of the electric field to focus on a specific location and the non-cytotoxicity of the combination treatment in HEK293 cells, this approach presents a means to achieve a cancer-specific effect in a safe manner.
There are different forms of programmed cell death reported in recent studies, such as apoptosis, autophagy, and programmed necrosis.41 Induction of apoptosis has been recognized as a major form of cell death and response to anticancer agents.42 As we know, both the nuclear morphology alterations and the activation of caspase-3 are typical characteristics of cell apoptosis. Consistent with the results of the MTT assay, Hoechst and immunofluorescence analyses demonstrated that PANC-1 cells treated with curcumin alone exhibited mild apoptotic cell death, while combination treatment with PEF and curcumin was more effective in inducing apoptotic bodies along with activation of caspase-3. Consistently, a synergistic effect in increasing apoptosis rate was also observed by Annexin V-FITC/PI staining when cells were co-treated with PEF and curcumin. Our results suggest that apoptosis plays an important role in the enhanced anticancer effects of combination treatment with PEF and curcumin.
Apoptosis occurs through two main signaling pathways, the extrinsic death receptor-mediated pathway or intrinsic mitochondria-mediated pathway, which are activated by initiator caspase-8 and -9, respectively. A critical enzyme involved in both pathways is the effector caspase-3, which results in cleavage of a number of substrate proteins essential for cell growth.43 Moreover, the Bcl-2 family proteins, including pro-apoptotic protein Bax and antiapoptotic protein Bcl-2, play a crucial role in the activation of caspases and the regulation of apoptosis. Studies have shown that the ratio of Bax to Bcl-2 determines the susceptibility of cancer cells to apoptosis.44 Here, we found that the Bax/Bcl-2 ratio in curcumin-treated cells was further increased by PEF exposure. In addition, the levels of active caspase-8, -9 and the downstream of active caspase-3 were also markedly increased in combination treatment comparing to that of either treatment alone. This was in agreement with the results of immunofluorescence staining. These results suggest that PEF synergistically enhanced curcumin-induced apoptosis through both intrinsic and extrinsic pathways in PANC-1 cells. Furthermore, it has been reported that curcumin alone can trigger apoptosis through both pathways in pancreatic cancer,17,45 indicating that PEF might be an assisted agent for curcumin.
In view of earlier studies that demonstrated facilitated molecular uptake under PEF stimulation,46 we examined the effect of PEF on curcumin uptake efficacy. In the combination treatment, the increased cellular uptake of curcumin under PEF exposure was clearly observed, and this provided direct evidence of enhanced anticancer effect. The exposure of cells to external electric field is known to result in a change in the transmembrane potential. When the transmembrane potential of cells exceeds its threshold value, the permeability of cell membrane is increased due to the formation of pores, a process called electroporation.47 However, when the transmembrane potential is lower than its threshold value, extracellular molecules are incorporated into the cells via endocytosis.26,46 The transmembrane potential is theoretically given by ΔVm =1.5×E×a×cosθ; where E is the intensity of the applied electric field, a is the cell radius, and θ is the angle between the radial vector for a given location on cell membrane and the vector of electric field.48 Thus, exposure of PANC-1 cells with a diameter of ≈34 μm to electric field strength of 60 V/cm used in our experiment leads to an induced transmembrane potential ΔV=153 mV, which is below the breakdown threshold of mammalian cell (200–1,500 mV).49 It suggests that the observed increase of curcumin uptake into cells did not involve electroporation, but rather endocytosis under low intensity PEF treatment. Therefore, this non-invasive electro-endocytosis combined with curcumin could be effective and safe for treating cancers. Further studies are needed to evaluate the effects of pulse duration as well as frequency on cytotoxicity enhancement of curcumin in the future. We believe that this approach can be extended to other PEF therapy in fighting cancer, thereby widening the therapeutic window.
Conclusion
We show for the first time that the antiproliferative and pro-apoptotic effects of low-dose curcumin were strengthened when PANC-1 cells were exposed to non-invasive PEF in a non-contact manner. Our results demonstrated that PEF synergized with curcumin in inducing cell death via activation of extrinsic and intrinsic caspase pathways. Also, pro-apoptotic Bax and antiapoptotic Bcl-2 proteins were shown to be involved. Moreover, increased uptake of curcumin into cells under PEF exposure in combination treatment clearly gives evidence for enhanced cytotoxic effects. These findings may provide support to develop an application of non-invasive electric field for cancer therapy.
Acknowledgments
We would like to thank Technology Commons in College of Life Science, National Taiwan University for use of the flow cytometry system, and the staff of the imaging core at the First Core Labs, National Taiwan University Hospital for technical assistance. This work was supported by grants from the Ministry of Science and Technology (MOST105-2112-M-002–006-MY3; CY Chao) and Ministry of Education (NTU-ICRP-103R7560-2; CY Chao) of Taiwan, Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Fryer RA, Galustian C, Dalgleish AG. Recent advances and developments in treatment strategies against pancreatic cancer. Curr Clin Pharmacol. 2009;4(2):102–112. | ||
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. | ||
Manson MM. Cancer prevention – the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;9(1):11–18. | ||
Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. | ||
Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68(18):7283–7292. | ||
Bachmeier BE, Mohrenz IV, Mirisola V, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29(4):779–789. | ||
Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer. 2009;100(9):1425–1433. | ||
Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39(1):56–68. | ||
Masuelli L, Benvenuto M, Fantini M, et al. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J Biol Regul Homeost Agents. 2013;27(1):105–119. | ||
Bush JA, Cheung KJ, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271(2):305–314. | ||
Moragoda L, Jaszewski R, Majumdar AP. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001;21(2A):873–878. | ||
Uddin S, Hussain AR, Manogaran PS, et al. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene. 2005;24(47):7022–7030. | ||
Shim JS, Lee HJ, Park SS, Cha BG, Chang HR. Curcumin-induced apoptosis of A-431 cells involves caspase-3 activation. J Biochem Mol Biol. 2001;34(3):189–193. | ||
Magalska A, Sliwinska M, Szczepanowska J, Salvioli S, Franceschi C, Sikora E. Resistance to apoptosis of HCW-2 cells can be overcome by curcumin- or vincristine-induced mitotic catastrophe. Int J Cancer. 2006;119(8):1811–1818. | ||
Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. 2008;26(4):289–297. | ||
Zhao Z, Li C, Xi H, et al. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway. Mol Med Rep. 2015;12(4):5415–5422. | ||
Balasubramanian S, Eckert RL. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J Biol Chem. 2007;282(9):6707–6715. | ||
Premanand C, Rema M, Sameer MZ, Sujatha M, Balasubramanyam M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest Ophthalmol Vis Sci. 2006;47(5):2179–2184. | ||
Magalska A, Brzezinska A, Bielak-Zmijewska A, Piwocka K, Mosieniak G, Sikora E. Curcumin induces cell death without oligonucleosomal DNA fragmentation in quiescent and proliferating human CD8+ cells. Acta Biochim Pol. 2006;53(3):531–538. | ||
Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40(3):720–727. | ||
Miklavcic D, Corovic S, Pucihar G, Pavselj N. Importance of tumour coverage by sufficiently high local electric field for effective electrochemotherapy. Eur J Cancer Suppl. 2006;4(11):45–51. | ||
Snoj M, Cemazar M, Slekovec Kolar B, Sersa G. Effective treatment of multiple unresectable skin melanoma metastases by electrochemotherapy. Croat Med J. 2007;48(3):391–395. | ||
Fulimoto T, Maeda H, Kubo K, et al. Enhanced anti-tumour effect of cisplatin with low-voltage electrochemotherapy in hamster oral fibrosarcoma. J Int Med Res. 2005;33(5):507–512. | ||
Shankayi Z, Firoozabadi SM, Hassan ZS. Optimization of electric pulse amplitude and frequency in vitro for low voltage and high frequency electrochemotherapy. J Membr Biol. 2014;247(2):147–154. | ||
Antov Y, Barbul A, Mantsur H, Korenstein R. Electroendocytosis: exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromolecules. Biophys J. 2005;88(3):2206–2223. | ||
Novac BM, Banakhr FA, Smith IR, et al. Demonstration of a novel pulsed electric field technique generating neither conduction currents nor joule effects. IEEE Trans Plasma Sci. 2014;42(1):216–228. | ||
Frelinger AL, Gerrits AJ, Garner AL, et al. Modification of pulsed electric field conditions results in distinct activation profiles of platelet-rich plasma. PLoS One. 2016;11(8):e0160933. | ||
Robinson VS, Garner AL, Loveless AM, Neculaes VB. Calculated plasma membrane voltage induced by applying electric pulses using capacitive coupling. Biomed Phys Eng Express. 2017;3(2):025016. | ||
Kunwar A, Barik A, Pandey R, Priyadarsini KI. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochim Biophys Acta. 2006;1760(10):1513–1520. | ||
Ramachandran RP, Madhivanan S, Sundararajan R, Lin CWY, Sankaranarayanan K. An in vitro study of electroporation of leukemia and cervical cancer cells. In: Sundararajan R, editor. Electroporation-Based Therapies for Cancer: From Basics to Clinical Applications. Cambridge: Woodhead Publishing Ltd; 2014:161–183. | ||
Camarillo IG, Xiao F, Madhivanan S, et al. Low and high voltage electrochemotherapy for breast cancer: an in vitro model study. In: Sundararajan R, editor. Electroporation-Based Therapies for Cancer: From Basics to Clinical Applications. Cambridge: Woodhead Publishing Ltd; 2014:55–102. | ||
Hottinger AF, Pacheco P, Stupp R. Tumor treating fields: a novel treatment modality and its use in brain tumors. Neuro Oncol. 2016;18(10):1338–1349. | ||
Harakawa S, Inoue N, Hori T, et al. Effects of a 50 Hz electric field on plasma lipid peroxide level and antioxidant activity in rats. Bioelectromagnetics. 2005;26(7):589–594. | ||
Hara A, Ogawa Y. Electric field therapy apparatus. US Patent. 1989;No. 4802470. | ||
Parasramka MA, Gupta SV. Synergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cells. J Oncol. 2012;2012:709739. | ||
Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J Biol Chem. 2010;285(33):25332–25344. | ||
Osterman CJ, Lynch JC, Leaf P, et al. Curcumin modulates pancreatic adenocarcinoma cell-derived exosomal function. PLoS One. 2015;10(7):e0132845. | ||
Saczko J, Kamińska I, Kotulska M, et al. Combination of therapy with 5-fluorouracil and cisplatin with electroporation in human ovarian carcinoma model in vitro. Biomed Pharmacother. 2014;68(5):573–580. | ||
Vásquez JL, Gehl J, Hermann GG. Electroporation enhances mitomycin C cytotoxicity on T24 bladder cancer cell line: a potential improvement of intravesical chemotherapy in bladder cancer. Bioelectrochemistry. 2012;88:127–133. | ||
Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833(12):3448–3459. | ||
Ferreira CG, Span SW, Peters GJ, Kruyt FA, Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 2000;60(24):7133–7141. | ||
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. | ||
Raisova M, Hossini AM, Eberle J, et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117(2):333–340. | ||
Youns M, Fathy GM. Upregulation of extrinsic apoptotic pathway in curcumin-mediated antiproliferative effect on human pancreatic carcinogenesis. J Cell Biochem. 2013;114(12):2654–2665. | ||
Rosemberg Y, Korenstein R. Incorporation of macromolecules into cells and vesicles by low electric fields: induction of endocytotic-like processes. Bioelectrochem Bioenerg. 1997;42(2):275–281. | ||
Kotnik T, Frey W, Sack M, Haberl Meglič S, Peterka M, Miklavčič D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015;33(8):480–488. | ||
Debruin KA, Krassowska W. Modeling electroporation in a single cell. I. Effects of field strength and rest potential. Biophys J. 1999;77(3):1213–1224. | ||
Gowrishankar TR, Pliquett U, Lee RC. Dynamics of membrane sealing in transient electropermeabilization of skeletal muscle membranes. Ann N Y Acad Sci. 1999;888:195–210. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.