Back to Journals » International Journal of Nanomedicine » Volume 18
Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications
Authors Tian J, Han Z, Song D, Peng Y, Xiong M, Chen Z, Duan S , Zhang L
Received 13 October 2023
Accepted for publication 16 December 2023
Published 23 December 2023 Volume 2023:18 Pages 7923—7940
DOI https://doi.org/10.2147/IJN.S444582
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Eng San Thian
Jiaqi Tian,1,* Zhengpu Han,1,2,* Dandan Song,1 Yanjie Peng,1 Min Xiong,3 Zhen Chen,2 Shuyin Duan,4 Lin Zhang1,5
1Clinical Medical Research Center for Women and Children Diseases, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan, People’s Republic of China; 2School of Public Health, Weifang Medical University, Weifang, People’s Republic of China; 3School of Public Health, North China University of Science and Technology, Tangshan, People’s Republic of China; 4School of Public Health, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, People’s Republic of China; 5Key Laboratory of Birth Defect Prevention and Genetic Medicine of Shandong Health Commission, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lin Zhang, Clinical Medical Research Center for Women and Children Diseases, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University, Jinan, 250001, People’s Republic of China, Tel +86-531-68795046, Email [email protected] Shuyin Duan, School of Public Health, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, 250001, People’s Republic of China, Tel +86-13419581411, Email [email protected]
Abstract: Exosomes are nano-sized membrane vesicles that transfer bioactive molecules between cells and modulate various biological processes under physiological and pathological conditions. By applying bioengineering technologies, exosomes can be modified to express specific markers or carry therapeutic cargo and emerge as novel platforms for the treatment of cancer, neurological, cardiovascular, immune, and infectious diseases. However, there are many challenges and uncertainties in the clinical translation of exosomes. This review aims to provide an overview of the recent advances and challenges in the translation of engineered exosomes, with a special focus on the methods and strategies for loading drugs into exosomes, the pros and cons of different loading methods, and the optimization of exosome production based on the drugs to be encapsulated. Moreover, we also summarize the current clinical applications and prospects of engineered exosomes, as well as the potential risks and limitations that need to be addressed in exosome engineering, including the standardization of exosome preparation and engineering protocols, the quality and quantity of exosomes, the control of drug release, and the immunogenicity and cytotoxicity of exosomes. Overall, engineered exosomes represent an exciting frontier in nanomedicine, but they still face challenges in large-scale production, the maintenance of storage stability, and clinical translation. With continuous advances in this field, exosome-based drug formulation could offer great promise for the targeted treatment of human diseases.
Keywords: engineered exosomes, drug delivery, clinical application, preparation strategy
Introduction
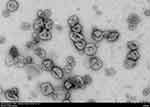
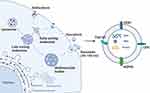
Exosomes are nano-sized membrane vesicles secreted by cells with a diameter ranging from 30–150 nm (Figure 1). They were first discovered in the culture supernatant of in vitro sheep reticulocytes. At first, exosomes are hypothesized to transfer unnecessary proteins between cells during cell growth and development, which serves as vehicles for the disposal of cellular metabolic “wastes”.1,2 Subsequent studies demonstrate that cells that generate small vesicles through endocytosis can fuse to form early endosomes, and the latter gradually mature into late endosomes. As the late endosomes produce numerous intraluminal vesicles (ILVs) and evolve into multivesicular bodies (MVBs), the newly generated ILVs are then released extracellularly along with the MVBs, fusing with the plasma membrane (Figure 2).3 These vesicles contain various active substances, including nucleic acids, proteins, and lipids, and can transmit information between cells to regulate pathological and physiological activities.4–9 Compared with commonly used drug carriers such as liposomes and inorganic mesoporous materials, exosomes exhibit superior biocompatibility, targeting ability, low toxicity, and high transmission efficiency. Specifically, they are free of side effects caused by material aggregation, making them a highly promising drug carrier.10,11 Recent studies show that bioengineering techniques can modify exosomes to enhance their drug-carrying capacity, significantly expanding their clinical potential and scope. As a result, exosomes have become a hot point in clinical medicine and biomedical research, and they are now supposed to be a crucial and smart tool for understanding the molecular mechanism of diseases and serve as an emerging opportunity for clinical and translational studies.
Engineered exosomes are natural exosomes treated with bioengineering techniques to enhance their drug-loading efficiency, targeting ability, and resistance to clearance by the body.12 Typically, engineered exosomes do not differ significantly in size or shape from naturally occurred exosomes. However, their cargo or contents may vary depending on the purpose of the research. For example, drugs can be encapsulated inside or on the surface of exosomes through genetic engineering and chemical modification, while drug-loaded exosomes have broad applications in tumor therapy and immune regulation. Currently, drug formulations based on engineered exosomes are being tested for cancer, cardiovascular disease, and neurodegenerative disorders.13–16 In addition, engineered exosomes can also be combined with nanomaterials such as metal nanoparticles and graphene to improve drug delivery efficiency, thereof expanding the application range of existing classic drugs.17,18 However, there is no unified standard for exosome drug loading strategies in practical applications, and even the same preparation method lacks a unified experimental scheme. Therefore, it is essential to systematically sort out a widely accepted approach for the preparation of drug-loaded exosomes.
To summarize new methods in the field and provide researchers with reliable experimental schemes, we will detail the preparation and drug loading methods of engineered exosomes in this review, then discuss the advantages and disadvantages of different preparation methods and their applicable conditions, and provide application cases on drug-loaded exosomes in treating specific diseases.
Drug Loading into Exosomes: Methods and Strategies
Endogenous Loading Method
Endogenous loading is an engineering method based on parent cells. This method involves modifying donor cells and introducing target molecules through direct transfection and co-incubation. After the donor cells are loaded with target molecules, the MVBs can deliver active molecules to extracellular vesicles via ILVs, as shown in Figure 2.19,20 Notably, the co-incubation method is one of the most widely used drug loading strategies, and they put the drugs and donor cells together, which induces new biological characteristics in exosomes by altering culture conditions and the incubation environment of donor cells. Critically, this method involves selecting suitable donor cells, such as fibroblasts and hematopoietic stem cells, and then incubating them with drugs suspended in the culture medium. Under appropriate stimulation, which will induce the release of exosomes, the drug-loaded exosomes will then be isolated and purified using techniques such as ultracentrifugation, polymer-based precipitation, immunoaffinity capture, and microfluidics.
The endogenous loading method has a wide spectrum of cargoes that can be loaded, among which the most common bioactive molecules are RNAs, proteins, small nucleic acids, and small molecules. Studies have shown that paclitaxel (PTX) can be isolated from mesenchymal stem cell (MSC)-derived exosomes, and the paclitaxel-loaded exosomes are verified to be with strong anti-proliferative activity and are more effective and biocompatible than PTX alone.21,22 However, the endogenous loading method based on drug and donor cell co-incubation still faces challenges in verifying the drug-loading efficacy. Also, it is high-cost and has difficulty selecting the most suitable donor cells and drugs.
By encapsulating the desired drugs or expressing them on the membrane of exosomes, transfection is another commonly used endogenous drug-loading method to deliver monomeric compounds or nucleic acids into donor cells.23,24 The transfection process can be achieved through chemical, electroporation, or virus vector-mediated arms, and the procedures are summarized as collecting donor cells, transfecting the required cargo into donor cells, collecting exosomes in the supernatant, removing cell debris and large molecular impurities, and finally harvesting drug-loaded exosomes. Practically, multiple factors must be considered when using the transfection method to load drugs, which includes the type of target cells, the properties of the required cargo drugs (such as size, charge, and hydrophilicity), and the desired therapeutic effects. Attention must also be paid to the transfection process to ensure cell health and exosome yield. It should be noted that preparing drug-loaded exosomes requires strict experimental design and verification to determine the most suitable cargo molecules and experimental conditions. Wen et al25 transfected miRNA-144 into bone marrow mesenchymal stem cells and successfully prepared miRNA-144-loaded exosomes. By applying them to heart cells, they found that these exosomes can improve myocardial cell apoptosis under hypoxic conditions through the PTEN/Akt pathway.
Exogenous Loading Method
Exogenous loading refers to the use of membrane penetration strategies to load drugs directly into pre-separated exosomes. It can be used to load small molecule drugs, proteins, and even nanomaterials.26–28 Compared with endogenous loading methods, exogenous loading has a lower technical threshold and more alternatives, and it is currently a widely used and common method for engineering exosomes to load drugs. Generally, exogenous loading methods include drug co-incubation, ultrasonic incubation, electroporation incubation, saponin treatment, freeze-thaw cycles, extrusion, and transfection, which are summarized in Figure 3.
The drug co-incubation method is a common drug loading strategy where purified exosomes are incubated with drugs at room temperature. This procedure involves precisely weighing the drug in a volumetric flask and dissolving it in an appropriate buffer using ultrasound before diluting it. Then, the exosome suspension is diluted, followed by the addition of an equal volume of a drug solution. After being thoroughly mixed, the suspension is incubated in a shaker at constant temperature for a period, and then the drug-loaded exosomes are harvested. Sun et al29 found that the curcumin-loaded exosomes can protect mice from lipopolysaccharide-induced septic shock, and exosomes encapsulated with curcumin also increase drug stability and bioavailability in vivo.
Based on the pulsed-focused ultrasound technology, the ultrasonic incubation method can temporarily enlarge the pores and fissures in the exosome membrane to facilitate drug entry. This specific procedure involves treating purified exosomes and drug solution with ultrasound in the ice-water bath. The ultrasound conditions are commonly set as 20% amplitude, 5 seconds on, 5 seconds off, and 1-minute cycles for a total of 6 cycles with 2-minute intervals between cycles. The sample is then removed and allowed to recover for 1 hour in a 37°C incubator. Subsequently, the sample is centrifuged at 120,000 g for 70 minutes at 4°C to remove the supernatant and free drug. After washing with phosphate-buffered saline (PBS), the drug-loaded exosome suspension is centrifuged again for 70 minutes and resuspended in an appropriate volume of PBS to obtain the drug-loaded exosome. With the wide application of this technology, Alptekin et al30 demonstrated an enhanced drug delivery of exosomes treated with ultrasound, and those exosomes can successfully transport the drugs to the stroke area of the brain without harming the normal brain structure.
Similarly, using transient current pulses, the electroporation method can create a pore in the exosome membrane through which drug molecules are engulfed. In detail, the exosomes are mixed with the desired drug in an electroporation buffer before placing them in an electroporation cuvette. The mixture is pressurized at 4°C, where the required voltage, pulse, and time are selected based on the most effective preparation parameters for the drug and equipment used. The mixture is then incubated for 30 minutes in a water bath at 37°C until the exosome membrane has fully recovered before further purifying the exosomes. Yan and colleagues31 utilized milk exosomes to develop an effective miRNA-31-5p delivery system, which encapsulated miRNA-31-5p mimics via electroporation, and they demonstrated that exosomes had increased cellular uptake level of miRNA-31-5p and improved anti-degradation properties. Additionally, other researchers32,33 show that miRNA-loaded exosomes can significantly improve endothelial cell function by promoting angiogenesis in vitro and enhancing diabetic wound healing in vivo.
Saponin is a commonly used surfactant that acts as a membrane permeabilizer. Through the saponification process, the saponin method induces the formation of small pores in lipid membranes, thereby facilitating the entry of hydrophilic and non-permeable molecules into exosomes with improved drug loading efficiency. The specific procedure for the saponin method includes exosome purification, followed by incubation with saponin and drugs for 10 minutes. Again, the purification procedure is performed through low permeability dialysis to obtain drug-loaded exosomes. Utilizing the saponin method, Wang et al34 successfully encapsulated the anticancer drug PTX into exosomes and revealed that these drug-loaded exosomes significantly enhanced the cytotoxicity of cisplatin (CDDP) on gastric cancer cells and reversed the CDDP resistance both in vitro and in vivo. Similarly, Thakur et al35 prepared exosomes loaded with doxorubicin (DOX) using the saponin method based on mouse liver cells. The resulting DOX-loaded exosomes considerably enhanced the DOX uptake in tumor cells with notably increased antitumor effects.
Based on the principle that lowering the temperature below 0°C would result in the freezing and volume expansion of water inside exosomes, the freeze-thaw method can encapsulate drugs into exosomes by enlarging the exosome membrane pore size. Upon meeting the threshold of the expansion stress, fissures are developed in the exosome membrane structure. They subsequently increase the temperature of the suspension, resulting in the melting of ice into water, which then enters the interior of the exosomes through pores or capillaries on the surface of the membrane structure. As the expansion stress decreases, pores on the exosome membrane contract. The specific procedure involves mixing exosomes and drugs before rapidly freezing at −80°C and thawing at room temperature for at least 3 cycles to load drugs into exosomes. Ebrahimian et al36 successfully loaded sesamin into MSC-derived exosomes using the freeze-thaw method, and they showed that the exosome nanodrug could specifically target breast cancer cells to release sesamin and induce cell apoptosis. Surprisingly, the efficacy of this drug was validated to be safe for human beings in vivo and held promising potential as an effective treatment for breast cancer.
The membrane extrusion method involves mixing exosomes with drug molecules and passing them through a nanofiltration membrane filter to create fissures in the exosome membrane for drug loading. The specific procedure involves dissolving drugs in an appropriate solvent before slowly adding them to exosomes. The mixture is then extruded using devices such as a manual extruder or microgrinder to encapsulate drugs inside exosomes. Finally, excess solvent and unencapsulated drugs are removed through centrifugation or filtration to obtain pure drug-loaded exosomes. Liang et al37 used the extrusion method to co-package exosomes with drugs and miRNA-21 inhibitors that can be stably released to inhibit tumor growth and metastasis.
The exosome transfection method is the process of introducing exogenous DNA or RNA fragments into exosomes to obtain new phenotypes. The common methods for exosome transfection include electroporation, cationic polymer-based transfection, and chemical transfection. Electroporation uses a pulsed electric field to apply voltage to exosomes, resulting in the opening of the exosome membrane and allowing drugs to enter the exosomes. Electroporation requires the collection of exosomes from the culture medium, followed by purification of the obtained exosomes through ultracentrifugation or other methods. The transfection complex is prepared by packaging the exosomes with DNA or RNA. Finally, electroporation parameters are adjusted to ensure optimal transfection efficiency. Parameters such as voltage, pulse duration, and electrode spacing need to be optimized according to cell type and exosome type. Yang et al38 proposed a new method for bladder cancer treatment using the electroporation method to prepare drug-loaded exosomes. By loading quantum dots into stem cell-derived exosomes, they found that quantum dot-loaded exosomes had higher targeting specificity for bladder cancer cells with minimal side effects, making them a potential precision treatment for bladder cancer. To transfect klotho plasmid into MSC-derived exosomes, Chen et al39 first transfected klotho plasmid into MSCs, then used electroporation to load adenosine kinase (ADK) siRNA into the exosomes, and finally successfully obtained klotho/ADK siRNA-loaded exosomes. Results of flow cytometry assays demonstrated that klotho/ADK siRNA-loaded exosomes could effectively target endothelial progenitor cells (EPCs), and EPCs that engulfed these exosomes were able to enhance the secretion of pro-angiogenic factors and adenosine, thereby promoting endothelial cell proliferation and migration.
The cationic polymer-based transfection method is an emerging strategy for drug-loading into exosomes in this field. In general, cationic polymers such as polyethyleneimine (PEI) and poly-L-lysine (PLL) are commonly used transfection reagents, and they can form stable complexes with DNA sharing ideal transfection efficiency.40 The principle of cationic polymer-based transfection can be explained as the cationic polymer with a positive charge attracts exosomes with a negative charge to form a complex, and the complex then introduces exogenous molecules into the interior of the exosomes. Zhupanyn et al41 compared the siRNA delivery efficiency of PEI-based nanoparticles and natural exosomes from different cell lines and found that PEI-modified exosomes had higher efficacy for gene expression and higher storage stability. Factors that affect transfection efficiency include cationic polymer concentration, transfection time, exosome source, and purity. However, the presence of cationic polymers may produce cytotoxicity and interfere with the stability and function of exosomes.
The chemical method is a promising approach to loading drugs onto the surface of exosomes through specific chemical reactions. For example, fatty acid modification or anion exchange resin hydrophobic-hydrophilic interaction principles can be utilized for drug loading into exosomes. Take azobenzene phenyl disulfide urea (ABP) as an extreme case, and it can be employed to bind drugs to the surface of exosomes covalently. Stham et al42 shows that RAD51 siRNA can be carried by exosomes through chemical methods, and the resulting exosomes are highly effective in silencing RAD51 in cancer cells. Familtseva et al43 has also observed siRNA being transported by exosomes to mouse aortic endothelial cells through fluorescence labeling.
Pros and Cons of Different Exosome Drug Loading Methods
Endogenous Loading Method
The advantages of the endogenous loading method for preparing therapeutic exosomes are attractive. First, these methods have high safety as endogenously loaded exosomes are produced through the natural secretion process of cells, which eliminates concerns about potential immune reactions or tumor formation when implanted in the body. Second, they are highly specific because they use exosomes that originate from autologous cells and share the same surface markers and membrane structure as their parent cells, which enables them to recognize and target specific cells through surface interactions. Third, they are highly controllable because they allow the composition of exosomes to be changed by manipulating the culture conditions of donor cells or using gene editing technology, which gives them flexibility and plasticity in controlling their composition and function. Lastly, they are diverse and multi-effective because they can carry various types of cargo, such as proteins, lipids, nucleic acids, and metabolites, and exert different biological effects on recipient cells.44,45 Therapeutic exosomes can originate from other types of cells, so the contents of exosomes from various cell sources vary, resulting in different effects on target cells. In clinical practice, the method of preparing exosomes can be selected based on the type of disease.46
However, those drug-loaded exosomes prepared through endogenous loading methods still have challenges in clinical applications.47–49 For example, the preparation of exosomes requires a lot of time and resources, making it difficult to achieve industrial-scale production. Additionally, the type and quantity of exosomes from different cell sources vary and may affect their therapeutic effects. Moreover, the stability of exosomes is poor since these exosomes are prone to rupture and degradation during storage and transportation. Due to the poor uniformity and complex content of exosomes, there are safety concerns, such as potential immune reactions and bacterial and viral contamination. Therefore, further improvements in preparation, storage, transportation, and safety are urgently needed for endogenous loading drugs into exosomes.
Exogenous Loading Method
Co-incubation is a common technical approach for the exogenous loading of drugs into exosomes. This method has several advantages, such as high drug-loading efficiency, low toxicity, strong controllability, and low cost.50–52 Co-incubation allows exosomes to protect and transport drugs to target cells stably and has better biocompatibility in the human body than traditional synthetic nanoparticles. Co-incubation also enables adjusting the surface proteins and contents of exosomes by changing the co-incubation conditions. Moreover, co-incubation is a simple and relatively economic process for preparing exosomes. However, co-incubation also has some disadvantages, such as requiring a high level of technical expertise and equipment support, having a limited drug loading capacity due to the small size of exosomes, and having poor storage stability, as exosomes are prone to aggregation and degradation during long-term storage.
The ultrasonic incubation method is a viable method for drug loading into exosomes with numerous advantages. One of them is that ultrasound enhances drug-loading efficiency by facilitating drug penetration into exosomes. Another one is that this method does not require any surface or chemical modification, which reduces the risk of toxic and immunogenic effects from modifications. Furthermore, this method has a wide range of applicability, as it can be used for different types of cells.53,54 However, this technique also has some limitations that need to be considered. For instance, ultrasound may damage the structure or viability of exosomes, which lowers the drug loading capacity. Also, preparing different batches of exosomes under different conditions may cause variations in size, distribution, drug loading amounts, and other characteristics, which affects the reproducibility of drug loading effects for different batches of exosomes.
Compared with other methods for drug loading into exosomes, the electroporation method has several advantages, such as strong controllability, high efficiency, and broad applicability.55,56 It allows adjusting various parameters, such as voltage, current, and pulse, to achieve the desired quantity and quality of exosomes. It also extracts exosomes with high yields and can prepare diverse sources of exosomes from different types of cells. However, this method also has some drawbacks, such as high cost, high technical threshold, strict control, potential damage, and complex experiments.10,57 In detail, it requires specialized and costly electroporation instruments that pose a high technical challenge, and it needs technical support and experimental experience to control the pulse parameters and processing time. Besides, it may induce damage to exosomes, especially the membrane structure, leading to irreversible rupture and lowering the quality and yield of the extracted exosomes. Moreover, it needs a sterile environment and sufficient cell numbers, which increases the experiment’s complexity and difficulty.
The saponin method has simple steps and low equipment requirements compared to the ultrasound and freeze-thaw methods. Also, this method has high loading efficiency, which enables an efficient loading of drugs within exosomes. Moreover, it does not interfere with the biological function of exosomes, such as cell signaling and immune regulation. However, the drawbacks of this method are also well documented by previous studies,58,59 which are mainly low stability, high toxicity, and additional purification. It may affect the exosomes’ stability, causing the release of loaded substances to mix with membrane fragments, which degrades the biological activity of loaded substances. It also uses saponin, a surfactant that has potential physical toxicity, causing cell death or toxic effects on organisms. Furthermore, it damages the exosome membrane, which requires additional purification steps to remove membrane fragments and residual surfactants.
The freeze-thaw cycle method can effectively encapsulate drugs into exosomes without causing damage to their structures, and it also avoids using any organic solvents or chemical crosslinking agents, which makes it more environmentally friendly, economical, and easy to operate. Moreover, this method uses temperature differences to regulate drug loading and release rates, which enhances drug loading efficiency.60,61 However, the shortages of this method are low production efficiency, exosome instability, and limited applicability. Besides, this method requires a long preparation time due to the continuous cooling and heating process, which may disrupt exosome stability and quality, causing the loss of their original function. Furthermore, it is only suitable for certain types of drugs and requires careful consideration of drug adaptability issues.
The extrusion method provides better control over the yield and quality of drug-loaded exosomes and produces relatively pure specimens. Compared with other exosome preparation techniques, the extrusion method is relatively gentler and can reduce the physical damage to cells in the process. Furthermore, it is also easy to standardize and verify through repeated experiments, which makes this method highly repeatable. Still, there are some unavoidable drawbacks of this method, which include high equipment requirements, low production efficiency, and limited application. Besides, this method also requires specialized membrane filtration equipment, which increases the burden on the laboratory. The low production efficiency of this method is caused by step-by-step screening and purification procedures, which intrinsically limit the number of exosomes produced. Moreover, some types of cells are not suitable for the extrusion method because they secrete unstable exosomes.62,63
Transfection is a promising method for preparing drug-loaded exosomes with several benefits over other methods. For example, the transfection method has higher purity and stability, and it can directly load biologically active substances such as drugs and siRNAs into exosomes without needing other media. Also, it has better biocompatibility and lower immunogenicity than traditional nanoparticles such as liposomes, which results in lower potential side effects.64,65 Nonetheless, the drawbacks of this method can be shown as the relatively complex process, difficult control, and uncertain effectiveness. Besides, it consumes more time and effort and may also be difficult to control the encapsulation efficiency and drug release rate.
The Optimal Drugs for Encapsulation in Exosomes
Given the structure of exosomes that are constructed of phospholipid bilayer, they provide a strong affinity for hydrophobic compounds. Essentially, hydrophobic small molecules have strong lipophilicity and high lipid solubility, making them easily penetrable through cell membranes. However, the relatively low delivery efficiency in vivo is hampered by enzymes in the body that degrade those drugs, thus limiting their clinical applications.61,66 As a result of their inherent instability and low bioavailability, hydrophobic small molecule drugs require drug carriers for sustained benefit. The co-incubation of drugs and exosomes can encapsulate hydrophobic small molecule drugs either within exosomes or on the surface of the exosome bilayer, establishing exosome drug loading and improving water solubility and bioavailability while decreasing in vivo degradation. Furthermore, the cell-membrane-penetrating deficiencies of hydrophilic drugs can be overcome by fusing them with the exosome membrane. Facilitated by exosomes, larger proteins that are difficult to cross cell membranes can enter target cells through fusion.
In addition to the co-incubation strategy, the commonly used ultrasonic incubation method can break the exosome membrane and load hydrophilic drugs or larger protein molecules into exosomes.55,67 In this case, exosomes act as carriers to deliver drugs to target cells and regulate their biological functions. Therefore, using the ultrasonic incubation method to prepare exosomes loaded with hydrophilic drugs or larger protein molecules is a promising biological therapy. Similarly, electroporation is a molecular introduction technique that increases cell membrane permeability to introduce DNA, RNA, or other molecules into cells. It is extensively used to prepare nucleic acid-loaded exosomes,59,64,68,69 from which nucleic acid molecules can be efficiently introduced to cells through electroporation, and loading them into exosomes can prevent premature degradation and maximize their likelihood of arriving at the targeted cell.
The freeze-thaw cycle method for preparing drug-loaded exosomes has a wide range of applicability, including water-soluble drugs, low molecular weight compounds, protein and peptide drugs, as well as DNA or RNA nucleic acid drugs.70–73 Using the freeze-thaw cycle method to prepare drug-loaded exosomes can prevent related drugs from being degraded before reaching their target organs, increasing drug bioavailability and thereby improving therapeutic effects.
Different from the above-mentioned drug-loading strategies, the extrusion method is a straightforward and cost-effective approach for producing drug-loaded exosomes on a mass scale. Nevertheless, this method requires high pressure and can lead to drug oxidation and degradation. Thus, drugs with hydrophobicity, liposolubility, small molecular weight, and nucleic acids are ideal for the extrusion method.65,74 Usually, these drugs are encapsulated within the oil/water interface, which can effectively suppress the drugs from oxidation and degradation, thus enhancing their therapeutic effectiveness and stability.
The saponin method is a simple and low-cost approach for preparing drug-loaded exosomes, which is ideal for drugs with hydrophobic, biological macromolecular, hydrophilic, and high molecular weight characteristics.71,75 The biological function and structure of these drugs are optimally protected when they are encapsulated in exosomes, thereby enhancing their therapeutic effectiveness. Also, the transfection method for preparing drug-loaded exosomes is simple to operate and can be easily produced on a large scale. It is suitable for a wide range of molecules, including nucleic acids, proteins, peptides, hydrophobic molecules, and drugs with high molecular weights.75 When using the transfection method to prepare drug-loaded exosomes, it is important to consider the properties and intended applications of the drug to meet the purpose of the study better.
Clinical Application of Drug-Loaded Exosomes
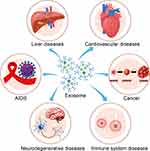
The past decade has witnessed the burgeoning and maturation of exosome drug delivery, a field that has evolved into a promising biological treatment modality. Contemporary techniques enable researchers to load therapeutic agents either within or onto the surface of exosomes, thereby facilitating targeted therapy with precision. Recent investigations underscore the broad applicability of engineered exosomes for drug delivery in the treatment of specific diseases, with its range of applications continually broadening (Figure 4, Table 1 and Table 2).
 |
Table 1 Summary of the Application of Engineered Exosome Drug Delivery in Specific Diseases |
 |
Table 2 Clinical Trials Registered in ClinicalTrial.gov Regarding Exosomes |
As previously discussed, drug encapsulation in exosomes can significantly enhance drug utilization and minimize side effects. For instance, familial hypercholesterolemia, a prevalent genetic disorder characterized by dysfunctional low-density lipoprotein receptor (LDLR) and elevated blood cholesterol levels, poses an increased risk of cardiovascular diseases. In a study, Li et al92 loaded normal Ldlr into exosomes and administered them to mice with an LDLR defect. The results showed that exosomes carrying the Ldlr gene reduced blood cholesterol levels, improved vascular function, and decreased the formation of atherosclerotic plaques. In another study by Wan et al,93 they pioneered the packaging of the CRISPR/Cas9 system into exosomes and then introduced them into liver cancer cells. Surprisingly, these specific exosomes effectively inhibited the growth and invasion of liver cancer cells without causing significant toxicity to healthy cells, which underscores the potential of exosome-based drug delivery in liver disease treatment.
As a leading global cause of death, cardiovascular disease is often associated with focal vascular inflammation, ischemia, and necrosis. Exosomes carrying miRNA or siRNA have been shown to regulate inflammatory responses and are demonstrated to promote vascular regeneration and repair. For instance, Gao et al94,95 extracted exosomes from the cultured cardiac progenitor cells and found reduced infarct areas after injecting those exosomes into a pig model of acute myocardial infarction. In another study by Cao et al,96 it was demonstrated that exosomes derived from MSCs, rich in miRNA-125b-5p, can repair kidney damage caused by ischemic injury. Specifically, once these exosomes are incorporated into damaged renal tubular cells, they can inhibit p53 expression, thereby preventing apoptosis and promoting cellular regeneration.
Neurodegenerative diseases are marked by lethal nerve cell death and dysfunction.97 The blood-brain barrier, however, prevents large molecule drugs from penetrating brain tissue, thus limiting their effectiveness in treating these diseases. In contrast, exosomes have shown promise in repairing brain tissues since they can mitigate neuroinflammation and damage by controlling the production and release of bioactive molecules. These molecules can not only regulate immune cell activity but suppress inflammatory responses and foster neuronal growth and repair.98 Nevertheless, it’s worth noting that an increase in exosome biogenesis under asthmatic conditions has been linked to pulmonary inflammation.99 Studies have shown that exosomes derived from MSCs can reduce the expression of β-amyloid precursor protein enzyme 1 (BACE1) by carrying miRNA-29c-3p, which leads to decreased production of β-amyloid at the protein level and thus highlight a potential solution for Alzheimer’s disease.100 Furthermore, exosomes can promote neuronal growth and differentiation via the Wnt/β-catenin signaling pathway, thereby improving conditions in Alzheimer’s disease.
Rheumatoid arthritis and systemic lupus erythematosus are common autoimmune diseases for which effective treatments are currently lacking. However, exosomes, capable of carrying anti-inflammatory molecules, offer a promising avenue for the precise delivery and treatment of these diseases. As stated above, MSCs are a type of adult stem cell, and they possess multi-differentiation characteristics and exhibit immunomodulatory and anti-inflammatory properties.101 Studies show that exosomes derived from MSCs can disseminate bioactive molecules and play crucial roles in immunomodulation and regeneration.102 Interestingly, those exosomes not only mimic the therapeutic impact of MSCs but also adeptly evade issues related to immune rejection, which highlights the potential of exosomes as a novel therapeutic strategy in the management of autoimmune diseases.
Different from autoimmune diseases, acquired immune deficiency syndrome (AIDS) is a perilous infectious disease caused by the human immunodeficiency virus (HIV). Studies103 have shown that exosomes could be used to inhibit the replication of HIV or kill the virus by transporting antiviral drugs or gene editing systems. Specifically, exosomes have the potential to carry antiviral proteins and RNAs to inhibit the virus’s replication and transmission. By regulating the host immune response, those exosomes enhance host resistance to HIV-1. Shrivastava et al104 developed a zinc finger protein named ZFP-362 that could target the HIV-1 promoter through exosome transporting. By binding to the active structural domain of DNA methyltransferase 3A, ZFP-362 induced stable, long-term epigenetic inhibition of HIV-1. Besides, delivering ZFP-362 through exosomes also resulted in the inhibition of HIV replication in mouse bone marrow, spleen, and brain, and transporting recombinant ZPAMt through exosomes silenced HIV-1-related epigenetic characteristics.
Cancer cells, characterized by high proliferation, self-renewal, stemness, metastatic potential, and drug resistance, pose a significant challenge to anticancer drugs. However, exosomes offer a promising solution by directly targeting cancer cells to deliver drugs since they can bypass processing by immune cells.105 This property of exosomes not only enhances the sensitivity of cancer cells to chemotherapy drugs but also amplifies the potency of anticancer drugs. A recent study by Fu et al106 has demonstrated the potential of this approach. By utilizing exosomes, they successfully delivered siRNAs to pancreatic cancer cells, where the siRNA-loaded exosomes were found to inhibit KRAS expression, thereby suppressing cancer cell proliferation and metastasis.
Advantages and Limitations
Compared to existing reviews on engineered exosomes, which primarily focus on exosome preparation, isolation, purification, and their applications in the treatment of diseases, there is a lack of systematic and comprehensive summarization on exosome drug loading strategies and their advantages and disadvantages, as well as an analysis of the applicability of different drug loading strategies. In this paper, we systematically outline the drug-loading strategies for different engineered exosomes, compare the advantages, disadvantages, and applicability of each drug-loading method, and discuss the potential for their use in treating specific diseases. The content provides a comprehensive summary of current research in this field and offers emerging clinical research ideas and important references for future research. Nonetheless, there is no unified standard for evaluating the drug-loading efficacy of engineered exosomes, so we do not discuss it in detail. Hopefully, we believe that, with the development of this field, exosome-based drug loading and delivery strategy will become an insightful choice for targeted biological therapy.
Although this review provides an overview of the recent advances and challenges in the translation of engineered exosomes for drug delivery, there are still some limitations and uncertainties that need to be addressed. First, the production and purification of exosomes are time-consuming and costly, and there is a lack of standardized protocols and quality control criteria for exosome engineering. Second, the loading efficiency and drug release kinetics of exosomes are influenced by various factors, such as the type and size of drugs, the loading methods, and the exosome source and stability. Third, the biodistribution, pharmacokinetics, and immunogenicity of exosomes are not fully understood, and there is a need for more in vivo studies and clinical trials to evaluate the safety and efficacy of exosome-based drug formulations. Fourth, the ethical and regulatory issues of exosome engineering are complex and challenging, and there is a need for clear and consistent guidelines and policies for exosome research and development. Therefore, future studies should focus on improving the production, characterization, and optimization of engineered exosomes, as well as exploring their mechanisms of action, therapeutic potential, and possible risks in various diseases.
Significance and Prospects
To sum up, engineered exosomes have emerged as a novel and promising platform for targeted drug delivery due to their natural origin, low toxicity, and excellent biocompatibility. Various methods have been explored to load drugs into exosomes, which include endogenous loading approaches based on donor cell engineering and direct exogenous loading techniques. Each drug loading strategy has its own pros and cons regarding encapsulation efficiency, exosome yield, scalability, cargo type, and ease of operation. To achieve optimal drug delivery, researchers should be careful in selecting the drug-loading method, which is tailored based on the physicochemical properties of the drug payload and intended therapeutic application. While still facing challenges in large-scale production, storage stability, and clinical translation, engineered exosomes represent an exciting frontier in nanomedicine. With continuous advances in this field, exosome-based drug formulations shine an emerging light on the targeted therapy of cancer, neurological, cardiovascular, immune, and infectious diseases.
For prospects of engineered exosomes as completely new drug delivery systems, here we list the critical future directions for research, including optimizing exosome isolation and purification methods, standardizing exosome characterization techniques, elucidating exosome uptake and trafficking mechanisms, developing efficient exosome loading strategies, functionalizing exosome surface with targeting ligands, enhancing exosome stability, and testing exosome toxicity. These continuous efforts will help advance the field of exosome-based therapeutics and pave the way for clinical applications.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 82003405), and the Natural Science Foundation of Shandong (ZR2020QH290), and the Taishan Scholar Program of Shandong Province (No. tsqn202211359).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi:10.1016/0092-8674(83)90040-5
2. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes. J Biol Chem. 1987;262:9412–9420. doi:10.1016/S0021-9258(18)48095-7</INTDOI>
3. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi:10.1126/science.aau6977
4. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789–804. doi:10.7150/thno.18133
5. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–6934. doi:10.2147/IJN.S264498
6. Kilchert C, Wittmann S, Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat Rev Mol Cell Biol. 2016;17:227–239. doi:10.1038/nrm.2015.15
7. Ahmadi M, Jafari R, Mahmoodi M, Rezaie J. The tumorigenic and therapeutic functions of exosomes in colorectal cancer: opportunity and challenges. Cell Biochem Funct. 2021;39:468–477. doi:10.1002/cbf.3622
8. Shaban SA, Rezaie J, Nejati V. Exosomes derived from senescent endothelial cells contain distinct pro-angiogenic miRNAs and proteins. Cardiovasc Toxicol. 2022;22:592–601. doi:10.1007/s12012-022-09740-y
9. Mahbubfam S, Rezaie J, Nejati V. Crosstalk between exosomes signaling pathway and autophagy flux in senescent human endothelial cells. Tissue Cell. 2022;76:101803. doi:10.1016/j.tice.2022.101803
10. Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894–906. doi:10.1016/j.jconrel.2020.10.020
11. Peng H, Ji W, Zhao R, et al. Exosome: a significant nano-scale drug delivery carrier. J Mater Chem B. 2020;8:7591–7608. doi:10.1039/D0TB01499K
12. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi:10.7150/thno.52570
13. Wang Q, Li T, Yang J, et al. Engineered exosomes with independent module/cascading function for therapy of parkinson’s disease by multistep targeting and multistage intervention method. Adv Mater. 2022;34:2201406. doi:10.1002/adma.202201406
14. Lai J, Huang C, Guo Y, Rao L. Engineered extracellular vesicles and their mimics in cardiovascular diseases. J Control Release. 2022;347:27–43. doi:10.1016/j.jconrel.2022.04.046
15. Kugeratski FG, Kalluri R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021;288:10–35. doi:10.1111/febs.15558
16. Zhang M, Hu S, Liu L, et al. Engineered exosomes from different sources for cancer-targeted therapy. Sig Transduct Target Ther. 2023;8:1–20. doi:10.1038/s41392-023-01382-y
17. Huang W, Yu Y, Yang C, et al. Aptamer decorated magnetic graphene oxide nanoparticles for effective capture of exosomes. Chem Eng J. 2022;431:133849. doi:10.1016/j.cej.2021.133849
18. Zhou Y-G, Mohamadi RM, Poudineh M, et al. Interrogating circulating microsomes and exosomes using metal nanoparticles. Small. 2016;12:727–732. doi:10.1002/smll.201502365
19. Li T, Li X, Han G, et al. The therapeutic potential and clinical significance of exosomes as carriers of drug delivery system. Pharmaceutics. 2023;15:21. doi:10.3390/pharmaceutics15010021
20. Bui S, Dancourt J, Lavieu G. Virus-free method to control and enhance extracellular vesicle cargo loading and delivery. ACS Appl Bio Mater. 2023;6:1081–1091. doi:10.1021/acsabm.2c00955
21. Abas BI, Demirbolat GM, Cevik O. Wharton jelly-derived mesenchymal stem cell exosomes induce apoptosis and suppress EMT signaling in cervical cancer cells as an effective drug carrier system of paclitaxel. PLoS One. 2022;17:e0274607. doi:10.1371/journal.pone.0274607
22. Kalimuthu S, Gangadaran P, Rajendran RL, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.01116
23. Yang Z, Li X, Gan X, et al. Hydrogel armed with Bmp2 mRNA-enriched exosomes enhances bone regeneration. J Nanobiotechnol. 2023;21:119. doi:10.1186/s12951-023-01871-w
24. Pan X, Yang L, Wang S, Liu Y, Yue L, Chen S. Semaglutide alleviates inflammation-Induced endothelial progenitor cells injury by inhibiting MiR-155 expression in macrophage exosomes. Int Immunopharmacol. 2023;119:110196. doi:10.1016/j.intimp.2023.110196
25. Wen Z, Mai Z, Zhu X, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:36. doi:10.1186/s13287-020-1563-8
26. Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. doi:10.1016/j.impact.2020.100261
27. Das CK, Jena BC, Banerjee I, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16:24–40. doi:10.1021/acs.molpharmaceut.8b00901
28. Ferreira D, Moreira JN, Rodrigues LR. New advances in exosome-based targeted drug delivery systems. Crit Rev Oncol/Hematol. 2022;172:103628. doi:10.1016/j.critrevonc.2022.103628
29. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi:10.1038/mt.2010.105
30. Alptekin A, Khan MB, Ara R, et al. Pulsed Focal ultrasound as a non-invasive method to deliver exosomes in the brain/stroke. bioRxiv. 2021. doi:10.1101/2021.02.03.429621
31. Yan C, Chen J, Wang C, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Delivery. 2022;29:214–228. doi:10.1080/10717544.2021.2023699
32. He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021;11:e468. doi:10.1002/ctm2.468
33. Lou R, Chen J, Zhou F, Wang C, Leung C-H, Lin L. Exosome-cargoed microRNAs: potential therapeutic molecules for diabetic wound healing. Drug Discovery Today. 2022;27:103323. doi:10.1016/j.drudis.2022.07.008
34. Wang Y, Wang Y, Qin Z, et al. The role of non-coding RNAs in ABC transporters regulation and their clinical implications of multidrug resistance in cancer. Expert Opin Drug Metab Toxicol. 2021;17:291–306. doi:10.1080/17425255.2021.1887139
35. Thakur A, Sidu RK, Zou H, Alam MK, Yang M, Lee Y. Inhibition of glioma cells’ proliferation by doxorubicin-loaded exosomes via microfluidics. Int J Nanomed. 2020;15:8331–8343. doi:10.2147/IJN.S263956
36. Ebrahimian M, Hashemi M, Etemad L, Salmasi Z. Thymoquinone-loaded mesenchymal stem cell-derived exosome as an efficient nano-system against breast cancer cells. Iran J Basic Med Sci. 2022;25:723–731. doi:10.22038/IJBMS.2022.64092.14116
37. Liang G, Zhu Y, Ali DJ, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol. 2020;18:10. doi:10.1186/s12951-019-0563-2
38. Yang J, Wang Q, Xing T, et al. Engineered exosome-mediated cobalt sulfide quantum dot targeted delivery for photothermal and chemodynamic anticancer therapy. J Drug Delivery Sci Technol. 2023;83:104441. doi:10.1016/j.jddst.2023.104441
39. Chen W, Yang M, Bai J, et al. Exosome-modified tissue engineered blood vessel for endothelial progenitor cell capture and targeted siRNA delivery. Macromol Biosci. 2018;18. doi:10.1002/mabi.201700242
40. Kim N-H, Choi S-H, Kim C-H, Lee CH, Lee TR, Lee A-Y. Reduced MiR-675 in exosome in H19 RNA-related melanogenesis via MITF as a direct target. J Invest Dermatol. 2014;134:1075–1082. doi:10.1038/jid.2013.478
41. Zhupanyn P, Ewe A, Büch T, et al. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J Control Release. 2020;319:63–76. doi:10.1016/j.jconrel.2019.12.032
42. Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signaling. 2013;11:88. doi:10.1186/1478-811X-11-88
43. Familtseva A, Chaturvedi P, Kalani A, Tyagi N, Tyagi S. A link between mitophagy and apoptosis in endothelial cells: exosomal delivery of Mfn-2 siRNA. FASEB J. 2015;29:
44. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi:10.1016/j.addr.2016.02.006
45. Huyan T, Li H, Peng H, et al. Extracellular Vesicles - Advanced Nanocarriers in Cancer Therapy: progress and Achievements. Int J Nanomed. 2020;15:6485–6502. doi:10.2147/IJN.S238099
46. Haraszti RA, Didiot M-C, Sapp E, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi:10.3402/jev.v5.32570
47. Richter M, Vader P, Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv Drug Deliv Rev. 2021;173:416–426. doi:10.1016/j.addr.2021.03.020
48. Alqurashi H, Ortega Asencio I, Lambert DW. The emerging potential of extracellular vesicles in cell-free tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2021;27:530–538. doi:10.1089/ten.TEB.2020.0222
49. Allahverdiyev AM, Parlar E, Dinparvar S, Bagirova M, Abamor EŞ. Current aspects in treatment of breast cancer based of nanodrug delivery systems and future prospects. Artif Cells Nanomed Biotechnol. 2018;46:S755–S762. doi:10.1080/21691401.2018.1511573
50. Nazimek K, Bryniarski K. Increasing the therapeutic efficacy of extracellular vesicles from the antigen-specific antibody and light chain perspective. Front Cell Dev Biol. 2021;9:790722. doi:10.3389/fcell.2021.790722
51. Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi:10.1080/10717544.2020.1748758
52. Takahashi Y, Takakura Y. Extracellular vesicle-based therapeutics: extracellular vesicles as therapeutic targets and agents. Pharmacol Ther. 2023;242:108352. doi:10.1016/j.pharmthera.2023.108352
53. Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015–1028. doi:10.7150/thno.30853
54. Derkus B, Emregul E. Ultrasonics-Assisted Effective Isolation and CharacterizationCharacterization of ExosomesExosomes from Whole Organs. In: Mavromoustakos T, Tzakos AG, Durdagi S, editors. Supramolecules in Drug Discovery and Drug Delivery: Methods and Protocols. New York, NY: Springer US; 2021:25–34. doi:10.1007/978-1-0716-0920-0_3
55. Xi X-M, Cheng-Meng S-JX, Lu R. Drug loading techniques for exosome-based drug delivery systems. Die Pharmazie. 2021;76:61–67. doi:10.1691/ph.2021.0128
56. Pofali P, Mondal A, Londhe V. Exosome as a Natural Gene Delivery Vector for Cancer Treatment. Curr Cancer Drug Targets. 2020;20:821–830. doi:10.2174/1568009620666200924154149
57. Zhang M, Zang X, Wang M, et al. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: recent advances and challenges. J Mater Chem B. 2019;7:2421–2433. doi:10.1039/C9TB00170K
58. Mishra A, Singh P, Qayoom I, Prasad A, Kumar A. Current strategies in tailoring methods for engineered exosomes and future avenues in biomedical applications. J Mater Chem B. 2021;9:6281–6309. doi:10.1039/D1TB01088C
59. Zhang Y, Li J, Gao W, Xie N. Exosomes as Anticancer Drug Delivery Vehicles: prospects and Challenges. FBL. 2022;27:293. doi:10.31083/j.fbl2710293
60. Dao A, Kushwaha R, Kumar A, Huang H, Banerjee S. Engineered exosomes as a photosensitizer delivery platform for cancer photodynamic therapy. ChemMedChem. 2022;17:e202200119. doi:10.1002/cmdc.202200119
61. Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi:10.1038/aps.2017.12
62. Narayanan E. Exosomes as Drug Delivery Vehicles for Cancer Treatment. Curr Nanosci. 2020;16:15–26. doi:10.2174/1573413715666190219112422</INTDOI>
63. Sun Z, Yang J, Li H, et al. Progress in the research of nanomaterial-based exosome bioanalysis and exosome-based nanomaterials tumor therapy. Biomaterials. 2021;274:120873. doi:10.1016/j.biomaterials.2021.120873
64. Zhang Y, Liu Q, Zhang X, et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J Nanobiotechnol. 2022;20:279. doi:10.1186/s12951-022-01472-z
65. Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–543. doi:10.1016/j.jconrel.2022.05.027
66. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi:10.1016/j.canlet.2015.10.020
67. Feng Q, Zhang Y, Fang Y, et al. Research progress of exosomes as drug carriers in cancer and inflammation. J Drug Targeting. 2023;31:335–353. doi:10.1080/1061186X.2022.2162059
68. Burgio S, Noori L, Marino Gammazza A, et al. Extracellular vesicles-based drug delivery systems: a new challenge and the exemplum of malignant pleural mesothelioma. Int J Mol Sci. 2020;21:5432. doi:10.3390/ijms21155432
69. Amiri A, Bagherifar R, Ansari Dezfouli E, Kiaie SH, Jafari R, Ramezani R. Exosomes as bio-inspired nanocarriers for RNA delivery: preparation and applications. J Transl Med. 2022;20:125. doi:10.1186/s12967-022-03325-7
70. Yao Y, Jiang Y, Song J, et al. Exosomes as Potential Functional Nanomaterials for Tissue Engineering. Adv Healthcare Mater. 2023;12:2201989. doi:10.1002/adhm.202201989
71. Shi Y, Guo S, Liang Y, et al. Construction and Evaluation of Liraglutide Delivery System based on Milk Exosomes: a New Idea for Oral Peptide Delivery. Current Pharm Biotechnol. 2022;23:1072–1079. doi:10.2174/1389201022666210820114236
72. Sen S, Xavier J, Kumar N, Ahmad MZ, Ranjan OP. Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech. 2023;13:101. doi:10.1007/s13205-023-03521-2
73. Bashyal S, Thapa C, Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J Control Release. 2022;348:723–744. doi:10.1016/j.jconrel.2022.06.011
74. Khosravi N, Pishavar E, Baradaran B, Oroojalian F, Mokhtarzadeh A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: a promising biomimetic nanoplatforms for cancer theranostics. J Control Release. 2022;348:706–722. doi:10.1016/j.jconrel.2022.06.026
75. Huang Y, Liu Z, Li N, et al. Parkinson’s disease derived exosomes aggravate neuropathology in SNCA*A53T Mice. Ann Neurol. 2022;92:230–245. doi:10.1002/ana.26421
76. Pascucci L, Coccè V, Bonomi A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi:10.1016/j.jconrel.2014.07.042
77. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi:10.1016/j.nano.2015.10.012
78. Lou G, Chen L, Xia C, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39. doi:10.1186/s13046-019-1512-5
79. Du J, Wan Z, Wang C, et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021;11:8185–8196. doi:10.7150/thno.59121
80. Dumontel B, Susa F, Limongi T, et al. Nanotechnological engineering of extracellular vesicles for the development of actively targeted hybrid nanodevices. Cell Biosci. 2022;12:61. doi:10.1186/s13578-022-00784-9
81. Wang J, Liu Y, Zhang Y, Li X, Fang M, Qian D. Targeting exosomes enveloped EBV-miR-BART1-5p-antagomiRs for NPC therapy through both anti-vasculogenic mimicry and anti-angiogenesis. Cancer Med. 2023. doi:10.1002/cam4.5941
82. Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116:255–263. doi:10.1161/CIRCRESAHA.116.304360
83. Youn S-W, Li Y, Kim Y-M. Modification of cardiac progenitor cell-derived exosomes by miR-322 provides protection against myocardial infarction through Nox2-dependent angiogenesis. Antioxidants (Basel). 2019;8:18. doi:10.3390/antiox8010018
84. Wang Q, Zhang L, Sun Z, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosome-encapsulated arginine-glycine-aspartate (RGD) hydrogels boost therapeutic efficacy of cardiac repair after myocardial infarction. Mater Today Bio. 2021;12:100171. doi:10.1016/j.mtbio.2021.100171
85. Chen S, Tang Y, Liu Y, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52:e12669. doi:10.1111/cpr.12669
86. Liang Y, Xu X, Li X, et al. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl Mater Interfaces. 2020;12:36938–36947. doi:10.1021/acsami.0c10458
87. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi:10.1038/nbt.1807
88. Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi:10.1038/mt.2011.164
89. Didiot M-C, Hall LM, Coles AH, et al. Exosome-mediated delivery of hydrophobically modified siRNA for Huntingtin mRNA silencing. Mol Ther. 2016;24:1836–1847. doi:10.1038/mt.2016.126
90. Riazifar M, Mohammadi MR, Pone EJ, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13:6670–6688. doi:10.1021/acsnano.9b01004
91. Lu X, Xu G, Lin Z, et al. Engineered exosomes enriched in netrin-1 modRNA promote axonal growth in spinal cord injury by attenuating inflammation and pyroptosis. Biomater Res. 2023;27:3. doi:10.1186/s40824-023-00339-0
92. Li Z, Zhao P, Zhang Y, et al. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics. 2021;11:2953–2965. doi:10.7150/thno.49874
93. Wan T, Zhong J, Pan Q, Zhou T, Ping Y, Liu X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022;8:eabp9435. doi:10.1126/sciadv.abp9435
94. Gao L, Gregorich ZR, Zhu W, et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018;137:1712–1730. doi:10.1161/CIRCULATIONAHA.117.030785
95. Gao L, Wang L, Wei Y, et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. 2020;12:eaay1318. doi:10.1126/scitranslmed.aay1318
96. Cao J-Y, Wang B, Tang -T-T, et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11:5248–5266. doi:10.7150/thno.54550
97. Olufunmilayo EO, Gerke-Duncan MB, Holsinger RMD. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants. 2023;12:517. doi:10.3390/antiox12020517
98. Wu Y, Li J, Zeng Y, et al. Exosomes rewire the cartilage microenvironment in osteoarthritis: from intercellular communication to therapeutic strategies. Int J Oral Sci. 2022;14:40. doi:10.1038/s41368-022-00187-z
99. Almohammai A, Rahbarghazi R, Keyhanmanesh R, Rezaie J, Ahmadi M. Asthmatic condition induced the activity of exosome secretory pathway in rat pulmonary tissues. J Inflamm. 2021;18:14. doi:10.1186/s12950-021-00275-7
100. Jahangard Y, Monfared H, Moradi A, Zare M, Mirnajafi-Zadeh J, Mowla SJ. Therapeutic effects of transplanted exosomes containing miR-29b to a rat model of Alzheimer’s disease. Front Neurosci. 2020;14:564. doi:10.3389/fnins.2020.00564
101. Ahmadi M, Mahmoodi M, Shoaran M, Nazari-Khanamiri F, Rezaie J. Harnessing normal and engineered mesenchymal stem cells derived exosomes for cancer therapy: opportunity and challenges. Int J Mol Sci. 2022;23:13974. doi:10.3390/ijms232213974
102. Rezaie J, Nejati V, Mahmoodi M, Ahmadi M. Mesenchymal stem cells derived extracellular vesicles: a promising nanomedicine for drug delivery system. Biochem Pharmacol. 2022;203:115167. doi:10.1016/j.bcp.2022.115167
103. Hamilton W, Weiss RA, Wain–Hobson S, et al. The origins of acquired immune deficiency syndrome viruses: where and when? Philos Trans R Soc London Ser B. 2001;356:867–876. doi:10.1098/rstb.2001.0863
104. Shrivastava S, Ray RM, Holguin L, et al. Exosome-mediated stable epigenetic repression of HIV-1. Nat Commun. 2021;12:5541. doi:10.1038/s41467-021-25839-2
105. Rezaie J, Etemadi T, Feghhi M. The distinct roles of exosomes in innate immune responses and therapeutic applications in cancer. Eur J Pharmacol. 2022;933:175292. doi:10.1016/j.ejphar.2022.175292
106. Fu Z, Zhang X, Zhou X, et al. In vivo self-assembled small RNAs as a new generation of RNAi therapeutics. Cell Res. 2021;31:631–648. doi:10.1038/s41422-021-00491-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.