Back to Journals » International Journal of Nanomedicine » Volume 18
Engineered Plant-Derived Nanovesicles Facilitate Tumor Therapy: Natural Bioactivity Plus Drug Controlled Release Platform
Authors Chen X, Ji S, Yan Y, Lin S, He L, Huang X, Chang L, Zheng D , Lu Y
Received 12 April 2023
Accepted for publication 19 June 2023
Published 22 August 2023 Volume 2023:18 Pages 4779—4804
DOI https://doi.org/10.2147/IJN.S413831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Xiaohang Chen,1,2 Shuaiqi Ji,1,2 Yuxiang Yan,1 Shuoqi Lin,1,2 Lianghang He,1,2 Xiaoyu Huang,2 Lin Chang,2 Dali Zheng,1 Youguang Lu1,2
1Fujian Key Laboratory of Oral Diseases, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Preventive Dentistry, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, People’s Republic of China
Correspondence: Youguang Lu; Dali Zheng, Email [email protected]; [email protected]
Abstract: Tumors are the second-most common disease in the world, killing people at an alarming rate. As issues with drug resistance, lack of targeting, and severe side effects are revealed, there is a growing demand for precision-targeted drug delivery systems. Plant-derived nanovesicles (PDNVs), which arecomposed of proteins, lipids, RNA, and metabolites, are widely distributed and readily accessible. The potential for anti-proliferative, pro-apoptotic, and drug-resistant-reversing effects on tumor cells, as well as the ability to alter the tumor microenvironment (TME) by modulating tumor-specific immune cells, make PDNVs promising anti-tumor therapeutics. With a lipid bilayer structure that allows drug loading and a transmembrane capacity readily endocytosed by cells, PDNVs are also expected to become a new drug delivery platform. Exogenous modifications of PDNVs enhance their circulating stability, tumor targeting ability, high cell endocytosis rate, and controlled-release capacity. In this review, we summarize PDNVs’ natural antitumor activity, as well as engineered PDNVs as efficient precision-targeted drug delivery tools that enhance therapeutic effects. Additionally, we discuss critical considerations related to the issues raised in this area, which will encourage researchers to improve PDNVs as better anti-tumor therapeutics for clinic applications.
Keywords: plant-derived nanovesicles, cancer therapy, natural bioactivity, drug delivery platform, engineered EVs, targeted precision therapy
Graphical Abstract:
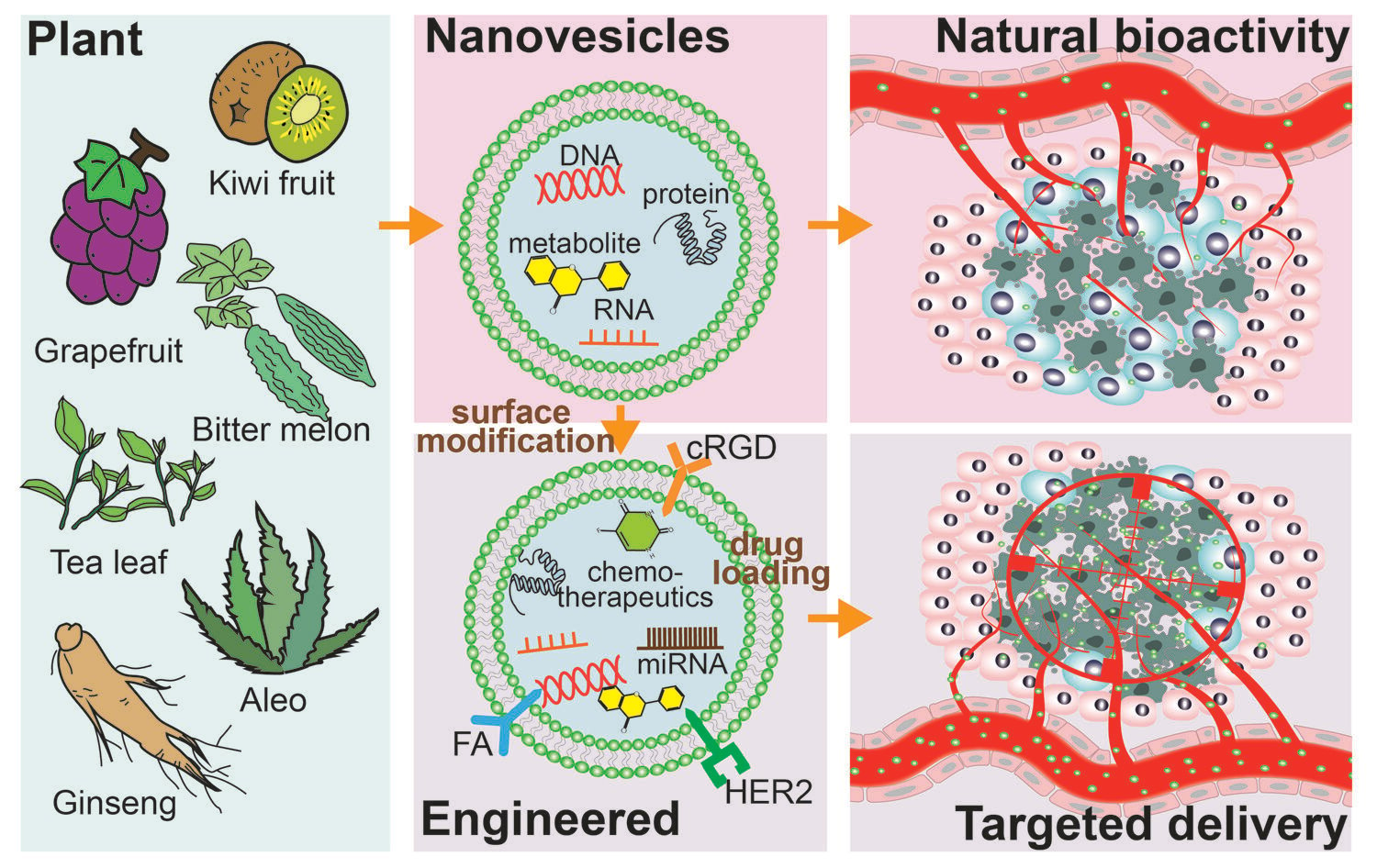
Introduction
Tumors are the second leading cause of death worldwide, with approximately 1670 deaths per day in the United States and 7700 deaths per day in China.1,2 The fundamental reason why cancers are still considered “frightening” is the difficulty of early tumor detection and the variety of issues present in tumor treatments, including but not limited to the following: (1) Surgical resection is the primary form of treatment, but it is unable to remove the tiny potential unlocated tumor foci that may become a fatal hidden danger. In addition, some tumors that are detected may be missed by surgical indicator, and thus require combination with radiotherapy and other means for removal.3,4 (2) Chemotherapeutic drugs lack targeting and enter the body with the blindness of “one thousand killed, eight hundred lost”, resulting in severe side effects such as lowered immunity and other issues.5 (3) Although emerging immunotherapy has been proven to be effective for some tumors, many “cold tumors” are ‘insensitive’ to immunotherapy.6 (4) Following drug therapy, the mutating tumor cells will progressively develop drug resistance ability, resulting in drug treatment failure.7 Accordingly, there is a growing demand for more efficient targeted tumor treatment, particularly in precision-targeted drug delivery systems.8,9
The field of targeted tumor therapy has seen many exciting advancements in the precision drug delivery system, particularly in the area of nanomaterials.10 (1) Through surface functionalization, nanomaterials can possess highly precise targeting delivery capabilities;11 (2) Encapsulation by nanomaterials can protect drugs from degradation and clearance in vivo, enhancing drug stability and bioavailability;12 (3) Nanomaterials can enhance penetration and retention efficiency, further improving drug penetration efficiency and specificity;13 (4) Regulating the structure and composition of nanomaterials can achieve efficient drug release, among other benefits.14 As a novel emerging drug delivery tool, exosomes derived from animals possess advantages including natural origin, good biocompatibility, natural targeting ability, and biodegradability.15 However, they currently face challenges such as low yield and high costs.16 Therefore, PDNVs, also known as plant derived exosomes like nanoparticles, have become an alternative option, as they offer similar advantages and are easier to produce at a lower cost.17
PDNVs are extensively distributed in a range of plants and function as cellular messengers that control plant cell differentiation and defense against viral invasion.18,19 PDNVs are less expensive than vesicles derived from mammals due to more accessible source.20 On the one hand, composed of proteins, lipids, RNA, and metabolites, PDNVs have shown excellent natural bioactivity as therapeutics, particularly in terms of antitumor bioactivity that directly controls cell proliferation, apoptosis, and even ameliorates tumor drug resistance.21,22 Furthermore, PDNVs can also alter the TME by activating tumor immunity and regulating tumor-associated fibroblasts and microorganisms. On the other hand, with a lipid bilayer structure that allows them to cross the blood-brain barrier and load hydrophilic and hydrophobic drugs, PDNVs are expected to be valuable drug delivery vehicles.23 Interestingly, studies have demonstrated that the fate of PDNVs in vivo may have some specificity, including the propensity for distinct PDNVs to travel to various organs, and many PDNVs with antitumor effects have negligible harmful effects on normal cells.24,25 However, these characteristics still fall short of making PDNVs the perfect tool for precise and efficient targeted delivery.
Fortunately, PDNVs contain a large number of lipids and proteins on their surface, which offer abundant opportunities for further modifications, such as co-incubation and co-extrusion, to improve the efficacy of PDNVs and create precise drug delivery tools.26,27 Based on this, PDNVs have the potential to become smart drug delivery tools with high circulating stability, specific tumor targeting, tumor tissue penetration, and a high tumor cell uptake rate.23 In this review, we systematically summarize how to utilize the benefits of PDNVs, how to modify and load drugs into PDNVs, how to match the precision targeting delivery need of the PDNVs system, and the opportunities and challenges that this field faces. First, we summarize the natural anticancer activities of PDNVs and consider their benefits of PDNVs as drug delivery tools in terms of their specific structures and components. Then, we discuss attempts to build ideal drug delivery platforms through engineering modifications and drug loading. Finally, we focused on the bottlenecks facing PDNVs as a potential anti-tumor strategy, which will serve as inspiration for further research and the advancement of PDNVs in the clinic settings.
Composition: The Components of PDNVs
Plant-derived nanovesicles (PDNVs) are biological nanovesicles secreted by plant cells, composed of cell components wrapped in a membrane, including proteins, lipids, RNA, and metabolites.22 The content and proportion of these components vary depending on factors such as plant species, and growth conditions, so different PDNVs also exhibit different effects in the biomedical field.28 It has been demonstrated that PDNVs can fuse with the cell membrane, releasing their components into recipient cells, achieving information transfer and material transfer.29
PDNVs have multiple biological functions and play an important role in plant growth, development, and response to environmental stress.26,30 The proteins and RNA in PDNVs can regulate the physiological processes of cellular metabolism, proliferation, and differentiation.18 In addition, PDNVs also contain secondary metabolites, such as flavonoids and alkaloids, which can act as signaling molecules or antioxidants, contributing to cells’ resistance to external environmental stress.30 Studies have shown that the bioactive components in PDNVs have a wide range of potential applications in the body, including antitumor, anti-inflammatory, and regenerative activities, suggesting they could be used as a novel type of drug formulation.31 At the same time, PDNVs can be used as an effective drug carrier, realizing targeted delivery and controlled release of drugs through their membrane structure and component features.26
Bioactivity: PDNVs as an Anti-Tumor Therapeutic
Numerous studies have confirmed the natural bioactivity of PDNVs as disease therapeutics, particularly in anti-tumor therapy. (Table 1 and Figure 1) PDNVs were shown to not only play roles in anti-tumor effects that directly regulate the behavior of tumor cells, including proliferation, apoptosis, metabolism, and drug resistance capacity; but also play roles in the regulation of tumor-associated immune cells, fibroblasts, and microorganisms for improving the therapeutic effects of drugs, all of which inhibit tumor progression and invasiveness.32,33 Quite remarkably, Xiao et al isolated PDNVs from 11 edible fruits and vegetables, resolved the internal miRNAs by illumina sequencing, and found that the highly expressed miRNAs were mainly closely associated with inflammatory responses and cancer-related pathways.34
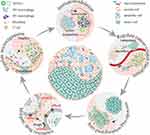 |
Figure 1 Natural anti-tumor bioactivities of PDNVs. (Grey areas represent untreated tumor tissue, while pink areas represent PDNV-treated tumor tissue). |
 |
Table 1 Natural Antitumor Activity of PDNVs |
Direct: Regulation of Tumor Cell Biological Behavior
Tumor cells exhibit a variety of biological traits that make them a formidable “villain”, including an unlimited ability for replication and proliferation, resistance to apoptosis, abnormal metabolism, and drug resistance mutations.54,55 Numerous studies already conducted have demonstrated that the ability of PDNVs directly controls these tumor cell behaviors.
Inhibition of Tumor Cell Proliferation
For the organism to operate normally, cells must proliferate and differentiate in a normal manner.56 Tumor cells, in contrast to normal tissue cells, possess a self-sufficient growth signaling system that may self-stimulate continuous proliferation, leading to uncontrolled growth.57 The anti-proliferative ability of PDNVs in tumor cells, which inhibits tumor progression, is exemplified in numerous studies. Stanly et al discovered that the microvesicles (MVs) and nanovesicles (NVs) from four citrus species, C. sinensis, C. limon, C. paradisi, and C. aurantium, particularly suppressed the proliferation of the lung, skin, and breast cancer cells. The PDNVs’ induction of cell cycle arrest at the G2/M checkpoint and blockage of the G0/G1 and G2/M cell cycle transitions may be critical for this anti-proliferative capacity. Meanwhile, the cell cycle proteins CyclinB1 and CyclinB2 are expressed at lower levels, whereas the cell cycle inhibitor Cdkn1a (p21) is expressed at higher levels, which may explain the cell cycle arrest effects. Additionally, they found that tumor cells had undergone cleavage poly(ADP-ribose) polymerase 1 (PARP-1) and had decreased levels of intercellular adhesion molecule 1 and histone protease expression, as well as suppression of the protein kinase B (Akt) and extracellular-signal-regulated kinase (ERK) signaling pathways that promote proliferation.32
Bitter melon-derived NVs may block U251 glioma cells from proliferating, migrating, and invading through the downregulation of matrix metallopeptidase 9 (MMP9) and the participation of highly expressed miR5813 in the phosphatidylinositol-3-kinase (PI3K)/AKT pathway.36 Maize-derived NVs also demonstrated more potent anti-proliferative effects on colon cancer cells when coupled with RAW264.7 than they did alone.49 Although NVs from both coffee and asparagus can inhibit the proliferation of hepatocellular carcinoma cells in a dose-dependent manner, it is currently impossible to determine which efficacy is more potent due to the different quantification methods employed in the two studies.48,58 These findings suggest that, although the precise mechanism of action governing the anti-proliferative effect of PDNVs may vary depending on the plant source, PDNVs are predicted to be an effective tool for controlling tumor cell proliferation. The specific mechanism of action of various PDNVs that have an anti-proliferative effect on tumor cells still needs to be further investigated. This is of great significance in discovering certain patterns or new drug targets.
Promotion of Tumor Cell Apoptosis
Apoptosis, which is necessary for the removal of sick or damaged cells, is a highly important program for the body to maintain healthy growth and development.59 Cancer cells have acquired apoptosis-related proteins that alter how damage or abnormalities are detected in order to prevent apoptosis signaling activation.60 The two main apoptosis-related proteins that are altered in cancer cells are decreased expression of Caspases 3, 8, and 9, a family of proteases that are essential for apoptosis, and increased expression of B-cell lymphoma-2 (Bcl-2), which exerts its survival-promoting function by inhibiting the release of mitochondrial cytochrome C in response to a range of apoptotic stimuli.61,62 Therapies for treating tumors that target aberrant apoptosis in tumor cells include radiotherapy, chemotherapy, hormone treatment, and thermotherapy, among others. However, they come with severe side effects on healthy tissues.63
The pro-apoptotic effect of PDNVs has been well documented, and it may result from a variety of factors. One of them might work primarily by activating pro-apoptotic gene expression, producing more reactive oxygen species (ROS), and hastening the start of apoptosis.46 Chen et al, demonstrated that after 4 hours of incubation, Camellia sinensis derived NVs treated MCF-7 cells and 4T1 cells, in which the total ROS content was 14.2 and 9.3 times greater than the untreated cells, increased nitric oxide (NO) by 5.2 and 3.5 times, respectively.44 Fingerroots-derived NVs disrupted the redox balance in the colorectal cancer cell lines HT-29 and HCT116 by upregulating cysteinase, lowering glutathione levels, inducing the expression of the pro-apoptotic genes caspase 3 and 9, increasing ROS production, and promoting apoptosis.42 Lemons-derived NVs have also been demonstrated to have anticancer properties, decreasing the growth of stomach cancer cells in a dose-dependent manner, reducing cell proliferation, and increasing apoptosis. The anti-cancer properties are connected to considerable stimulation of ROS generation by these NVs, an effect that is almost entirely undone by the ROS inhibitor N-acetylcysteine. In contrast, ROS increases growth arrest and DNA damage inducible protein alpha (GADD45a), which stops the gastric cancer cell cycle in the S phase and encourages apoptosis.38 Although the precise mechanism causing this ROS generation boost is unknown, several studies imply that it might be caused by miRNA in PDNVs.43
Mitigating Drug-Resistant Behavior
The poor prognosis of malignant tumors is partially a result of the development of drug resistance.64 The continued development of cutting-edge therapies such as adjuvant nanoparticle therapy and the next generation of drugs, cannot stop the evolution of novel drug resistance mutations.65 Simply put, malignancies present complex drug resistance mechanisms. Tumor cells can be genetically manipulated to inhibit drug absorption, cause immediate metabolism of the drug after uptake, or excrete through excretory proteins.66 Fortunately, PDNVs will be a strategy to mitigate tumor drug resistance partly because with a lipid bilayer structure that makes it easier for cells to uptake.
One of the most commonly used chemotherapeutic drugs for cancer is 5-fluorouracil (5-FU), yet tumor cells frequently develop resistance to 5-FU due to the inflammatory TME, metabolic enzymes, and cancer stem cells.67 In tumor cells, the nucleotide- binding oligomerization domain, leucine- rich repeat and pyrin domain- containing 3 (NLRP3) inflammatory vesicles are directly related to 5-FU resistance.68 Yang et al found that bitter melon derived NVs could activate intracellular ROS, suppress the expression of NLPR3, inhibit the proliferation of CAL-27 cells, and that all 11 of the miRNAs contained in these NVs may be involved in the regulation of this protein. More than 70% of CAL-27 resistant 5-FU cells underwent apoptosis with the aid of bitter melon derived NVs, which was three times more efficient than 5-FU alone.35 However, because bitter melon derived NVs were unable to properly target tumor areas in vivo, the study employed peritumor injections, which could be problematic in clinical therapeutic applications.
PDNVs can mitigate drug resistance in tumor cells not only by regulating inflammatory vesicles, but also by efficiently transporting drug-carrying vesicles or dissipating intracellular energy. However, these mechanisms may vary depending on the source of PDNVs. Xiao et al used heparin-modified lemon-derived NVs to successfully transport doxorubicin (DOX) into DOX-resistant cells. This was mainly achieved through caveolin-mediated endocytosis (main), macropinocytosis (secondary), and clathrin-mediated endocytosis. Lemon derived NVs in particular can downregulate the expression of cotintegrin-1 (CAV-1) while inducing a reduction in downstream adenosine triphosphate (ATP) generation, greatly limiting drug efflux, and efficiently dissipates intracellular energy.37 This suggests that PDNVs are a promising tool for improving drug resistance offering advantages such as increased drug uptake and decreased drug efflux.
Altered Cellular Metabolism
Cancer frequently stifles the production of metabolic enzymes in order to rewire its metabolic machinery to fulfill the constant energy needs for improved proliferation and survival.53 PDNVs have been demonstrated to change the metabolic behavior of cells, as have many natural substances. Acetyl coenzyme A carboxylase (ACACA, ACC) is overexpressed in a number of tumor types. Proteomic analysis of rectal cancer cells treated with citrus limon-derived NVs showed a considerable downregulation of acetyl-CoA carboxylase 1 (ACACA) and altered lipid metabolism. Additionally, silencing of ACACA in cells reduced proliferation.40
Indirect: Remodeling the Tumor Microenvironment
Immunomodulation
Immune cells can become tumor-promoting helpers that can generate pro-survival, pro-migration, and anti-detection molecules, promoting tumor growth and spread when tumor cells hold them captive in the complicated TME.69 Although some anti-inflammatory drugs, such as aspirin and statins, significantly lower the risk of developing cancer and dying from it, some pro-inflammatory cytokines or stimulants, such as transforming growth factor-β (TNF-β) and cyclic GMP-AMP synthase - stimulator of interferon gene (cGAS-STING) pathway activators, can significantly increase the effectiveness of tumor therapy by encouraging immune cell infiltration into tumor tissues. This suggests that inflammation is a “double-edged sword”.70 Fortunately, PDNVs play a role in immunomodulation, including controlling macrophage polarization and the amount of pro- or anti-inflammatory factors secreted by immune cells.
There are many studies on PDNVs regulating the release of inflammatory cytokines in immune cells.71 Zhang et al isolated NVs from ginger and found that vesicles could increase the expression of anti-inflammatory factors (Interleukin (IL)-10 and IL-22) and decrease the expression of pro-inflammatory factors (TNF-α, IL-6 and IL-1β) to reduce inflammation associated with colitis. It has a repairing effect on the damaged intestinal mucosa and prevents the formation of colitis associated-tumors.45 Zhu et al also found that tea-derived NVs could achieve the above immunomodulation effect by reducing the production of ROS. Meanwhile, these tea-derived NVs also effectively suppressed inflammatory bowel responses by increasing the diversity and overall abundance of gut microbiota, which may also help prevent or treat inflammatory bowel disease and colitis-associated tumors.47 At the same time, PDNVs components like miRNA may be strongly linked to this anti-inflammatory response.72
Notably, the regulatory effect of PDNVs on macrophages is expected to cause “cold tumors”, which are resistant to immunotherapy, to turn into “hot tumors”, greatly increasing efficacy.33 Studies have shown that some tumors only weakly respond to immune checkpoint inhibitor therapy, mainly because these TME, commonly referred to as “cool tumors”, have limited T cell infiltration. Activating “cold tumors” into “hot tumors” is one strategy for increasing anti-tumor immunity throughout the body. Contrary to popular belief, Cao’s team found that macrophage polarization is also directly correlated with immunotherapy sensitivity. They discovered that ginseng-derived NVs may alter macrophage polarization both in vitro and in vivo by facilitating the transition of macrophage phenotype from pro-inflammatory M1 to anti-inflammatory M2 through activation of toll-like receptor 4 (TLR4) and myeloid differentiation factor 88 (MyD88) signaling via ceramide and associated proteins. Through immunomodulation, the impact is anticipated to greatly slow the growth of melanoma in mice.46 They also found that while using ginseng-derived NVs can slow the growth of “cold tumors” like breast cancer, it cannot guarantee long-term mouse survival. A positive result can be shown from the combination therapy using programmed cell death protein 1 (PD-1) mAb and ginseng-derived NVs. In comparison to PD-1 therapy alone, they discovered that this approach dramatically raised the number of CD45 immune cells, significantly decreased the M2/M1 ratio in the tumor, and stimulated CD8 T cell activation. These findings imply that ginseng-derived NVs may primarily regulate CD4+ and CD8+ T lymphocyte activation, as the effect of combination therapy was significantly diminished when these cells were removed. GDNPs have a synergistic effect on PD-1 mAb therapy and can alter tumor-associated macrophages (TAMs) to boost the release of chemokine (C-X-C motif) ligand 5 (CXCL5) and CXCL9 to attract CD8 T lymphocytes into the tumor bed. This combined therapy approach may enable the body to develop tumor immunity, which may also prevent metastasis and recurrence of the disease.33 This combination drug delivery approach offers fresh perspectives on how to treat tumors without inflicting systemic toxicity.
In order to further investigate the specific mechanism of action of PDNVs on immune regulation, Liu et al conducted a more in-depth study.52 They found that not only ginseng-derived NVs can remodel the tumor microenvironment, but also Artemisia-derived nanovesicles can reprogram tumor-associated macrophages, greatly enhancing the efficacy of PD-L1 inhibitors. This effect may be mainly driven by the mitochondrial DNA (mtDNA) in the vesicles, which activates the cyclic GMP-AMP synthase/stimulator of interferon genes pathway (cGAS-STING) pathway in tumor-associated macrophages, awakening them from a pro-tumor state and promoting their transition to an anti-tumor state, ultimately leading to tumor regression (Figure 2). These findings further support the potential application of PDNVs as an immunomodulatory agent. However, it is important to note that this mechanism may not be present in all PDNVs, and further research is needed to explore the mechanisms of other PDNVs.
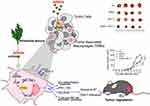 |
Figure 2 The natural anti-tumor bioactivity of PDNVs. Notes: Nanovesicles isolated from Artemisia contain a high amount of mtDNA, which can regulate tumor-associated macrophages through the cGAS/STING pathway and further modulate tumor progression. When combined with PD-L1 inhibitors, it significantly enhances the therapeutic effect. ***indicates P<0.001). Copyright 2014. Reproduced with permission from Liu J, Xiang J, Jin C, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnology. 2023;21(1):78.52Abbreviations: mtDNA, mitochondrial DNA; cGAS/STING, cyclic GMP-AMP synthase/Stimulator of Interferon Genes pathway; PD-L1, Programmed Death Ligand-1. |
Regulation of Tumor-Associated Fibroblasts
Tumor-associated fibroblasts (CAFs) play roles in regulating tumor cell invasion and metastasis, immune evasion, angiogenesis, and chemoresistance. They are a dominant and diverse cell type in TME, prevalent in most solid tumors.73,74 Kim et al found that tumor-associated fibroblasts were inhibited by Dendropanax morbifera derived NVs using a 3D microfluidic system for a cancer metastasis model. They discovered that Dendropanax morbifera derived NVs dramatically decreased the expression of genes linked to motility and extracellular matrix, including integrins and collagens, and prevented the formation of CAFs in a dose-dependent manner. For instance, under the influence of NVs, genes linked to the development of cancer, including TGF-β, can be lowered more than 5 times. The research also demonstrated that plant defense proteins, such as peroxidase in Dendropanax morbifera derived NVs, can lower the amount of hydrogen peroxide as well as raise ROS levels in the TME that can weaken tumor progression and metastatic ability.28 The concentration-dependent inhibitory action of PDNVs on CAFs has not been demonstrated in vivo, and additional research is required to determine whether PDNVs share these characteristics.
Regulation of Microenvironmental Microorganisms
Tumor occurrence and development have been linked to the presence of microorganisms in the TME, which play a role in the growth, invasion, metastasis, and drug resistance of tumors.75 The oral cavity, gastrointestinal system, respiratory tract, and other areas were thought to be the primary locations for these germs in the past.76 Numerous recent studies have also confirmed the existence of bacteria in organs like the breast that are not directly related to the outside environment. PDNVs are also a useful weapon for plants to fend off the invasion of outside bacteria and viruses. Studies on the regulation of oral flora and intestinal microbial flora by PDNVs are prevalent.77,78
The importance of bacteria in promoting tumor metastasis is demonstrated by a study that showed the disruption of gastrointestinal microbiota enhances breast cancer metastasis in a mouse model.79 Antibiotic treatment can disturb the balance of the gastrointestinal microbiota, increasing the chances of tumor metastasis. In mice, oral administration of tea-related substances, such as tea tree blossoms and tea-derived NVs, significantly increased the diversity and richness of the gastrointestinal microbial community, preserving the homeostasis of the intestinal flora. Furthermore, the researchers observed a significant reduction in breast cancer growth and metastasis. However, additional correlation analysis and verification are still required to determine whether this is due to the function of NVs in promoting gut flora homeostasis.44,47
Advantages: PDNVs are Suitable as Drug Delivery Vehicles
Trans-Biological Barrier Capacity
Plant-derived miRNAs have been found in human bloodstream, which can control the expression of specific genes in humans.80 According to Xu et al, ginseng-derived NVs can efficiently transmit miRNAs into BMSCs, encouraging them to differentiate into nerve cells.29 These studies reaffirm the ability of PDNVs to communicate with other species and across biological barriers. PDNVs are endocytosed by cells in an energy-dependent manner, as evidenced by reduced absorption when exposed to sonication or boiling. Various PDNVs are endocytosed by cells in different ways, for example, ginger-derived NVs can be endocytosed by colon 26 and HT-29 cells through phagocytosis and by primary hepatocytes through micropinocytosis.81
The poor solubility of many drugs is a primary barrier to their bioavailability and effectiveness.82 Interestingly, curcumin delivered by PDNVs is more stable and has a higher blood concentration. Clinical experiments have also shown that PDNVs can contribute naturally to the bioavailability of various hydrophobic and insoluble substances in the body, facilitating their entry into mammalian cells and aiding in the preservation of human health. The blood-brain barrier prevents 99% of drugs from crossing it, making it difficult to treat brain-related disease.83 Wang’s team showed that grapefruit-derived NVs loaded with DOX can treat glioma through the blood-brain barrier by tail vein administration.23 Grapefruit derived NVs loaded with miR-17 modified by folic acid (FA) can also be rapidly delivered to the brain by intranasal administration and selectively endocytosed by GL-26 tumor cells.84 Moreover, when grapefruit-derived NVs were used as drug delivery vehicles to inject pregnant mice through the tail vein, they were found to be unable to cross the placental barrier, suggesting good biocompatibility.24 Many studies have also demonstrated the good transdermal effect of PDNVs, which may be convenient for treating skin tumor or diseases.85
Natural Targeting Ability
Studies have demonstrated that different PDNVs may have a natural targeting ability, preferring to go to specific organs or cells.25 Moreover, numerous studies have shown that PDNVs have anti-cancer properties while having minimal harmful effects on healthy cells, particularly in inducing pro-apoptotic effects.24 For example, in A498 and A549 cancer cells, garlic-derived NVs differentially slowed down the cell cycle of tumor cells in the S phase, increased the expression of the pro-apoptotic genes Caspase 3 and 9, and decreased the expression of the anti-apoptotic gene Bcl-2 by 60%, with no adverse impact on healthy cells. Furthermore, at low dosages, garlic-derives NVs even induced fibroblast proliferation, indicating good biocompatibility.41 The pro-apoptotic effect of citrus limon derived NVs is primarily responsible for the regulation of the tumor necrosis factor (TNF)-related apoptosis-inducing ligand receptor (TRAIL-R), which induces cancer cell death by influencing the autocrine secretion of cells. In healthy cells, this protein is not expressed, which partially explains the selection-specific pro-apoptotic action of NVs.39
The targeting potential of PDNVs in vivo depends on several factors, including their composition, mode of administration, and the organism status.22,27 (1) The lipid composition of PDNVs may play a role in determining their preference to remain in the intestine or go to the liver. For example, ginger-derived NVs are rich in phosphatidic acid and more likely to stay in the intestine, while grapefruit-derived NVs containing phosphatidylcholine may target the liver.86 (2) The mode of administration also affects the destination of PDNVs. Intraperitoneal and tail vein injection of PDNVs may result in their accumulation in liver and spleen, while oral administration leads to their residence mainly in the gastrointestinal tract.46 Macrophages and stem cells are the primary targets of NVs after oral administration, and green tea-derived NVs can be selectively endocytosed by macrophages via galactose receptor-mediated endocytosis.47,87(3) The status of the receptor cells also plays a role in the destination of PDNVs. Mice fed an ethanol diet retained more NVs from ginger in the liver, while animals fed a non-alcoholic diet had more NVs in the colon.88 These observations suggest that the surface of PDNVs and cells may interact through some unique, yet undiscovered mechanisms. Further functionalization of PDNVs with cell-specific targeting capabilities and more natural targeting studies are necessary to improve drug delivery targets.
High Economic Benefit
Mammalian-origin derived extracellular vesicles (EVs) have been criticized in the field due to their high cost and poor production.89 In contrast, PDNVs may be a more cost-effective drug delivery systems because they are simpler to access, require less time for culture, and can save researchers’ time. Some academics have estimated the economic gain of using PDNVs. For example, the average amount of cabbage-derived NVs per gram was 1.504×1011 particles, while the retail price for per gram of cabbage was about $0.00137.17 Additionally, Li et al reported that they could handle 3 L (equivalent to 300 cell culture dishes (150 mm)) ginger-derived juice as a raw material in 1 hour.90 These examples illustrate the potential economic efficacy of employing PDNVs as drug delivery tools. Furthermore, researchers have shown that the bitter melon NVs had protein and RNA contents more than 100 times lower than those of the bitter melon juice, yet the effect was the same. This suggests a potent ability of PDNVs.35
Optimizating: Engineered PDNVs as Drug Delivery Platform to Amplify the Therapy Effects
The miRNAs, proteins, lipids, and metabolites present in PDNVs make them a natural source of anticancer therapeutic agents. Compared to single natural compounds or miRNAs, PDNVs offer a natural “shield” for these agents, with relatively good biocompatibility and bioavailability. In addition to the therapeutic cargo they carry, the inherent lipid bilayer vesicle structure of PDNVs allows for further optimization and upgrading by enabling drug loading and modification, making them more desirable anti-tumor tools (Table 2).
 |
Table 2 Engineered PDNVs Amplify Anti-Tumor Effects |
Preparatory Steps: Surface Functionalized Modification
The field of mammalian-derived EVs has seen several successful examples of surface functional modification, many of which can be applied to PDNVs for enhancing therapeutic efficacy. Popular examples include co-incubation, post-incubation co-extrusion, and sonication. The simplest modification approach involves co-incubating NVs with aptamers at low temperature, often for more than 12 hours.37,85,91 Researchers have also used catalysts, such as N-ethyl-N’-(3-(dimethylamino)propyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS) to boost efficiency.37 Passive methods such as ultrasound may improve modification efficiency, but their potential impact on content leakage remains to be explored. For example, Zhang et al used vortexing and sonication to optimize the pharmacokinetic features of asparagus-derived NVs with PEG modification for prolonged cycling stability.58
Notably, contrary to the mainstream mammalian-derived EVs studies, many researchers have frequently “reconstituted” PDNVs by primarily using their lipids to prepare lipid-rich membranes. They then co-incubate them with tumor-associated targeting receptors, such as folate receptors, and pass them through 200 nm polycarbonate membranes to form folate-targeting membrane-like NVs structures.84,94 The plentiful phospholipids or cholesterol of PDNVs can serve as lipophilic anchors for these receptors during co-incubation, incorporating the receptors into the lipid bilayer. In addition, researchers have effectively modified HA to the surface of reconstituted grapefruit-derived reconstituted NVs loaded with DOX by incubating the lipids-rich membranes with polyetherimide (PEI) and HA1 at 4°C for an extended period of time after sonication.91 Composite membrane vesicles can also be created by mixing PDNVs with animal-derived membranes for more natural in vivo tumor targeting capabilities. For example, grapefruit-derived NVs and plasma membranes were combined via co-extrusion, and the resulting hybrid NVs could target inflammatory tumor tissues.98
In the field of mammalian cells -derived EVs, the technique of transfecting source cells to highly express specific proteins or RNAs before isolation and obtaining EVs rich in related targets is in full swing, but there is no pertinent attempt information in the field of PDNVs.83,99 The biological activity of proteins, safety, and cross-disciplinary collaboration are crucial factors when producing human proteins in plant cells. When modifying the surface of PDNVs, the lower number of modifiable sites due to their lipid-rich and protein-poor composition than EVs obtained from animals cells may affect the modification efficiency. Moreover, no agreed-upon standards exist for evaluating the effectiveness of various modification tactics or the appropriate modification strategies for particular PDNVs components.
Additionally, the protein corona effect is a well-known phenomenon that occurs when extracellular vesicles interact with proteins in a biological system, resulting in the formation of a layer of protein coating.100,101 This protein corona is formed due to the interaction between the surface bioactive functional groups of the extracellular vesicles, such as hydroxyl and amino groups, and the proteins.101 Various factors, including the size, shape, surface charge, and protein concentration of the extracellular vesicles, influence the formation of the protein corona.102,103 It remains unknown whether PDNVs, which have a different composition from mammalian-derived extracellular vesicles and contain a richer lipid component, will form this protein corona. Addressing this issue is crucial because the formation of the protein corona in vivo may significantly impact the distribution and drug delivery efficiency.104 Therefore, further investigation is needed to elucidate the protein corona effect in PDNVs and its potential implications for their therapeutic applications.
Preparatory Steps: Loading of Therapeutic Cargo
Researchers in the field of PDNVs have successfully loaded numerous drugs, such as chemotherapeutic drugs, anti-inflammatory drugs, miRNAs, siRNAs, DNA expression vectors, and proteins, into PDNVs using co-incubation, sonication, and post-incubation co-extrusion.24 The most significant benefit of this drug-loading strategy is its ability to significantly lower drug toxicity and increase efficacy. For example, Zhang et al constructed ginger-derived NVs loaded with DOX having FA ligands with FA targeting ability. These ginger-derived NVs loaded with DOX had stronger therapeutic effects than DOX loaded in liposomes and could greatly reduce the cardiotoxicity of DOX.105 Wang et al discovered that when grapefruit-derived NVs co-incubated with methotrexate (MTX) for 2 hours, the therapeutic efficacy was substantially higher than that of MTX alone, and the effect was significantly increased.106
Efficiently drug loading into PDNVs remains a problem that researchers in this field are working to solve. Studies have shown that the loading efficiency of DOX into PDNVs can be improves by using layer-by-layer fucoidan/polylysine functional ginger-derived NVs and co-extruding DOX with the lipid membrane, which resulted in a loading efficiency of up to 73.7 ± 5.7%.105 Additionally, ultrasound can help positively charged DOX efficiently attach to negatively charged lipids by electrostatic interaction, resulting in an efficiency of up to 95.9 ± 0.26% for loading Dox.94 Furthermore, patching DOX-loaded heparin-based nanoparticles (DNs) onto the surface of natural grapefruit NVs (NV-DN2), catalyzed by EDC/NHS, resulted in a 4-fold higher loading efficiency of NV-DN2 compared to conventional methods of encapsulating DOX, with an efficiency of 0.014–0.48 g DOX/1 g of EVs.23
Loading miRNAs into PDNVs with the help of additional reagents has also led to promising results. For example, Zhuang et al used polyethyleneimine (PEI) as an effective enhancer for nucleic acid delivery and found that the capacity of the grapefruit-derived NVs to carry miR-17 increased from 5.91±0.6% to 86.2±5.7% in the presence of PEI. However, neutralizating grapefruit-derived NVs significantly reduced the harmful effects of PEI.84,107 Transfection reagents on the market have also been shown to be effective in loading miRNAs into PDNVs. Del Pozo-Acebo also showed that the relative expression of miRNA was at least 600-fold higher after loading miRNA into Broccoli derived NVs using transfection reagent.96 After 4 hours of incubation transfection reagents and miR-184 with 1×1011 Cabex derived NVs at 37°C, You et al found a 667,000-fold rise in miRNA levels of Cabex derived NVs, indicating a sizable quantity of miRNA loading.17 However, the metrics for evaluating loading efficiency vary, making it difficult to identify key factors affecting loading efficiency, such as miRNA length, efficiency of transfection reagents, and length of the incubation period.
There are also many factors that can affect loading efficiency, such as the ratio of PDNVs to drug. Zeng et al found that mixing 300 μg/mL indocyanine green (ICG) with 200 μg/mL aloe-derived NVs (1 mL) and incubating at 37°C for 30 minutes produced better results than other ratios.85 When multiple drugs are loaded, there may be a “competitive” relationship between the drugs, and Zeng et al proposed using π-π stacking interactions to turn DOX and ICG into “friends”, making the relationship “cooperative” and improving drug delivery efficiency.95 However, loading of large molecules remains the bottleneck, and techniques such as electroporation may damage the membrane structure of PDNVs. Further studies are needed to resolve the impact of recipient cell type, drug delivery method, molecular weight size, source of plant cells, loading co-incubation time, and drug solubility on loading efficiency.
Goals: Constructing an Ideal Drug Delivery Platform with Following Characteristics
The ideal tumor drug delivery system should have the following five characteristics:108 (1) High circulatory stability. Where the drug delivery system is not recognized and eliminated by the body’s mononuclear phagocytes and complement system, increasing its chances of reaching the tumor site.109 (2) Cancer targeting ability. Allowing drugs to specifically target and accumulate in tumor tissues, reducing the potential for side effects on healthy tissues.97 (3) Penetration ability. Enabling the drug delivery system to penetrate the fibrous capsule of tumors.13 (4) Easily endocytosis by tumor cells. A drug delivery system facilitates cellular uptake of the drug, which is essential for effective drug delivery.11 (5) Controlled drug release. Ensuring the drug is successfully released into the cells after passing through the drug delivery system.110 (Figure 3)
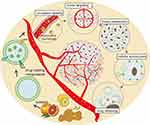 |
Figure 3 Engineered PDNVs as ideal antitumor drug delivery systems. Abbreviations: HER2, Human Epidermal growth factor Receptor 2; cRGD, arginine-glycine-aspartate cyclic peptide; FA, folic acid. |
PDNVs can serve as natural drug delivery platforms with some targeting ability, the ability to cross biological barriers, and the ability to be easily endocytosed by cells. However, in order to become an ideal drug delivery system, PDNVs must possess each of these five traits one at a time to improve effectiveness and enhance bioavailability. Furtunately, PDNVs are a promising paradigm, and with the right modifications, PDNVs can overcome these challenges and become an ideal effective drug delivery system.
Improving Circulation Stability
After oral administration, PDNVs are resistant to the gastric acid environment and do not activate complement bodies like C3a, which makes them a promising new approach for delivery drugs to the gastrointestinal tract. However, when administered intravenously PDNVs are quickly cleared from the body due to the activation of the complement system.44 According to studies, co-administrationof grapefruit-derived NVs (GDNVs) after exosomes isolated from the patient’s blood can reduce the accumulation of GDNVs in the liver and redirect them to the lungs. This may be due to the critical roles played by CD36 and IGFR1 receptors in blood exosome-mediated inhibition of GDNVs entry into hepatic macrophages.111
After tail vein injection, asparagus-derived NVs tend to accumulate most frequently the liver and spleen, where are two fundamental parts of the mononuclear macrophage system. Polyethylene glycol (PEG) can be used to bind and protect the cell membrane, effectively preventing recognition by scavenger receptors and prolonging the circulation time.58 Heparin has also been utilized to reduce phagocytosis by the immune system and improve the stability and bioavailability of PDNVs in vivo due to its strong anti-complement activation capabilities. Niu et al modified the surface of PDNVs with heparin, resulting in a significantly prolonged half-life of DOX to about 69.3 hours and a substantially higher amount of drug in tumor tissue compared to a single tail vein injection of DOX23 (Figure 4). Unlike traditional nanomaterials,112,113 PDNVs have a reported size of around 200nm and are cleared from the body primary due to their surface characteristics. However, the detailed mechanism of PDNVs clearance is not yet fully understood.
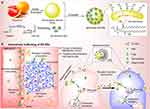 |
Figure 4 The schematic of engineered grapefruit-derived NVs as biomimetic drug delivery tools. (a) cRGD and ADH-DOX were modified on heparin and self-assembled into PH-sensitive heparin-modified nanovesicles (DNs). DNs were assembled with extracellular vesicles purified from grapefruit to synthesize biomimetic extracellular vesicles (EV-DNs). (b) The process and situation of endocytosis of EV-DNs in cells. Abbreviations: cRGD, arginine-glycine-aspartate cyclic peptide; DOX, doxorubicin; ADH, adipic acid dihydrazide; EVs, Extracellular vesicles; DNs, DOX-loaded heparin-based nanoparticles. Note: Reprinted with permission from Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. Copyright 2021 American Chemical Society.23 |
Targeting Delivery to Tumors
Precise targeted delivery is crucial in achieving the goal of precision medicine. PDNVs have shown potential selective effects on tumor cells, such as pro-apoptosis and anti-proliferation, while exhibiting only minor toxicity on normal cells. However, they do not naturally target tumor tissues in vivo. Although there are many targeted drugs available on the market for tumor therapy, such as erlotinib, drug resistance remains a challenge. To address this, researchers have focused on modifying PDNVs to target specific proteins that are highly expressed in tumor cells, such as FA, human epidermal growth factor receptor 2 (HER2), and Epidermal Growth Factor Receptor (EGFR) (Figure 5).114 For example, modifying cRGD on the surface of PDNVs to recognize integrin αvβ3 receptors, which enriched on the surface of tumor cells, significantly improved targeting efficiency compared to unmodified NVs.23,87
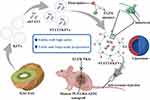 |
Figure 5 Engineered PDNVs targeted drug delivery platform. EGFR ligand-modified kiwifruit-derived extracellular vesicles are used as a siSTAT3 delivery platform for the treatment of cancer. Abbreviations: KEVs, kiwi fruit extracellular vesicles; STAT3, Signal Transducer and Activator of Transcription 3; EGFR, Epidermal Growth Factor Receptor. Notes: Reproduced from Huang H, Yi X, Wei Q, et al. Edible and cation-free kiwi fruit derived vesicles mediated EGFR-targeted siRNA delivery to inhibit multidrug 785 resistant lung cancer. J Nanobiotechnology. 2023;21(1):41.114 |
FA is a high-affinity ligand for the folate receptor and is highly expressed in many tumors but almost negligible in non-tumor cells.115 Currently, the most popular approach for surface modification of PDNVs is to use FA as a targeted switch. For example, modifying ginger NVs with folate receptor targeting resulted in a 2.8-fold increase in targeting efficiency compared to unmodified ginger-derived NVs.94 This approach has also been successfully applied to grapefruit-derived NVs to target GL-26 brain tumors.24,84 Additionally, cholesterol-coupled pRNA-3WJ can be used to modify NVs with cancer-specific ligands for targeting cancer cells and solid tumors. Ginger-derived NVs have been functionalized using arrowtail pRNA-3WJ and FA for ligand display, enable systematic gene targeting delivery to tumor sites.90
The increased expression of HER2 is a hallmark of HER2+ breast cancer, which promotes cancer cell proliferation and aggressiveness. Tang et al used Cell-SELEX to select an aptamer, hyaluronan 1 (HA1) that targets HER2-overexpressing breast cancer cells. With the aid of HA1, the effectiveness of PDNVs targeting breast cancer was dramatically increased.91 Similarly, p-selectin, a tumor-associated cell adhesion molecule, has been identified as a potential cancer-specific delivery targets for colon cancer therapy, as it is present on cell membranes of tumor-adjacent endothelial cells and on secretory α-granules of platelets.116 Zhang et al selected rockweed polysaccharide, a polysaccharide with high affinity for p-selectin, as a modifier of PDNVs to target tumors. This approach was at least 4.4-fold more efficient than the unmodified one in accumulating in tumors. Upon delivery to the tumor microenvironment, high local concentrations of hydrogen peroxide and free radicals trigger the degradation of rockweed polysaccharides, exposing PDNVs to penetrate tumor tissue.105
Targeting tumor tissue with a single protein change may not offer a competitive advantage, which is why researchers have employed membrane vesicles encasing PDNVs in the presence of several proteins. As tumor tissues frequently display inflammation, it may be possible to modify switches on the surface of PDNVs to target inflammatory regions. Studies have demonstrated that inflammatory chemokine receptors found on the surface of leukocytes can target inflammatory tissues, among others.117 To improve the ability of PDNVs to target inflammatory tumor sites, Wang et al wrapped plasma membranes of activated T cells coated with inflammatory chemokine receptor enrichment on the surface of grapefruit-derived NVs and enhanced their homing to inflammatory tissues due to the high expression of chemokines at inflammatory sites. The targeting effect could be significantly inhibited by blocking lymphocyte function-associated antigen 1 (LFA-1) or CXC chemokine receptor 1 (CXCR1) and CXCR2 on their surface. By tracing, they found that this strategy effectively targeted tumor tissues and significantly inhibited tumor growth.98
Target-modified PDNVs have been shown to significantly reduce toxic side effects such as cardiotoxicity of chemotherapeutic drugs.105 However, it should be noted that different tumors have different labels, and current studies have focused on locally localizable tumors. More challenging in the clinical setting are potential tumor metastases, and more promising results would be achieved if targeting could be validated with a model of systemic multiple metastases. In addition, it has been demonstrated that more efficient accumulation at the tumor site can be achieved by targeting the tumor vasculature, which has not yet been attempted.
Promoting Tissue Penetration
Many drug delivery systems that target tumor tissues are unable to penetrate deeply into those tissues and must instead remain on the surface. Niu et al demonstrated that their method of patching heparin nanoparticles loaded with Dox onto the surface of grapefruit-derived NVs (NV-DN2) has good tissue penetration and can carry out transcytosis effectively, both in vitro and in vivo. They found that PKH67-NV and PKH67-NV-DN2 could successfully penetrate hCMEC/D3 cells into LN229 cells in an in vitro blood-brain barrier (BBB) model, and the bionic system NV-DNs could successfully cross the BBB/BBTB to target glioma cells by combining the benefits of NVs and DNs, such as αvβ3 receptor-mediated cytokinesis and membrane fusion. Moreover, NV-DN2 was found to successfully pass the BBB in an in vivo tumor model, with its red fluorescence accumulating in intracranial tumors.23 In addition, many studies have shown that PDNVs have a transdermal effect. For example, aloe-derived NVs are effectively skin-permeable and could be used as a non-invasive transdermal drug delivery strategy for skin tumors.85
Enhancing Cellular Uptake
To effectively deliver drugs to tumor cells, PDNVs must endocytosed by the cells, and the uptake rate is particularly important. Xiao et al created a heparin-cRGD-EVs-doxorubicin (HERD) that was able to enter DOX-resistant cancer cells via caveolin-mediated endocytosis (primary), macrophage-mediated endocytosis (secondary), and coagulase-mediated endocytosis (final), showing good cellular uptake. Surface-modified PDNVs have also been shown to have better endocytosis by cells.37 These uptake pathways are important physiological channels for cellular uptake of EVs for cellular communication and are not drug transporters, so the potential for drug resistance is likely to be low. Endocytosis can be further improved by modifying positively charged substances on the surface of NVs. For example, modifying positively charged poly-lysine on the surface of PDNVs can make them more readily available for cellular uptake.105
Optimizing Drug Release
After the successful uptake of PDNVs by cells, it is essential that the drug within the NVs can be released to achieve its therapeutic effect. Several studies suggest that PDNVs have a natural PH-responsive drug release ability, which is favorable in the acidic tumor microenvironment. Zhang et al co-incubated DOX with ginger-derived NVs to form a membrane-like structure and found that such vesicles had a stronger therapeutic effect and a better ability to release drugs in response to PH compared to liposome-loaded DOX. This natural PH responsiveness may be due to the significant reduction in the negative charge on the surface of NVs and reduced electrostatic adsorption with the drug under tumor-acidic conditions.93 Changes in PH alter the potential on the surface of PDNVs, and when PH is acidic, the zeta potential is usually positive, around 2.84, while in neutral zeta potential, the zeta potential is around −25mv.88 Yi et al also found that about 88% of DOX was released from tea-derived NVs (TDNVs) under acidic conditions at pH=5.0, while at pH=6.0 and 7.5, about 80% and 70% of DOX was released from TDNVs after 25h. These results suggest that the main driving force for maintaining the stability of DOX-loaded TDNVs is the electrostatic interaction between DOX and TDNVs. As acidic conditions may lead to a decrease in negative charge on TDNVs, the binding between TDNVs and DOX is weakened, and more DOX is dissociated from TDNVs as the pH decreases.93 PH-responsive drug release behavior is a favorable property for in vivo antitumor applications.
In addition to the successful loading and release of chemotherapeutic drugs in PDNVs, many researchers have also tried loading RNA and proteins in PDNVs and obtained better results. Zhang et al selected biotin, as a contender and found that ginger-derived NVs carrying biotinylated eYFP DNA expression vectors expressed YFP protein in A549 cells with an efficiency comparable to Lipofectamine 2000 transfection. In addition, ginger-derived NVs carrying biotinylated proteins such as anti-CD4 or anti-CD8 antibodies significantly enhanced the transfection efficiency of CD4+ or CD8+ T cells in splenocytes, as well as the expression of fluorescein in these cells. This approach efficiently delivered luciferase functional siRNAs to the cells and efficiently inhibited gene expression.24 Additionally, You et al evaluated the efficiency of Cabex-derived NVs in delivering miRNA into cells. After co-incubation of the miRNA-loaded Cabex-derived NVs with colon cancer cells for 72 h, the levels of miRNA in the cells before and after administration were measured by real-time fluorescence quantitative PCR, revealing a 246,000-fold increase in miRNA.17 In addition, studies have also shown that external forces such as photothermal can enhance the release of PDNVs drugs at the tumor site.95,118
Key Consideration of PDNVs as Anti-Tumor Tools
The Mode and Interval of Drug Administration May Affect the Therapeutic Effect of PDNVs
Studies have shown that different modes of administration of NVs result in different therapeutic effects. For example, camellia sinensis-derived NVs activate the C3 complement system by tail vein injection and exhibit some hepatotoxicity, while oral administration has been shown to have a modulating effect on intestinal flora without exhibiting toxicity. Co-administration can also affect the therapeutic effect of NVs. For example, injecting plasma exosomes followed by grape-derived NVs -loaded with DOX and PTX can enhance their accumulation in tumor tissue and lungs via CD36 and insulin-like growth factor 1 receptor (IGFR1) -mediated pathways and inhibit their uptake by hepatic Kupffer macrophages, thereby enhancing the targeting of PDNVs.111 D. morbifera and P. densiflora sap-derived NVs showed strong cytotoxic effects on tumor cells, and the combination of both NVs was more effective than single NVs treatment, with a 4-fold and 3-fold reduction in IC50 of A431 cells, respectively, but further studies are needed to elucidate the mechanism of therapeutic effects.25 A caveat is that the timing of NVs administration is also critical, and studies have shown that milk-derived EVs may reduce primary tumor growth but accelerate tumor metastasis in mice with colon and breast cancer cells.119 Whether some PDNVs also have this negative effect needs to be explored further.
Source of PDNVs Affects Therapeutic Effect
The choice of PDNVs as an anti-tumor source is still an open question. In the above summary, we have seen various PDNVs with anti-tumor effects, but it is worthwhile to summarize and consider which PDNVs have the strongest effect of certain PDNVs on certain tumors. For example, ginger-derived NVs, which interfere with macrophages, preferentially induce the expression of the antioxidant gene heme oxygenase-1 (HO-1) and the anti-inflammatory cytokine IL-10; whereas grapefruit, ginger and carrot-derived NVs promote the activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2).120 Additionally, NVs derived from different parts of the same plant, such as roots and leaves, can have different effects. For example, Dendropanax morbifera leaf-derived NVs have better whitening and anti-melanin effects than the leaves.
Standards for the standardized isolation and production of PDNVs should be established as soon as possible. For example, different seasons of harvesting grapefruit and ginger may lead to the isolation of different constituents, and different varieties of plants may have varying compositions.121 Additionally, NVs isolated by different methods may have different sizes, and there are significant differences in the contents of NVs of different sizes. For example, NVs of citrus origin contain more organic acids and amino acids, while microvesicles contain more sugars and sugar derivatives, which can lead to different effects.32 These differences pose a challenge to the batch stability of the isolated substances.
Most current studies have obtained supernatants from roots, stems, branches, and leaves of plants by grinding or squeezing in a mixture mode. Boccia et al found that their tumor-promoting apoptotic activity was isolated from cultivated root systems of P. sagittatum in vitro, an approach that may enable the engineering of PDNVs as drug delivery systems and facilitate standardized production for clinic use, avoiding differences due to plant lot, species, and origin.51 Woith et al found that NVs could also be isolated from dried plants, possibly because NVs are stable enough to withstand the process of water loss, but whether there is a difference in effect is still unknown.122
EVs Isolation and Preservation
The isolation of PDNVs is a common bottleneck in the field of EVs research, and although PDNVs may have a higher economic impact, they face many problems, including aggregation during ultra-high speed centrifugation, particularly when the amount of vesicles is large.123 Scholars have used various methods to improve the yield of PDNVs, and reviews and reflections have been conducted.31 For example, the use of buffer pads can reduce aggregation due to long ultracentrifugation time, resulting in a 2-3-fold increase in yield.90 Reducing the centrifugation time and decreasing the centrifugation speed has also been shown to significantly reduce the aggregation of PDNVs. Chen’s group found that centrifugation times of 20 minutes and at lower centrifugation speeds increased the yield of NVs while avoiding aggregation.50,85 The use of buffers containing polyethylene glycol can maintain the integrity of NVs by wrapping them up to form a mesh network. Centrifugation methods based on polyethylene glycol-6000 (PEG6000) can also greatly improve the yield of NVs, with the authors indicating that 60–90% of ginger-derived NVs can be collected.124 The yield of ginger-derived NVs is further improved by 4–5 times when PEG precipitation is performed at low pH conditions (pH 4 and 5), and the authors indicated that this approach may not affect their biological activity.125 Chen’s team also developed an electrophoresis-based approach to collect PDNVs under an electric field, which may be easier to carry out in various laboratories compared to ultra-high-speed centrifuges, reducing the operating time.38
The preservation of the collected PDNVs is also an important topic that lacks standardization. Kim et al resuspended Dendropanax morbifera leaf-derived NVs in pure water stored them at various temperatures for about a month to assess the changes they underwent. They found that vesicles were less aggregated and more stable in size at −20 °C, but all samples underwent a loss in protein amount.126 Freeze-drying may be a better means of preserving samples because this method did not significantly change morphology, RNA, or protein content over longer periods of time, whereas repeated freeze-thawing may be lead to fusion of vesicles. Interestingly, it has been suggested that preservation conditions should be different for different PDNVs, which may be related to their charge and composition, but there is no definite law yet. It is also worth considering whether to prepare some preservation media specifically according to the nature of vesicles.
Conclusion
In conclusion, various lipids, proteins, RNAs, and metabolites carried on PDNVs have also shown excellent anti-tumor activities, including direct inhibition of tumor cell proliferation, potentiation apoptosis, and mitigation drug resistance behavior, as well as anticipated anti-tumor effects by changing the TME. Based on the unique lipid bimolecular structure of PDNVs, they have a natural trans-biological barrier structure that includes the blood-brain barrier and the skin barrier and is very simple for cells to uptake. Additionally, PDNVs may be taken up specially by some cells and organs, although the mechanism is unknown. PDNVs are more economical than those from animals. PDNVs are also natural drug delivery tools that allow drugs to be loaded onto NVs by co-incubation, and better loading may be accomplished with the cooperation of some pro-drug delivery tools. Furthermore, methods to improve the circulation stability, tumor targeting, tissue penetration, and cellular uptake of NVs are anticipated in order to achieve the precise targeting of drugs for release in tumor cells.
Future research on plant-derived nanovesicles (PDNVs) should focus on improving the purity, stability, and yield during isolation and preservation, optimizing biological activity and dosages, enhancing drug loading efficiency and surface modification of PDNVs, and developing effective evaluation methods to ensure their safety and efficacy for clinical applications. At least 2 clinical trials about PDNVs are currently being conducted, although the fact that there is still more work to be done. Researchers have paid a lot of attention to this tiny vesicle, and one day its great power in anti-tumor applications may emerge.
Funding
This work was supported by the National Natural Science Foundation of China (82272868 and 82173180) and Fujian Medical Innovation Foundation [grant number: 2020CXA049].
Disclosure
The authors declare that they have no competing interests.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA. 2021;71(1):7–33.
2. Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590.
3. Gulack B, Nussbaum D, Keenan J, et al. Surgical resection of the primary tumor in stage IV colorectal cancer without metastasectomy is associated with improved overall survival compared with chemotherapy/radiation therapy alone. Dis Colon Rectum. 2016;59(4):299–305.
4. Abraha I, Aristei C, Palumbo I, et al. Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma. Cochrane Database Syst Rev. 2018;10(10):Cd002102.
5. Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74(10):2663–2668.
6. Jenkins L, Jungwirth U, Avgustinova A, et al. Cancer-associated fibroblasts suppress CD8+ T-cell infiltration and confer resistance to immune-checkpoint blockade. Cancer Res. 2022;82(16):2904–2917.
7. Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19(7):405–414.
8. Akhavan O, Ghaderi E. Graphene nanomesh promises extremely efficient in vivo photothermal therapy. Small. 2013;9(21):3593–3601.
9. Ouyang C, Zhang S, Xue C, et al. Precision-guided missile-like DNA nanostructure containing warhead and guidance control for aptamer-based targeted drug delivery into cancer cells in vitro and in vivo. J Am Chem Soc. 2020;142(3):1265–1277.
10. Dutta B, Barick KC, Hassan PA. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv Colloid Interface Sci. 2021;296:102509.
11. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124.
12. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–1094.
13. He B, Sui X, Yu B, Wang S, Shen Y, Cong H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. 2020;27(1):1474–1490.
14. Sun Y, Zheng L, Yang Y, et al. Metal-organic framework nanocarriers for drug delivery in biomedical applications. Nanomicro Lett. 2020;12(1):103.
15. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–759.
16. Li YJ, Wu JY, Liu J, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021;19(1):242.
17. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332.
18. Di Gioia S, Hossain MN, Conese M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020;15(1):1096–1122.
19. Lian MQ, Chng WH, Liang J, et al. Plant-derived extracellular vesicles: recent advancements and current challenges on their use for biomedical applications. J Extracell Vesicles. 2022;11(12):e12283.
20. Urzì O, Raimondo S, Alessandro R. Extracellular vesicles from plants: current knowledge and open questions. Int J Mol Sci. 2021;22(10):1.
21. Kim SQ, Kim KH. Emergence of edible plant-derived nanovesicles as functional food components and nanocarriers for therapeutics delivery: potentials in human health and disease. Cells. 2022;11(14):1.
22. Ly NP, Han HS, Kim M, Park JH, Choi KY. Plant-derived nanovesicles: current understanding and applications for cancer therapy. Bioact Mater. 2023;22:365–383.
23. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492.
24. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867.
25. Kim K, Yoo HJ, Jung JH, et al. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J Funct Biomater. 2020;11(2):1.
26. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31.
27. Cong M, Tan S, Li S, et al. Technology insight: plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108.
28. Kim K, Jung JH, Yoo HJ, et al. Anti-metastatic effects of plant sap-derived extracellular vesicles in a 3D microfluidic cancer metastasis model. J Funct Biomater. 2020;11(3):1.
29. Xu XH, Yuan TJ, Dad HA, et al. Plant Exosomes As Novel Nanoplatforms for MicroRNA Transfer Stimulate Neural Differentiation of Stem Cells In Vitro and In Vivo. Nano Lett. 2021;21(19):8151–8159.
30. Woith E, Guerriero G, Hausman JF, et al. Plant extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int J Mol Sci. 2021;22(7):1.
31. Feng J, Xiu Q, Huang Y, Troyer Z, Li B, Zheng L. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Adv Mater. 2023;2023:e2207826.
32. Stanly C, Alfieri M, Ambrosone A, Leone A, Fiume I, Pocsfalvi G. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell Line. Cells. 2020;9(12):1.
33. Han X, Wei Q, Lv Y, et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30(1):327–340.
34. Xiao J, Feng S, Wang X, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. Peer J. 2018;6:e5186.
35. Yang M, Luo Q, Chen X, Chen F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnology. 2021;19(1):259.
36. Wang B, Guo XJ, Cai H, et al. Momordica charantia-derived extracellular vesicles-like nanovesicles inhibited glioma proliferation, migration, and invasion by regulating the PI3K/AKT signaling pathway. J Funct Foods. 2022;90(1):104968.
37. Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9(20):e2105274.
38. Yang M, Liu X, Luo Q, Xu L, Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnology. 2020;18(1):100.
39. Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–19527.
40. Raimondo S, Saieva L, Cristaldi M, Monteleone F, Fontana S, Alessandro R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J Proteomics. 2018;173:1–11.
41. Özkan İ, Koçak P, Yıldırım M, et al. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021;11(1):14773.
42. Wongkaewkhiaw S, Wongrakpanich A, Krobthong S, Saengsawang W, Chairoungdua A, Boonmuen N. Induction of apoptosis in human colorectal cancer cells by nanovesicles from fingerroot (Boesenbergia rotunda (L.) Mansf.). PLoS One. 2022;17(4):e0266044.
43. Potestà M, Roglia V, Fanelli M, et al. Effect of microvesicles from Moringa oleifera containing miRNA on proliferation and apoptosis in tumor cell lines. Cell Death Discov. 2020;6:43.
44. Chen Q, Li Q, Liang Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12(2):907–923.
45. Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340.
46. Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7(1):326.
47. Zu M, Xie D, Canup BSB, et al. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279:121178.
48. Kantarcıoğlu M, Yıldırım G, Akpınar Oktar P, et al. Coffee-derived exosome-like nanoparticles: are they the secret heroes? Turk J Gastroenterol. 2022;2022:1.
49. Sasaki D, Kusamori K, Takayama Y, Itakura S, Todo H, Nishikawa M. Development of nanoparticles derived from corn as mass producible bionanoparticles with anticancer activity. Sci Rep. 2021;11(1):22818.
50. Chen T, Ma B, Lu S, et al. Cucumber-derived nanovesicles containing cucurbitacin B for non-small cell lung cancer therapy. Int J Nanomedicine. 2022;17:3583–3599.
51. Boccia E, Alfieri M, Belvedere R, et al. Plant hairy roots for the production of extracellular vesicles with antitumor bioactivity. Commun Biol. 2022;5(1):848.
52. Liu J, Xiang J, Jin C, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnology. 2023;21(1):78.
53. Phan LM, Yeung SC, Lee MH. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol Med. 2014;11(1):1–19.
54. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674.
55. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
56. Cockburn K, Annusver K, Gonzalez DG, et al. Gradual differentiation uncoupled from cell cycle exit generates heterogeneity in the epidermal stem cell layer. Nat Cell Biol. 2022;24(12):1692–1700.
57. Yang L, Shi P, Zhao G, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8.
58. Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int J Nanomedicine. 2021;16:1575–1586.
59. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–193.
60. Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8(4):603–619.
61. Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16(2):99–109.
62. Imran M, Aslam Gondal T, Atif M, et al. Apigenin as an anticancer agent. Phytother Res. 2020;34(8):1812–1828.
63. Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395–417.
64. Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: an overview. Cancers. 2014;6(3):1769–1792.
65. Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomedicine. 2017;12:2957–2978.
66. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7(3):339–348.
67. Sethy C, Kundu CN. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: implication of DNA repair inhibition. Biomed Pharmacother. 2021;137:111285.
68. Feng X, Luo Q, Zhang H, et al. The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. CR. 2017;36(1):81.
69. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284.
70. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263.
71. Raimondo S, Urzì O, Meraviglia S, et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J Cell Mol Med. 2022;26(15):4195–4209.
72. Trentini M, Zanotti F, Tiengo E, et al. An apple a day keeps the doctor away: potential role of miRNA 146 on Macrophages treated with exosomes derived from apples. Biomedicines. 2022;10(2):1.
73. Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18(1):70.
74. Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol Cancer. 2021;20(1):154.
75. Song S, Vuai MS, Zhong M. The role of bacteria in cancer therapy - enemies in the past, but allies at present. Infect Agent Cancer. 2018;13:9.
76. Wong-Rolle A, Wei HK, Zhao C, Jin C. Unexpected guests in the tumor microenvironment: microbiome in cancer. Protein Cell. 2021;12(5):426–435.
77. Hansen LL, Nielsen ME. Plant exosomes: using an unconventional exit to prevent pathogen entry? J Exp Bot. 2017;69(1):59–68.
78. Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, de la Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68(20):5485–5495.
79. Fu A, Yao B, Dong T, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185(8):1356–1372.e1326.
80. Liu YC, Chen WL, Kung WH, Huang HD. Plant miRNAs found in human circulating system provide evidences of cross kingdom RNAi. BMC Genom. 2017;18(Suppl 2):112.
81. Fujita D, Arai T, Komori H, et al. Apple-derived nanoparticles modulate expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 cells. Mol Pharm. 2018;15(12):5772–5780.
82. Hatamie S, Akhavan O, Sadrnezhaad SK, Ahadian MM, Shirolkar MM, Wang HQ. Curcumin-reduced graphene oxide sheets and their effects on human breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2015;55:482–489.
83. Song H, Chen X, Hao Y, Wang J, Xie Q, Wang X. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J Nanobiotechnology. 2022;20(1):431.
84. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24(1):96–105.
85. Zeng L, Wang H, Shi W, et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J Nanobiotechnology. 2021;19(1):439.
86. Teng Y, Ren Y, Sayed M, et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652.e638.
87. Abdolahad M, Janmaleki M, Mohajerzadeh S, Akhavan O, Abbasi S. Polyphenols attached graphene nanosheets for high efficiency NIR mediated photodestruction of cancer cells. Mater Sci Eng C Mater Biol Appl. 2013;33(3):1498–1505.
88. Zhuang X, Deng ZB, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713.
89. Fan SJ, Chen JY, Tang CH, Zhao QY, Zhang JM, Qin YC. Edible plant extracellular vesicles: an emerging tool for bioactives delivery. Front Immunol. 2022;13:1028418.
90. Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8(1):14644.
91. Tang Z, Jun Y, Lv Y, et al. Aptamer-conjugated and doxorubicin-loaded grapefruit-derived nanovectors for targeted therapy against HER2(+) breast cancer. J Drug Target. 2020;28(2):186–194.
92. Yan W, Tao M, Jiang B, et al. Overcoming drug resistance in colon cancer by aptamer-mediated targeted co-delivery of drug and siRNA Using grapefruit-derived nanovectors. Cell Physiol Biochem. 2018;50(1):79–91.
93. Yi S, Wang Y, Huang Y, et al. Tea nanoparticles for immunostimulation and chemo-drug delivery in cancer treatment. J Biomed Nanotechnol. 2014;10(6):1016–1029.
94. Zhang M, Xiao B, Wang H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–1796.
95. Zeng L, Shi W, Wang H, et al. Codelivery of π-π stacked dual anticancer drugs based on aloe-derived nanovesicles for breast cancer therapy. ACS Appl Mater Interfaces. 2022;14(24):27686–27702.
96. Del Pozo-Aceb L, López de Las Haza MC, Tomé-Carneiro J, et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol Res. 2022;185:106472.
97. Xia W, Tao Z, Zhu B, et al. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int J Mol Sci. 2021;22(17):1.
98. Wang Q, Ren Y, Mu J, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–2529.
99. Chen X, Li H, Song H, et al. Meet changes with constancy: defence, antagonism, recovery, and immunity roles of extracellular vesicles in confronting SARS-CoV-2. J Extracell Vesicles. 2022;11(12):e12288.
100. Al-Ahmady ZS, Hadjidemetriou M, Gubbins J, Kostarelos K. Formation of protein Corona in vivo affects drug release from temperature-sensitive liposomes. J Control Release. 2018;276:157–167.
101. Heidarzadeh M, Zarebkohan A, Rahbarghazi R, Sokullu E. Protein Corona and exosomes: new challenges and prospects. CCS. 2023;21(1):64.
102. Tóth E, Turiák L, Visnovitz T, et al. Formation of a protein Corona on the surface of extracellular vesicles in blood plasma. J Extracell Vesicles. 2021;10(11):e12140.
103. Wu JY, Li YJ, Wang J, et al. Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein Corona. J Nanobiotechnology. 2021;19(1):405.
104. Hajipour MJ, Raheb J, Akhavan O, et al. Personalized disease-specific protein Corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015;7(19):8978–8994.
105. Zhang M, Yang C, Yan X, Sung J, Garg P, Merlin D. Highly biocompatible functionalized layer-by-layer ginger lipid nano vectors targeting p-selectin for delivery of doxorubicin to treat colon cancer. Adv Ther. 2019;2(12):1.
106. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–534.
107. Teng Y, Mu J, Hu X, et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7(18):25683–25697.
108. Sun Q, Sun X, Ma X, et al. Integration of nanoassembly functions for an effective delivery cascade for cancer drugs. Adv Mater. 2014;26(45):7615–7621.
109. Sultana S, Alzahrani N, Alzahrani R, et al. Stability issues and approaches to stabilised nanoparticles based drug delivery system. J Drug Target. 2020;28(5):468–486.
110. Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26(19):1.
111. Wang QL, Zhuang X, Sriwastva MK, et al. Blood exosomes regulate the tissue distribution of grapefruit-derived nanovector via CD36 and IGFR1 pathways. Theranostics. 2018;8(18):4912–4924.
112. Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323.
113. Fazaeli Y, Akhavan O, Rahighi R, Aboudzadeh MR, Karimi E, Afarideh H. In vivo SPECT imaging of tumors by 198,199 Au-labeled graphene oxide nanostructures. Mater Sci Eng C Mater Biol Appl. 2014;45:196–204.
114. Huang H, Yi X, Wei Q, et al. Edible and cation-free kiwi fruit derived vesicles mediated EGFR-targeted siRNA delivery to inhibit multidrug resistant lung cancer. J Nanobiotechnology. 2023;21(1):41.
115. Fernández M, Javaid F, Chudasama V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci. 2018;9(4):790–810.
116. Qi C, Wei B, Zhou W, et al. P-selectin-mediated platelet adhesion promotes tumor growth. Oncotarget. 2015;6(9):6584–6596.
117. Bonecchi R, Graham GJ. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front Immunol. 2016;7:224.
118. Akhavan O, Ghaderi E, Aghayee S, Fereydooni Y, Talebi A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J Mater Chem. 2012;22(27):13773–13781.
119. Samuel M, Fonseka P, Sanwlani R, et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat Commun. 2021;12(1):3950.
120. Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573.
121. Pocsfalvi G, Turiák L, Ambrosone A, et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J Plant Physiol. 2018;229:111–121.
122. Woith E, Melzig MF. Extracellular vesicles from fresh and dried plants-simultaneous purification and visualization using gel electrophoresis. Int J Mol Sci. 2019;20(2):1.
123. Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4:29509.
124. Kalarikkal SP, Prasad D, Kasiappan R, Chaudhari SR, Sundaram GM. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci Rep. 2020;10(1):4456.
125. Suresh AP, Kalarikkal SP, Pullareddy B, Sundaram GM. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS omega. 2021;6(27):17635–17641.
126. Kim K, Park J, Sohn Y, et al. Stability of plant leaf-derived extracellular vesicles according to preservative and storage temperature. Pharmaceutics. 2022;14(2):5.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
