Back to Journals » Clinical Ophthalmology » Volume 8
Endothelial distance after phakic iris-fixated intraocular lens implantation: a new safety reference
Authors Ferreira T , Portelinha J
Received 24 October 2013
Accepted for publication 18 November 2013
Published 17 January 2014 Volume 2014:8 Pages 255—261
DOI https://doi.org/10.2147/OPTH.S56484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Tiago Bravo Ferreira, Joana Portelinha
Egas Moniz Hospital, Lisbon, Portugal
Purpose: To evaluate the distance between the endothelial surface of the cornea to the anterior edge of an Artiflex® phakic intraocular lens (IOL) implant to improve the safety profile of this implant.
Methods: This is a retrospective clinical case series of 45 patients who had Artiflex phakic IOL implantation (Artiflex p-IOL) with a follow-up period of 3 years. A Pentacam HR imaging system was used to measure the distance from various points of the anterior edge of the Artiflex IOL to the endothelial surface of the cornea, which we called endothelial–IOL (E–IOL) distance, in 45 eyes. The E–IOL distances were assessed at 1, 3, 6, 12, 24, and 36 months. Corresponding correlations of central endothelial distance to temporal and nasal edges and center of the IOL anterior surface were tabulated.
Results: Mean follow-up was 21.39±11.28 months. A statistically significant reduction of the E–IOL distance was observed over the follow-up period (P<0.05), with the mean annual reduction being 24.70 µm. A strong positive correlation between the E–IOL distance of the edges of the IOL and the central distance was observed (correlation coefficients nasal/central: month 1, 0.905; month 36, 0.806; temporal/central: month 1, 0.906; month 36, 0.806; P<0.001). Moderate negative correlations were found between the spherical equivalent power of the implanted IOL and the E–IOL distance (correlation coefficients -0.271 to -0.412, P>0.05). For an E–IOL distance of the IOL edge >1,500 µm, the distance from the endothelium to the central point of the p-IOL optic should be a minimum of 1,700 µm to improve the safety profile for Artiflex p-IOL implantation and reduce the potential complication of accelerated endothelial cell loss.
Conclusion: After Artiflex IOL implantation, the mean annual reduction of the E–IOL distance was 25 µm. A negative correlation existed between the spherical equivalent power of the implanted IOL and the postoperative E–IOL distance. The minimum E–IOL distance from the center of the IOL to minimize the risk of endothelial cell loss was 1.7 mm. This distance, as is the 1.5 mm initially proposed by Baikoff, is a postoperative value. We cannot make that assumption for the preoperative evaluation, as the morphometry of the anterior chamber changes with the implant.
Keywords: phakic IOL, endothelium, iris-fixated phakic IOL
Introduction
Iris-fixated phakic intraocular lenses (p-IOLs) have been implanted in patients with myopia since 1986.1 The original biconcave design (Worst–Fechner lens) was altered in 1991 to a convex–concave format. In 1998, this type of lens was named the Artisan lens (Ophtec, Groningen, the Netherlands). Subsequently, different models were developed for hyperopia and astigmatism. In 2003, a foldable version of the Artisan lens, the Artiflex® (Ophtec), was introduced. This lens has the primary advantage that it can be introduced through a small incision (3.2 mm) with less induced astigmatism and faster visual recovery.2 More recently, a toric version of the Artiflex was introduced in Europe for management of spherocylindrical refractive errors.
Various studies have demonstrated that both the Artiflex and the Artisan offer predictable and stable postoperative refractive outcome.3–8 The long-term impact of anterior-chamber p-IOL implantation on corneal endothelial cell loss has been a matter of significant research and debate. As a result of numerous randomized clinical trials, the safety of Artisan and Artiflex IOLs are now well established, with reported endothelial cell losses of 4.8% at 6 months, 8.3% at 5 years, and 12.6% at 7 years, and long-term maintenance of the hexagonality and the cell coefficient of variation.7,9,10
In 2004, Baikoff et al proposed that the minimum safety distance between the edge of the optical zone of the p-IOL and the endothelium, as measured by anterior chamber optical coherence tomography (AC-OCT), should be greater than 1.5 mm to minimize the risk of endothelial cell loss.11
There are several devices with which clinicians can evaluate the final resting position of p-IOLs in the anterior chamber, including AC-OCT, ultrasound biomicroscopy, and Scheimpflug photography. The usefulness of AC-OCT in postoperative evaluation of the IOL position has been demonstrated in various studies.11–13 Notwithstanding the excellent image quality produced by ultrasound biomicroscopy, there are several limitations to its clinical practicality.14 It is uncomfortable for some patients, can be time-consuming, technically challenging to perform, and may carry the risk of inducing image distortion by direct compression onto the globe. On the other hand, advantages associated with Scheimpflug topography include its rapid image acquisition without direct physical contact. This type of photography, however, is limited by its requirement for clear optical media and the imprecision of certain images, due to the dispersion of light (which can be minimized using low-light conditions for image capture).15,16
The purpose of the present study was to evaluate the distance from the anterior edge of the Artisan p-IOL to the endothelial surface (endothelial distance [ED]) over a 3-year postoperative follow-up period. As a result of appropriate correlations from such findings, we were able to identify and establish new safety indices, which would assist clinicians in preoperative evaluations as well as in postoperative management, thus increasing the safety profile of Artiflex p-IOLs.
Materials and methods
Study population and design
This was a retrospective analysis of 45 eyes from 24 patients who underwent Artiflex p-IOL implantation in 2009–2012 for correction of high simple myopia and/or mixed myopic astigmatism. All surgeries were done at Egas Moniz Hospital between February 2009 and April 2012. The study followed the principles of the Declaration of Helsinki. Routine surgical informed consents were obtained from all patients preoperatively. The Centro Hospitalar de Lisboa Ocidental institutional review board approved the study.
Our chart review included patients aged over 21 years, who had stable refraction over the previous year, an anterior-chamber depth greater than 3.0 mm (from the endothelium to the anterior face of the crystalline lens), a scotopic pupil of less than 7.0 mm in diameter, and an endothelial cell density greater than 2,000 cells/mm2. Endothelial cell density was evaluated using a specular microscope in automated mode (SP-3000P; Topcon Medical Systems, Oakland, NJ, USA). The following were considered to be exclusion criteria on preoperative evaluations: the existence of anomalies in the iris, history of glaucoma or uveitis, and the presence of retinal lesions that would predispose the patient to retinal detachment. In accordance with the proposition by Baïkoff et al, a crystalline lens rise of greater than 600 μm also constituted an exclusion criterion in order to decrease the risk of iris-pigment dispersion.17
The evaluation of distances between the IOL and the endothelium was conducted using Scheimpflug photography (Pentacam HR; Oculus Optikgerate, Wetzlar, Germany). The Pentacam HR is an image-acquisition system for the anterior segment that uses a rotating camera that is based on the Scheimpflug principle. This system is capable of creating a three-dimensional map of the anterior segment of the eye, allowing for the measurement of the different safety distances prior to and following the implantation of p-IOLs. The camera maps the anterior and posterior surfaces of the cornea, capturing 50 images in 2 seconds, while moving around the ocular globe. In each image, the system evaluates 2,760 points, totaling 138,000 true elevation points. This technique allows for the creation of a three-dimensional image of the cornea and the anterior chamber.18
ED was evaluated from both the nasal and temporal edges and the center of the IOL in the meridian from 0°–180° (Figure 1). For each of these distances, we report a final number that was the average of three captured measurements of the Pentacam image. The same average was taken and reported on postoperative days 30 and 180 and the first, second, and third years. We established the evolving patterns of an increase or decrease in the distances between the corneal endothelium to the center of the optic and the nasal/temporal edges of the optic in relation to time. Correlations between the spherical equivalent power of the implanted IOL and the ED from the center and edges of the IOL were also established.
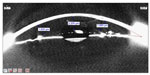  | Figure 1 Scheimpflug image showing the measurement of the endothelial distances from the edges and center of the intraocular lens. |
The intraocular lens
We used the already established guidelines of Ophthec in selecting the appropriate IOL-power to achieve the postoperative target refraction of emmetropia. The selected Artiflex p-IOL for our patients had a central 6.0 mm optic with polysiloxane and poly(methyl methacrylate) haptics (available in sphere −2.0 D to −14.5 D and in cylinder −1.0 D to −5 D).
Surgical technique
The surgical procedures were performed by two experienced surgeons (TF and AC). The method of implanting the Artiflex lens is similar to that of the Artisan lens, which has been published previously.19 In all cases, the primary clear corneal incision was 3.2 mm. The enclavation of the haptics in the iris was accomplished using a needle (Ophtec). The iridectomy was executed with scissors. For the toric lenses, the horizontal meridian was marked using an Elies pendulum marker (E Janach; Combined Medical Specialities, Johannesburg, South Africa) with the patient seated to avoid cyclotorsion. The axis of enclavation was marked intraoperatively using a Mendez marker (Duckworth and Kent, Baldock, UK) and an axis marker (Duckworth and Kent), according to the position suggested by the manufacturer. The haptics were enclaved in the iris in accordance with these markings.
Statistical analysis
All measurements were recorded in an Excel spreadsheet (Office 2010; Microsoft, Redmond, WA, USA). The statistical analyses were done using SPSS for Windows (version 16.0; IBM, Armonk, NY, USA). The normality of the measurements was confirmed using the Kolmogorov–Smirnov test. Statistically significant differences were established using one-way repeated-measures analysis of variance. Correlations were established in accordance with the Spearman correlation coefficient. To determine the safety measurement for the central ED (CED), 95% confidence intervals were calculated for a >1.5 mm distance between the endothelium and the edge of the IOL. The upper value of this interval was selected as the final reported distance value (nasal or temporal). Values of P<0.05 were considered statistically significant. The results are expressed as means ± standard deviation.
Results
Study population
A total of 45 eyes from 26 patients were evaluated. The average follow-up was 21.39±11.28 (12–42) months. The average age of the study patients was 32±4.6 years (26–40 years). In 22 eyes from 14 patients, a spherical Artiflex was implanted with mean IOL power of −10.00±1.18 D (−5.50 to −12.50 D). In 23 eyes from twelve patients, a toric Artiflex IOL was implanted, with mean IOL spherical power of −6.72±1.00 D (−4.50 to −8.00 D) and mean cylindrical power of −2.00±0.94 D (−1.00 to −3.50 D). The average CED was 2,670±316 cells/mm2 (2,222–3,109 cells/mm2) preoperatively and 2,531±451 cells/mm2 (1,988–3,199 cells/mm2) postoperatively (P=0.341). The average preoperative anterior chamber depth was 3.24±0.18 mm (3.08–3.58 mm). The average preoperative uncorrected distance visual acuity (UDVA) was 0.01±0.01 (0.01–0.05) decimal. The average preoperative corrected distance visual acuity (CDVA) was 0.77±0.26 (0.16–1.00) decimal. The postoperative UDVA was 0.84±0.17 (0.5–1.0) decimal, and the postoperative CDVA was 0.88±0.14 (0.5–1.0). The postoperative residual spherical equivalent was −0.21±0.32 D (0.00–0.25 D).
Evolving pattern of endothelial distance over time
The evolving pattern of the ED over the 3-year follow-up is depicted in Figure 2. A statistically significant reduction of the ED was noted over the course of the study for all of the examined distances (nasal ED [NED] month 1–month 36, P=0.039; temporal endothelial ED (TED) month 1–month 36, P=0.046; CED month 1–month 36, P=0.002). The average annual reduction was 20.23±3.43 μm for the NED, 28.6±3.21 μm for the TED, and 25.27±3.87 μm for the CED.
Correlation between the different endothelial distances
A statistically significant positive correlation was present over the course of the follow-up period between the ED from the edges of the IOL and the CED. The correlation coefficients for the different distances are given in Table 1.
With these results, for an ED from the edge of the IOL that was greater than 1,500 μm (according to the minimum safety distance between the edge of the p-IOL and the endothelium proposed by Baikoff11), safety values for the CED were established with 95% confidence intervals (Table 2).
Correlation between the endothelial distances and power of the IOL
Moderate negative correlations were found over the course of the follow-up between the spherical equivalent power of the implanted IOL and the EDs both from the center and edges of the IOL (Table 3).
Discussion
p-IOLs are considered to be a safe, effective, and stable method for the correction of moderate-to-high myopia. The effectiveness and safety of the spherical and toric Artiflex IOL have been demonstrated in various clinical studies.2,6–8 A concern following an anterior-chamber p-IOL implantation is the ongoing impact of the implant over time on the surrounding structures of the anterior segment, eg, the iridocorneal angle, the endothelium, and the crystalline lens. This clinical study sought to examine change in ED over 3 years following the implantation of the Artiflex IOL and to create a new safety measurement. This evaluation was performed with the intent of simplifying the postoperative monitoring of these patients.
Morphological alterations in the crystalline lens influence its relationship with iris-fixated lenses.20 With aging, in addition to the progressive increase in the thickness of the crystalline lens, there is also a movement of its anterior pole in the direction of the endothelium by approximately 18–20 μm annually.17,21 In the present study, a reduction in ED by an average of 25 μm annually was directly correlated with the annual anterior movement of the crystalline lens. One way to confirm if this movement is responsible for the reduction in ED could be to measure lens thickness with each postoperative distance measurement. This should be investigated in further studies. Knowledge of these data will allow us to calculate the time that the IOL can remain in the eye while maintaining a safe ED. Doors et al also described a linear reduction of 3.1 μm per year in the distance between the iris-enclaved IOL and the crystalline lens.22 As a result of our findings, we strongly recommend that clinicians include a morphometric study of the anterior chamber in patients who have had anterior chamber p-IOL implants during the follow-up period. This monitoring may enable the physician to identify individual patients who may have accelerated reduction of the ED and assist in taking the necessary preemptive measures. This may explain an early unexpected corneal decompensation in patients who have had anterior-chamber p-IOL implantation in previous published reports.
The relationship between the distance from the edge of the IOL to the endothelium and the loss of endothelial cells is known. In a study by Doors et al, a 1.37 mm distance from the edge of the IOL to the endothelium resulted in an annual loss of endothelial cells of 0.98%, whereas a 1.15 mm distance resulted in an annual loss of cells of 1.8%.22 The same authors described a model that was capable of predicting endothelial cell loss following the implantation of iris-fixated lenses in accordance with the distance from the edge of the IOL to the endothelium, as measured with AC-OCT.23
Baïkoff proposed a minimum endothelial safety distance of 1.5 mm between the edges of the IOL and the endothelium.11 Güell et al suggested a minimum CED of 2.0 mm. This distance, however, was determined arbitrarily.24 During the follow-up of these patients, the measurement of ED from the edges and center of the IOL is laborious. During the follow-up period, a strong positive correlation was observed between the ED from the edges of the IOL and the CED. For an ED from the edge of the IOL that is greater than 1.5 mm, a value of 1.7 mm was calculated as the minimum safe distance from the center of the IOL to the endothelium.
Higher p-IOL powers have a greater central thickness. Our data show moderate negative correlation of ED with increasing p-IOL power over time for both the central and peripheral distances. Surgeons may need to consider anterior-chamber depth of greater than 3.0 mm in implanting higher-power p-IOLs. In our future study, we will evaluate and report the direct correlation of the anterior-chamber depth in correspondence with the p-IOL power.
In conclusion, the average reduction in ED following the implantation of the Artiflex IOL is 25 μm per year. We recommend yearly morphometric evaluation of the anterior segment to include anterior-chamber depth and ED in routine postoperative follow-up of patients with anterior-chamber p-IOL implants. This evaluation is particularly important, and should be considered in children or older adults who may have more unique anterior segment structures whenever feasible.25,26 Given the strong correlation between the ED from the edges of the IOL and the CED, a CED greater than 1.7 mm appears sufficient to minimize the risk of endothelial cell loss.
Disclosure
The authors report no conflicts of interest in this work.
References
Fechner PU, van der Heijde GL, Worst JG. The correction of myopia by lens implantation into phakic eyes. Am J Ophthalmol. 1989;107(6):659–663. | |
Coullet J, Guëll JL, Fournié P, et al. Iris-supported phakic lenses (rigid vs foldable version) for treating moderately high myopia: randomized paired eye comparison. Am J Ophthalmol. 2006;142(6):909–916. | |
Stulting RD, John ME, Maloney RK, et al. Three-year results of Artisan/Verisyse phakic intraocular lens implantation. Results of the United States Food and Drug Administration clinical trial. Ophthalmology. 2008;115(3):464–472. | |
Budo C, Hessloehl JC, Izak M, et al. Multicenter study of the Artisan phakic intraocular lens. J Cataract Refract Surg. 2000;26(8):1163–1171. | |
Dick HB, Alió J, Bianchetti M, et al. Toric phakic intraocular lens: European multicenter study. Ophthalmology. 2003;110(1):150–162. | |
Dick HB, Budo C, Malecaze F, et al. Foldable Artiflex phakic intraocular lens for the correction of myopia: two-year follow-up results of a prospective European multicenter study. Ophthalmology. 2009;116(4):671–677. | |
Doors M, Budo CJ, Christiaans BJ, et al. Artiflex toric foldable phakic intraocular lens: short-term results of a prospective European multicenter study. Am J Ophthalmol. 2012;154(4):730–739. e2. | |
Ruckhofer J, Seyeddain O, Dexl AK, Grabner G, Stoiber J. Correction of myopic astigmatism with a foldable iris-claw toric phakic intraocular lens: short-term follow-up. J Cataract Refract Surg. 2012;38(4):582–588. | |
Saxena R, Boekhoorn SS, Mulder PG, Noordzij B, van Rij G, Luyten GP. Long-term follow-up of endothelial cell change after Artisan phakic intraocular lens implantation. Ophthalmology. 2008;115(4):608–613. | |
Benedetti S, Casamenti V, Benedetti M. Long-term endothelial changes in phakic eyes after Artisan intraocular lens implantation to correct myopia: five-year study. J Cataract Refract Surg. 2007;33(5):784–790. | |
Baïkoff G. Anterior segment OCT and phakic intraocular lenses: a perspective. J Cataract Refract Surg. 2006;32(11):1827–1835. | |
Baikoff G, Lutun E, Ferraz C, Wei J. Static and dynamic analysis of the anterior segment with optical coherence tomography. J Cataract Refract Surg. 2004;30(9):1843–1850. | |
Baikoff G, Lutun E, Wei J, Ferraz C. Contact between 3 phakic intraocular lens models and the crystalline lens: an anterior chamber optical coherence tomography study. J Cataract Refract Surg. 2004;30(9):2007–2012. | |
Pop M, Mansour M, Payette Y. Ultrasound biomicroscopy of the iris-claw phakic intraocular lens for high myopia. J Refract Surg. 1999;15(6):632–635. | |
Kohnen T, Cichocki M, Koss MJ. Position of rigid and foldable iris-fixated myopic phakic intraocular lenses evaluated by Scheimpflug photography. J Cataract Refract Surg. 2008;34(1):114–120. | |
Baumeister M, Bühren J, Kohnen T. Position of angle-supported, iris-fixated, and ciliary sulcus-implanted myopic phakic intraocular lenses evaluated by Scheimpflug photography. Am J Ophthalmol. 2004;138(5):723–731. | |
Baïkoff G, Bourgeon G, Jodai HJ, Fontaine A, Lellis FV, Trinquet L. Pigment dispersion and Artisan phakic intraocular lenses: crystalline lens rise as a safety criterion. J Cataract Refract Surg. 2005;31(4):674–680. | |
McAlinden C, Khadka J, Pesudovs K. A comprehensive evaluation of the precision (repeatability and reproducibility) of the Oculus Pentacam HR. Invest Ophthalmol Vis Sci. 2011;52(10):7731–7737. | |
Tahzib NG, Bootsma SJ, Eggink FA, Nuijts RM. Functional outcome and patient satisfaction after Artisan phakic intraocular lens implantation for the correction of myopia. Am J Ophthalmol. 2006; 142(1):31–39. | |
Koretz JF, Cook CA, Kaufman PL. Accommodation and presbyopia in the human eye. Changes in the anterior segment and crystalline lens with focus. Invest Ophthalmol Vis Sci. 1997;38(3):569–578. | |
Atchison DA, Markwell EL, Kasthurirangan S, Pope JM, Smith G, Swann PG. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008;8(4):29.1–20. | |
Doors M, Cals DW, Berendschot TT, et al. Influence of anterior chamber morphometrics on endothelial cell changes after phakic intraocular lens implantation. J Cataract Refract Surg. 2008;34(12):2110–2118. | |
Doors M, Berendschot TT, Webers CA, Nuijts RM. Model to predict endothelial cell loss after iris-fixated phakic intraocular lens implantation. Invest Ophthalmol Vis Sci. 2010;51(2):811–815. | |
Güell JL, Morral M, Gris O, Gaytan J, Sisquella M, Manero F. Evaluation of Verisyse and Artiflex phakic intraocular lenses during accommodation using Visante optical coherence tomography. J Cataract Refract Surg. 2007;33(8):1398–1404. | |
Alió JL, Toffaha BT, Laria C, Piñero DP. Phakic intraocular lens implantation for treatment of anisometropia and amblyopia in children: 5-year follow-up. J Refract Surg. 2011;27(7):494–501. | |
Pirouzian A, Ip KC. Anterior chamber phakic intraocular lens implantation in children to treat severe anisometropic myopia and amblyopia: 3-year clinical results. J Cataract Refract Surg. 2010;36(9):1486–1493. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




