Back to Archived Journals » Journal of Vascular Diagnostics and Interventions » Volume 2
Endovenous laser ablation for varicose veins: towards a personalized energy dose
Authors Varetto G, Guiot C, Destro M, Castagno C, Contessa L, Zan S, Garneri P, Rispoli P
Received 9 February 2014
Accepted for publication 16 June 2014
Published 17 September 2014 Volume 2014:2 Pages 85—90
DOI https://doi.org/10.2147/JVD.S62162
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Gianfranco Varetto,1 Caterina Guiot,2 Matteo Destro,1 Claudio Castagno,1 Luigi Contessa,1 Stefano Zan,1 Paolo Garneri,1 Pietro Rispoli1
1Division of Vascular Surgery, Department of Surgical Sciences, 2Department of Neuroscience, University of Turin, Turin, Italy
Background: Endovenous laser ablation is a minimally invasive procedure in the treatment of great saphenous vein insufficiency. Procedural criteria (energy delivered according to the selected fluence) could affect outcome after treatment, potentially improving the success rate and reducing complications. However, the optimal values of the required energy per unit volume are not known, but on the basis of clinical experience, a range of optimal speed of retraction of the laser fiber catheter should exist and strictly depend on the actual vein dimensions.
Methods: The study population included 21 patients. The equipment was a Diomed 30W® laser, wavelength 810 nm. Before treatment, three segments of the great saphenous vein were mapped and their diameter measured and recorded. The energy delivered to each segment was recorded as well as its relationship with vein diameter being evaluated for each vein segment.
Results: A 100% success rate was observed at 12-month follow-up assessment, the discomfort complaint 1 week after endovenous laser ablation by 19% of patients was always low (2 or 3 on a scale of pain of 10). On the basis of the actual result, which greatly improves our previous clinical experience, a range of effective values of speed of retraction of the laser fiber catheter (and of the energy per unit volume) is assessed, which strictly depends on the diameter of each segment of the vein.
Conclusion: The speed of retraction of the laser fiber catheter should be properly tailored, in order to deliver the right energy dose depending on the actual vein diameter. A real-time procedure can be easily performed using a simple mathematical nomogram.
Keywords: EVLA, great saphenous vein, fluence
Introduction
Chronic venous insufficiency, mainly affecting the great saphenous vein (GSV) and varicose veins, are often a source of discomfort, pain, loss of work days, disability, and reduced health-related quality of life.1–3 In the adult population, the incidence of chronic venous insufficiency is over 20%, and the condition affects women twice as often as men.4
Consistent with efforts to reduce post-treatment time and health care spending, minimally invasive techniques such as endovenous laser ablation (EVLA) have gained wider use. EVLA reportedly compares favorably with conventional surgery, coupled with a marked enhancement of quality of life after treatment.5 The success rate of EVLA is 88%–100%.6
Such high success rates have derived from a judicious selection of patients according to anatomic and clinical criteria. On the procedural side, however, it might be worth investigating how energy dosing-related variables (power, type of optic fiber, and fluence) impact on the outcome of laser treatment. Therapeutic effects are related to the fact that the laser energy is mainly converted into heat, inducing transmural vein wall destruction and irreversible obliteration of the vein. According to the recent review of Vuylsteke and Mordon,7 many heat-related processes occur and contribute to the result.
Interacting with water, heat induces a fast vaporization of the blood in the surrounding vascular region, and steam bubbles are created because of the high absorption of laser energy in blood.8 Such steam bubbles indicate that blood temperature passes the point of boiling at the site of the laser tip, thus transferring heat energy homogeneously to the inner vessel wall,9 which collapses entrapping the solid components of the blood without generating circulating thrombi.10 Histologic analysis at biopsy evidenced signs of coagulative necrosis in the intimal layer and the tunica media of the vein, together with cavities and separations compatible with a massive vaporization of their liquid content.10
However, the energy is at least partially absorbed by the hemoglobin, leading to clot formation and carbonization around the fiber tip and enhancing thermal conduction with a mechanisms resembling that of a “heat pipe.”11 Finally, direct contact between the fiber tip and the vein wall can accidentally provoke perivenous tissue destruction. All of them are, however, responsible for vein tissue damage, and its consequent fibroblast infiltration is a result of the injury response which leads to a replacement of the thrombus with collagen deposition. This replacement of the thrombus with collagen is necessary for eventual long-term success.12
The first two of the above mechanisms are related to a process which develops in the inner volume of the vein, and can therefore be thought of as “volume-dependent” effects, while direct contact would be a “surface-dependent” effect. In other terms, a range of “optimal values” for the energy per unit volume (J/v)opt should exist that is able to elicit the “right” vascular damage without any unwanted side effect. No direct measurements have been performed so far, but such a range can be assessed “ex-post,” based on the fact that previous treatments have been successful.
The energy J delivered by the laser depends on its power P and on the delivering time t. With P being fixed, t is the only variable which can be actually controlled through the speed of retraction vr. Accordingly, it makes sense to define as an independent variable the fluence or linear endovenous energy density (LEED). For example, the energy delivered for unit vessel length L, being:
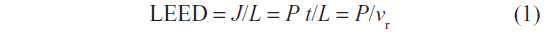
As previously stated, the operator can decide the actual LEED to be delivered during the procedure by varying the speed of retraction vr of the optic fiber catheter by paying attention to the position of the catheter and to the acoustic signal emitted by the laser equipment (normally one per second). Being the effects related to the volume of blood, we conclude that more attention should be paid to the patient’s vessel dimension, and that a correct and accurate procedure should take into account the actual vein diameter for dosing the delivered energy. In our previous experience, 274 patients (41 males, 233 females, mean age 52 years) were treated since 2005 with the same protocol described in this paper, with LEED values ranging between 70 and 80 J/cm. The medium follow-up was 26.6 months, the success rate was 95.2%, and failures were mainly detected in the first 6–12 months. Although non-systematically, we often took a single measurement of the vein diameter at the cross-junction of the GSV, and found a significant correlation between GSV diameter and therapeutic failure (P=0.048; 95% CI [confidence interval]).13
To investigate more closely such relationship, we designed the present study, where a limited number of patients was more accurately monitored through the EVLA procedure (diameters were measured at least in three sections, but prospectively, the whole vein can be mapped, and the LEED values and the corresponding speed of retraction vr of the optic fiber were reported), and their clinical procedural success were assessed.
Materials and methods
Model study
The therapeutic effects are supposed to be related to the ratio between the energy J delivered by the laser equipment to a given vascular segment, mainly converted into heat, and the blood volume V on which it is delivered. Expressing the volume in terms of the vessel diameter D:

Provided a range of optimal values of (J/v)opt is defined, which elicits the damaging effects on the vessel walls but have no undesired secondary effects for the patient, a range of optimal values of LEED, and specifically of retraction speed vopt, can be assessed depending on the patient’s vein diameter:

According to Equation 3, vopt is expected to be inversely proportional to the square of the vessel diameter through a “range” of coefficients k, which reflect the range of optimal energy delivered for unit volume able to induce the therapeutic effect.
Clinical study
Patients
The study population was 21 patients treated between January 2011 and July 2011 for GSV insufficiency. The inclusion criteria were vessel diameter <12 mm (according to Kim and Paxton14), incontinence of the saphenofemoral junction, distance between the skin surface and the GSV >0.5 cm, no vessel tortuosity or endoluminal material, and age >18 years. The exclusion criteria were large collaterals of the GSV, plexiform or tortuous saphenofemoral junction, lower limb vascular disease, inability to walk, pregnancy, breastfeeding, hypercoagulability syndromes, deep venous thrombosis, and poor general health condition.
In all, 21 EVLA procedures were performed. The case series comprised 18 (85.7%) women and three (14.3%) men; the mean age was 48 years (range, 42–68). The patients were categorized according to the Clinical-Etiology-Anatomy-Pathophysiology (CEAP) classification for chronic venous disease. “C” identifies clinical worsening stages of this pathology, from 1 to 6; all of our patients were C2, which is evidence of varicose veins.
The most frequent complaint before treatment was pain (n=17, 80.95%), followed by heaviness and fatigue (n=14, 66.6%), warmth (n=11, 52.4%), burning (n=7, 33.3%), swelling (n=7, 33.3%), itching (n=3, 14.3%), and tingling (n=3, 14.3%).
Preoperative phase
After obtaining informed consent, hemodynamic mapping was performed using a Biosound Esaote MyLab 25 ultrasound system with a multifrequency probe (5–12 MHz) in B-mode and the patient in the orthostatic position. The patient was then placed prone in a semilateral decubitus position, and the segment of the GSV to be treated was examined.
The geometry of the saphenous vein in our patients showed that the vein could be subdivided in three segments having a homogeneous diameter value. Consequently, the length of the vein was subdivided into three segments of equal length, which were then marked on the skin, for a total of 63 segments. The proximal segment begins approximately 2–3 cm after the saphenous-femoral junction, and ends about the middle of the thigh, the intermediate part of the vein anatomically includes the section of GSV from the middle to about distal third of the thigh, and the distal segment corresponds to the distal third of the thigh to about the proximal third of the leg (see Figure 1). The diameter of each segment was also measured.
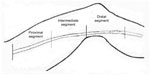  | Figure 1 Division into segments of great saphenous vein. |
Operative phase
The procedure was performed using local anesthesia (200 mg lidocaine in approximately 100 mL of saline) administered subcutaneously and inducing tumescence along the segment of vein undergoing EVLA. MAC (monitored anesthesia care) sedation (midazolam 0.02 mg/kg and remifentanil 0.025 μg/kg/min) was delivered during the procedure.
EVLA was performed with percutaneous access to the GSV using a diode laser (Diomed 30W®, Food and Drug Administration (FDA)-certified; wavelength, 810 nm; power, 12 W). An FDA-approved endovenous laser kit was employed, consisting in a 21 G needle, a 4 Fr sheath, a centimeter-scale catheter, and a 45 cm 0.018 steel wire.
The patient was initially positioned in the lateral decubitus position (anti-Trendeleburg) to facilitate cannulation of the GSV. Subsequently, EVLA was performed on the patient lying in the horizontal position without inclination.
During the procedure, using a continuous retraction protocol, the energy dose (in Joules) was recorded as the probe passed from one segment to the next. As guided by the centimeter scale or the acoustic signal, the operator was able to accurately adjust the pull-back speed.
Under our protocol, 100 J/cm are delivered empirically to the first 3 cm distal to the saphenous-femoral junction (to be sure that collapse is locally very effective), thus providing 300 J in this first segment. In the underlying segments, the dose is diminished empirically to 40 J/cm.
Following EVLA, compressive stocking 20–25 mmHg was prescribed for 4 weeks and antithrombotic treatment (enoxaparin 4,000 IU) for 10 days.
The recommended analgesic therapy was paracetamol 1 g as needed (up to 3 g per day).
Follow-up assessment
Postoperative clinical and diagnostic assessment was scheduled at 1 week, 1 month, 3 months, 6 months, and then yearly thereafter. Besides assessing persistent GSV obliteration, clinical examination sought to reveal possible local minor (pain and ecchymosis) and major (deep venous thrombosis, phlebitis, skin hyperpigmentation, erythema, and infection) complications. Pain was evaluated on a 10-point scale, where 10 is the greatest pain. Ecchymosis was evaluated on a 5-point scale, where 0 indicates no ecchymosis and 5 denotes extensive ecchymosis above and below the treated segment. The outcome was judged successful according to the criteria listed in Table 1.
  | Table 1 Procedural success criteria |
Statistical analysis
ANOVA (analysis of variance) was applied to test whether there was a statistically significant difference between the energy doses per unit length (LEED) delivered to the 63 vein segments and between their diameters.
Results
The success rate was 100%, without recanalization. No patients were lost to follow-up, whose mean duration was 10.3 months. No major complications were noted. Four (19%) patients reported experiencing mild pain (2 or 3 on a scale of 10), and 14 (66.6%) patients presented superficial ecchymosis (1 on a scale of 5) at the 1-week follow-up visit and equally affected all the GSV length treated. Pain and ecchymosis disappeared at subsequent control (1 month after EVLA).
The 21 GSV segments corresponding to the proximal part had a mean diameter value of 0.85 cm and a standard deviation (SD) of 0.19 cm, and were significantly (P<0.005) different from the 21 intermediate segments (mean diameter =0.63 cm, SD =0.11 cm) and (P<0.005) from the 21 segments in the distal part of the vein (mean diameter =0.69 cm, SD =0.16 cm). Similarly, also the energy delivered to the 21 proximal segments (mean value =678 J, SD =60 J) was significantly (P<0.005) larger than that delivered to the 21 intermediate segments (mean value =495 J, SD =129 J) and (P<0.005) to the distal segments (mean value =422 J, SD =75 J).
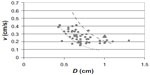
According to Equation 3, vopt is expected to be inversely proportional to the square of the vessel diameter. We plotted v versus D, using as unique data series the proximal, intermediate, and distal segments (see Figure 2).
The dotted and the dashed lines correspond to kmin =0.08 cm3/s and kmax =0.20 cm3/s and reflect the results of our clinical experience. The corresponding value for the energy per unit volumes are 191 and 76 J/cm3.
Far from introducing new unnecessary parameters, and aiming at simplifying the EVLA procedure, we proposed a simple prescription relating the only real “independent” parameter controlled by the operator (ie, the retraction speed vopt and the patient’s vein diameter). According to Figure 2, we estimated the k values corresponding to the lines which divided the experimental points into the 25th, 50th, and 75th percentiles. Assuming that data below the 25th and above the 75th percentiles were outliers, those within the range 25–75 were retained to define a “nomogram” (Table 2), indicating the suggested maximal and minimal retraction velocity v depending on the actual vein diameter.
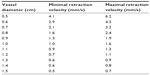  | Table 2 Nomogram relating the vessel diameter (cm) and the minimal and maximal velocity of retraction (cm/s) estimated as 25th and 75th percentiles of the experimental data |
Discussion
In our experience, EVLA for great saphenous varicose veins is a valid alternative to surgical stripping and compares favorably in long-term outcome. The advantage was that clinical success was achieved without the occurrence of local short-term complications such as deep venous thrombosis, phlebitis, skin hyperpigmentation, paresthesia, erythema, or infection. In our previous experience (274 patients treated with LEED values in the range between 70 and 80 J/cm unrelated to their GSV diameter), the success rate was 95.2%.13
In the present series, where the LEED administered to the patients was commensurated to their actual GSV diameter (mean LEED value of 60.3 J/cm in the first segment, reduced in the other segments to 43.9 and 37.6 J/cm), the clinical results were more satisfactory. Such results seem to realize a good balance between “underthreshold energy,” with the risk of recanalization (many authors have suggested using preset linear fluence thresholds to avert early post-procedural recanalization15), and “overthreshold energy,” with the risk of important side effects (the percentage of local skin and nervous complications following low LEED values have been reported elsewhere7,16).
A possible weak point is that vein diameters were measured before anesthesia, disregarding the effect of tumescence on the actual vein dimension during the EVLA procedure. However we consider such a theoretical effect negligible due to the small standardized amount of anesthetics used (100 mL) for each patient.
According to our reasoning, to determine the energy dose delivered during endovenous ablation, it is necessary to measure the overall delivered energy J, the energy per unit length LEED, and the energy per unit volume J/V. Both J and LEED are “natural” parameters to be controlled, since the energy dose is accurately assessable using the pulsed laser, which delivers 12 J per pulse. According to LEED=J/L=P/vr the energy dose delivered during endovenous ablation could be determined by the velocity of the laser retraction. Laser catheter retraction devices have been described,16 and currently available devices display in real time the energy dose delivered. In our experience, under the guidance of a centimeter-scale laser and preoperative skin mapping of the GSV, the energy being delivered to each segment reliably determined, and provided at least eight pulses are delivered to the first 3 cm of the most proximal segment and then diminished in the others, LEED could be easily evaluated. According to what was theoretically expected, LEED was found to be different for each segment and to be larger in the segments with larger diameters.
To evaluate the last parameter (eg, the energy per unit volume J/V), which is probably the most significant one to understand which physical processes occurred following laser energy deposition, no direct measurements were available, but a simple physical model based on the relationship between retraction velocity and vessel diameter afforded the indirect estimation. Namely, in all our treatments, the delivered energy per unit volume was between 74.1 and 296.5 J/cm3.
Unfortunately, such values cannot be compared at the moment with those eliciting the different physical processes (eg, plasma boiling and clot formation) responsible for vascular damage, but they may be useful as reference for further in vitro and in vivo investigation.
Moreover, future improvements can be devised. Following preoperative skin mapping of the GSV, sonographic evaluation of the diameters of each vascular segment, a simple “nomogram” (see Figure 2) or a software routine based on Equations 2 and 3 may be developed and used to pre-assess the optimal retraction velocity of the laser tip for each vascular segment to be treated. According to this optimal value, the standard intraoperative monitoring of the energy dose J and of LEED by means of a centimeter-scale catheter and guided by an acoustic signal emitted by the laser device, will enable the operator to estimate energy dosing to each segment.
Further studies on a larger series of cases, or a comparison between patient cohorts treated in the conventional way or according to the proposed protocol, are needed to confirm our results and to validate our operative proposal.
Conclusion
The novelty of our approach is that of definitely relating the energy delivered to the blood vessel to the actual dimension of the patient’s vein, defining a stricter range of values for the retraction velocity, allowing a kind of “treatment plan” for our patients which suits their actual vein dimension along the GSV undergoing EVLA.
Author contributions
Each author contributed equally to the research and drafting of the article.
Disclosure
The authors report no conflicts of interest in this work.
References
Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose vein and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(Suppl 5):2S–48S. | |
Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003;37(5):1047–1053. | |
Smith JJ, Guest MG, Greenhalgh RM, Davies AH. Measuring the quality of life in patients with venous ulcers. J Vasc Surg. 2000;31(4):642–649. | |
Carpentier PH, Maricq HR, Biro C, Ponçot-Makinen CO, Franco A. Prevalence, risk factors, and clinical patterns of chronic venous disorders of lower limbs: a population-based study in France. J Vasc Surg. 2004;40(4):650–659. | |
Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg. 2011;98(4):501–510. | |
Mundy L, Merlin TL, Fitridge RA, Hiller JE. Systematic review of endovenous laser treatment for varicose veins. Br J Surg. 2005;92(10):1189–1194. | |
Vuylsteke ME, Mordon SR. Endovenous laser ablation: a review of mechanisms of action. Ann Vasc Surg. 2012;26(3):424–433. | |
Proebstle TM, Lehr HA, Kargl A, et al. Endovenous treatment of the GSV with a 940 nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser generated steam bubbles. J Vasc Surg. 2002;35:29–36. | |
Proebstle TM, Sandhofer M, Kargl A, et al. Thermal damage of the inner vein wall during endovenous laser treatment: key-role of energy absorption by intravascular blood. Dermatol Surg. 2002;28:596–600. | |
Spreafico G, Giordano R, Piccioli A, et al. Histological damage of saphenous venous wall treated in vivo with radial fiber and 1470 nm diode laser. Ital J Vasc Endovasc Surg. 2011;18:241–247. | |
van der Geld CW, van den Bos RR, van Ruijven PW, Nijsten T, Neumann HA, van Gemert MJ. The heat-pipe resembling action of boiling bubbles in endovenous laser ablation. Lasers Med Sci. 2010;25:907–909. | |
Bush RG, Shamma HN, Hammond K. Histological changes occurring after endoluminal ablation with two diode lasers (940 and 1319 nm) from acute changes to 4 months. Lasers Surg Med. 2008;40(10):676–679. | |
Varetto G, Garneri P, Castagno C, et al. Ultrasound surveillance in endoluminal laser treatment for varicose veins. J Vasc Diagn. 2013;1:21–23. | |
Kim HS, Paxton BE. Endovenous laser ablation of the great saphenous vein with a 980 nm dioode laser in continuos mode: early tratment failures and successful repeat treatments. J Vasc Interv Radiol. 2006;17:1449–1455. | |
Timperman PE, Sichlau M, Ryu RK. Greater energy delivery improves treatment success of endovenous laser treatment of incompetent saphenous veins. J Vasc Interv Radiol. 2004;15(10):1061–1063. | |
Van den Bos RR, Kockaert MA, Neumann HA, Nijsten T. Technical review of endovenous laser therapy for varicose veins. Eur J Vasc Endovasc Surg. 2008;35(1):88–95. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.