Back to Journals » Clinical Ophthalmology » Volume 17
Endothelial Cell Loss Following Cataract Surgery Using Continuous Curvilinear Capsulorhexis or Precision Pulse Capsulotomy
Authors Vital MC, Jong KY , Trinh CE , Starck T, Sretavan D
Received 20 March 2023
Accepted for publication 25 May 2023
Published 16 June 2023 Volume 2023:17 Pages 1701—1708
DOI https://doi.org/10.2147/OPTH.S411454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mark C Vital,1 Kevin Y Jong,1 Clarise E Trinh,1 Tomy Starck,2 David Sretavan3
1Houston Eye Associates, Houston, TX, USA; 2UltraVision, San Antonio, TX, USA; 3Centricity Vision Inc., Carlsbad, CA, USA
Correspondence: Mark C Vital, Houston Eye Associates, 2855 Gramercy Street, Houston, TX, 77025, USA, Tel +1 713 668 6828, Fax +1 832 553-7154, Email [email protected]
Purpose: To compare endothelial cell density (ECD), percentage of hexagonal cells (%Hex) and coefficient of variation (CV) in cell size following lens cataract surgery with phacoemulsification performed using Continuous Curvilinear Capsulorhexis (CCC) or Precision Pulse Capsulotomy (PPC).
Patients and Methods: Sixty-seven subjects were randomly assigned to undergo lens cataract removal with the capsulotomy step performed using either CCC or PPC. Specular microscopy images were obtained pre-operatively, 1 month and 3 months after surgery. ECD, %Hex and CV were analyzed in a masked fashion by an independent reading center.
Results: The mean percentage ECD loss at 1 month was 11.5% in the CCC group and 12.3% in the PPC group (P = 0.818; t-test). At 3 months, the mean percentage ECD loss was 11.7% in the CCC group and 12.4% in the PPC group (P = 0.815; t-test). The mean %Hex at 1 month was 54.3% in the CCC group and 54.7% in the PPC group (P = 0.695; t-test). At 3 months, the mean %Hex was 56.2% in the CCC group and 54.7% in the PPC group (P = 0.278; t-test). The CV at 1 month was 34.4% in the CCC group and 34.3% in the PPC group (P = 0.927; t-test). At 3 months, the CV was 32.7% in the CCC group and 33.4% in the PPC group (P = 0.864; t-test).
Conclusion: No differences in ECD loss, %Hex and CV were observed between patients who received CCC or PPC. PPC use during cataract surgery does not result in any increased endothelial cell loss beyond that normally associated with this surgery.
Keywords: automated capsulotomy, zepto, corneal endothelium
Introduction
Corneal endothelial cells are critical for proper corneal stromal hydration and tissue transparency through the physiological action of their ionic pumps.1 Unfortunately, this critical cell population does not have regenerative potential, and significant endothelial cell loss through disease or trauma may trigger compensatory responses of cellular enlargement and/or migration of the remaining cells.2 Endothelial cell density (ECD) normally declines with age from 4000 cells/mm2 in childhood to a range of approximately 2250–2500 cells/mm2 by 80 years of age.3
Lens cataract surgery with phacoemulsification is associated with a 5–20% loss of corneal endothelial cells at 1–3 months after surgery.4–16 (Also reviewed in17) This endothelial cell loss is believed to be related to the ultrasound energy delivered during lens phacoemulsification and to fluidic turbulence during surgery.8,18,19 The ECD loss after cataract surgery typically does not pose a problem for the patient. However, more extensive endothelial cell loss resulting in an ECD of 600–800 cells/mm2 is associated with corneal decompensation and edema and is a serious complication that may require physician and surgical intervention such as penetrating or endothelial keratoplasty.2,3
The successful creation of an anterior capsulotomy provides the foundation for subsequent lens cataract removal and intracapsular lens implantation. Capsulotomy is currently typically performed using CCC, a manual procedure in which a capsular tear created by the surgeon is carefully extended in a circular pattern to create the desired opening.20,21 Given the skill required to create an appropriately sized and well-centered capsulotomy with even-spaced capsular overlap, laser-based capsulotomy methods22–27 as well as non-laser-based capsulotomy technologies such as Precision Pulse Capsulotomy (PPC) have been introduced to the market after FDA clearance.28–36
PPC is performed using an intraocular device comprising a small flexible suction cup that is used to secure an embedded nitinol capsulotomy ring onto the anterior lens capsule. Capsulotomy creation utilizes a short sequence of 12 energy pulses lasting a total of 4 milliseconds applied to the capsulotomy ring to create the rapid phase transition of water molecules in capsular tissue to create a tissue cutting force.28 The near-instantaneous creation of the entire circular capsulotomy opening has led to its proposed utility in difficult cases with intumescent white cataract where uncontrolled capsular extensions can lead to the Argentinian flag sign.37 Laboratory studies have demonstrated that PPC is associated with an intraocular temperature increase of only 2–3°C lasting for only 1–2 seconds that is unlikely to induce any endothelial cell damage. However, as with any cataract surgery instrument that delivers energy into the eye, endothelial cell safety must be clinically demonstrated. The present study was undertaken to test the hypothesis that cataract surgery with lens phacoemulsification performed using either CCC or PPC do not differ in post-operative ECD loss and other metrics of endothelial cell condition such as the percentage of hexagonal cells (%Hex) and the co-efficient of variation (CV) in cell size.38
Materials and Methods
Study Design
The current study was undertaken to determine whether PPC had deleterious effects on endothelial cell viability beyond that normally observed following routine lens cataract surgery with phacoemulsification. The study was designed as a prospective, randomized, multisite clinical trial with subjects receiving lens cataract surgery for age-related lens cataract removal randomly enrolled into either the PPC (interventional) or CCC (control) arms. A statistical power of 80% was set to test the hypothesis that ECD loss at 3 months following PPC cataract surgery was not greater than that following CCC cataract surgery, with a non-inferiority (NI) delta of 7.5% and an assumed standard deviation of 0.12.
Study data were obtained from specular microscopy images of the central corneal endothelium. Images were acquired at baseline prior to surgery and at 1 month and 3 months after surgery, all of which were read by an independent, third-party reading center. The primary endpoint was endothelial cell density loss at 3 months after surgery. Secondary endpoints included ECD loss at 1 month after surgery, as well as %Hex and CV at baseline, 1 month and 3 months. The 1- and 3-month time points were selected for analysis as ECD loss after cataract surgery manifests primarily during the first 3 months after surgery.39 The clinical study protocol used adhered to the basic principles of the Declaration of Helsinki and specifically to the tenants governing clinical research. The study protocol was reviewed and approved by WCG IRB (Puyallup, WA.) (Study Number 1309679). Informed written consent was obtained from all study subjects prior to participation in the study. This study was registered at ClinicalTrials.gov. under ID # NCT04882189. Study conduct was overseen by a contract research organization (Sierra Clinical Services; Wellington, FL.). Individuals interested in de-identified participant study data may contact the authors.
Subject Selection and Randomization
Subjects aged 50 years or older with age-related lens cataract with planned lens cataract removal with phacoemulsification were eligible to be enrolled. Subjects were excluded from enrollment for pre-existing corneal endothelium pathology, the presence of guttae, narrow angle glaucoma or advanced glaucoma, pseudoexfoliation, zonular abnormalities, corneal endothelial cell density less than 1800 cells/mm2, uveitis, anterior chamber depth less than 2.5mm or greater than 3.75mm, cataract grade LOCS II > 3, posterior polar cataract, prior ocular surgery in the study eye, history of medications with potential corneal endothelial cell toxicity, and participation in the prior 6 months or currently in another clinical study.
Subjects who were successfully screened were assigned to either the PPC (interventional) or CCC (control) arm using a computer-generated block randomization list maintained by a study coordinator masked to subject screening data. Subjects were assigned to the study arm according to the pre-specified block randomization list in the sequential order in which they passed the screening process.
Surgical Procedure
All surgeries were performed using a 2.4 mm primary corneal incision and the Centurion Vision System Phacomachine (Alcon, Inc.). DuoVisc (Alcon) was used as the Ophthalmic Viscosurgical Device in all surgeries. For subjects who received a CCC capsulotomy, a manual capsulorhexis was performed using capsulorhexis forceps. For subjects who received PPC capsulotomy, the PPC hand piece was prepared for surgery according to manufacturer’s instructions. The PPC silicone suction cup and nitinol ring were then inserted into the eye by the surgeon and suction applied through the PPC console to oppose the nitinol ring onto the anterior lens capsule. Energy was then applied using the console and the capsulotomy was created. Suction was then reversed, and the hand piece suction cup and nitinol ring removed from the eye. The remainder of the procedure including lens mobilization, lens phacoemulsification and IOL implantation was performed in a fashion duplicating that for subjects in the CCC arm according to the customary practice of the individual surgeon. In none of the cases did any instrument touch the endothelium nor was there any other type of endothelial trauma. The surgeries were conducted by three surgeons. Surgeon 1 with 25 years of experience in cataract surgery and more than 2000 cases using PPC, Surgeon 2 with 20 years of experience in cataract surgery and more than 1000 cases using PPC, Surgeon 3 with 30 years of experience in cataract surgery and approximately 100 cases using PPC.
Specular Microscopy
Specular microscope images were obtained prior to surgery, at 1 month and at 3 months after surgery for all study subjects. Specular microscopy was performed using a Konan specular microscope KSS-400 Series with image capture software Version 1.14. For each subject at each specular microscopy session, 3 central corneal images were obtained and submitted to the reading center (see below). The specular microscope and the technicians were certified by the reading center following acceptance of calibration images from each study site.
Specular Microscopy Image Reading Center
The analysis of specular microscopy images was performed by the Corneal Image Analysis Reading Center (CIARC) at the Case Western Reserve University and University Hospitals Cleveland Medical Center. CIARC utilizes a dual-grading reading method and all images were read and analyzed by two separate readers and adjudicated by a third reader as necessary, to ensure the validity of the image analysis.40–42 Study images were provided by the study sites to CIARC without identifying information through a web-based image management system.
Statistical Analysis
Biometric, demographic and other continuous data were summarized with descriptive statistics (N, mean, standard deviation, minimum, median, and maximum). Categorical variables were summarized with N and percentage. Point estimates and associated confidence intervals were also used. Categorical data between CCC and PPC groups were tested using the Chi-Square test, while the testing of means between CCC and PPC groups was performed using the appropriate t-test. Regression analysis was performed to test the relationship between ECD loss and cumulative dispersed energy (CDE). All analyses were performed using SAS software version 9.4. 95% confidence intervals (CI) were calculated for the mean ECD loss at 1 month and at 3 months for both the PPC and CCC groups.
Results
Patient Demographics
A total of 67 subjects who met the inclusion and exclusion criteria were enrolled in this study. Thirty-three subjects were randomly assigned to the CCC (control) arm and 34 assigned to the PPC (Interventional) arm. The mean age of the subjects in the CCC arm was 71.39 ± 7.99 years, while that of the PPC arm was 70.65 ± 5.66 years (P = 0.660; t-test). In the CCC group, 45.45% of subjects were female and 54.55% were male. In the PPC group, 47.06% were female and 52.94% were male (P = 0.895; Chi-Square).
Clinical Data and Visual Outcomes
The cataract grade for each subject was classified according to the LOCS II system. Within the CCC group, the breakdown was 6.1% grade 1, 63.6% grade 2, and 30.3% grade 3. Within the PPC group, the breakdown was 5.9% grade 1, 58.8% grade 2, and 35.3% grade 3. No statistically significant differences were found for the percentage of subjects with cataract grades 1, 2 or 3 between the CCC and PPC groups (P = 0.909, Ch-Square). The mean anterior chamber depth (ACD) (from the corneal endothelial surface to the anterior lens capsule) in the CCC group was 3.17 ±0.38 mm (range 2.6–4.1mm), while that in the PPC group was. 3.07±0.38mm (range 2.16–3.75 mm) (P = 0.303, t-test). The mean LogMAR uncorrected visual acuity (UCVA) and best corrected visual acuity (BCVA) for the CCC and the PPC groups at pre-op, 1 month, and 3 months were not significantly different from each other (Table 1).
 |
Table 1 Cataract Grade, ACD, UCVA and BCVA |
Endothelial Cell Density
There was no difference in the pre-operative mean ECD of subjects in the CCC arm compared to the PPC arm (CCC: 2534 ± 335 cells/mm2; PPC: 2522±345 cells/mm2), (P=0.0.8183, t-test). At 1 month, a mean ECD loss of 11.5% was observed in the CCC group and a mean ECD loss of 12.3% was observed in the PPC group (P = 0.818, t-test) (Table 1). At 3 months, a mean ECD loss of 11.7% was observed in the CCC group and a mean ECD loss of 12.4% was observed in the PPC group (P = 0.815, t-test) (Table 1). The percentage of ECD loss for both CCC and PPC subjects in the 25th, 50th and 75th percentiles were similar at both 1 and 3 months (Table 2). The upper bound of the 95% CI for ECD loss at 3 months after PPC was less than the NI delta of 7%; supporting the hypothesis that ECD loss at 3 months following PPC cataract surgery was not inferior to (ie, greater than) that observed following CCC cataract surgery.
 |
Table 2 ECD Loss at 1 and 3 Months After Cataract Surgery Using CCC or PPC |
ECD Loss as a Function of CDE
The percentage of ECD loss at 1 month in both the CCC and the PPC groups was linearly related to the CDE used for lens cataract removal. The mean CDE associated with the cataract surgeries performed using CCC was 5.23 ± 1.8 (range 2–10.2), while the mean CDE associated with the cataract surgeries performed using PPC was 6.24 ± 3.48 (range 2–17). There was no statistically significant difference found between the CDEs from the CCC and PPC groups (P = 0.149, t-test, 2-tailed, equal variance). Regression analysis revealed a linear relationship between the percentage of ECD loss at 1 month and CDE for both CCC and PPC cases with a linear regression equation of −1.5817*CDE (Standard Error 0.5629, t value 12.81, P = 0.0065). Based on this regression analyses, each unit increase in CDE is predicted to give rise to an approximately 1.6% increase in ECD loss at 1 month.
Percent Hexagonal Cells
The mean %Hex in specular images of the corneal endothelium obtained at pre-op, 1 month, and 3 months were not significantly different between the CCC and PPC groups (Table 3). The %Hex in the current study ranged from a pre-op value of ~58% to the 1 month and 3-month values that range from 54% to 56% and are in general agreement with that reported in the literature in a normal population and following cataract surgery with phacoemulsification.6,11 Separate analyses revealed no significant differences in the %Hex results obtained for the CCC group and the PPC group at pre-op, 1 month, and 3 months (Table 3).
 |
Table 3 The Mean Percentage of Hexagonal Cells and CV in Cell Size at Pre-op, 1 Month, and 3 Months in CCC and PPC Groups |
Coefficient of Variation of Cell Size
The CV in cell size determined from specular images of the corneal endothelium obtained at pre-op, 1 month, and 3 months were not significantly different between the CCC and PPC groups (Table 3). The CV in cell size in the current study ranged from a pre-op value range of ~32–36 and 1 month and 3-month values in the range of 32–36. These values are in general agreement with that reported in the literature in a normal patient population and following cataract surgery with phacoemulsification.6,11,43
Discussion
Cataract surgery with phacoemulsification is known to be associated with an average ECD loss in the range of 5.5% to 20% at 1 to 3 months after surgery.4–16 ECD loss after cataract surgery typically does not have major clinical sequelae when there is sufficient cellular reserve to maintain corneal health. However, as endothelial cells do not regenerate, greater cell loss resulting in ECDs of 600–800 cells/mm2 will cause endothelial dysfunction and lead to corneal decompensation. As ECD loss after cataract surgery is thought to arise in large part from the use of ultrasound energy during phacoemulsification, new cataract surgical technology such as PPC that involves energy delivery to the eye should be examined for endothelial cell safety. Results from the current prospective, randomized study using an independent image reading center demonstrated that the ECD loss after PPC capsulotomy during cataract surgery was no different to that observed when CCC was used as the capsulotomy method. Subjects in both the PPC and CCC study arms had a mean ECD loss of 11–12% ECD loss at 1 and 3 months after surgery, in line with results from published studies4–16 (Table 4).
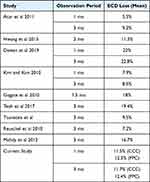 |
Table 4 Literature Survey with Mean Percentage of ECD Loss at 1 and 3 Months After Cataract Surgery with Phacoemulsification |
Insults to the corneal endothelium may lead to a decrease in the percentage of hexagonally shaped endothelial cells and an increase in the CV of cell size amongst the remaining cells as they enlarge to compensate for lost cells.1 In the current study, no differences were noted in both the %Hex and in CV between the CCC and PPC groups at any of the time points analyzed. These findings lend further support to the hypothesis that PPC automated capsulotomy by itself does not have any effects on EC condition and survival after cataract surgery. This is consistent with results from a previous laboratory study demonstrating that the energy delivered during PPC resulted only in a peak temperature change of +2°C lasting for a few seconds in the region of the corneal endothelium.28 This magnitude of temperature change was not expected to have any deleterious effects on cell viability and is in line with the results from the current prospective study in human subjects.
Conclusion
The percentage of ECD loss following cataract surgery performed using PPC was not statistically different to that found after cataract surgery performed with CCC at either the 1 or 3 month postoperative time points. Other parameters of EC health such as %Hex and CV of cell size were also not different between the 2 capsulotomy methods at these time points. PPC as an automated capsulotomy method has a demonstrated EC safety profile equivalent to CCC during cataract surgery.
Acknowledgments
The authors wish to thank Gerard Smits PhD for assistance with statistical analysis, the staff members at the clinical sites, and the staff at CIARC for their diligent support of this study.
Disclosure
TS reports no conflict of interest in this work. MCV is a consultant for Centricity Vision Inc., outside the submitted work. Dr KYJ reports personal fees from Centricity Vision Inc. during the conduct of the study. DS is a founder of and has financial interest in Centricity Vision Inc. In addition, DS has a patent on a capsulotomy ring design issued to owned by Centricity Vision Inc.
References
1. Gupta PK, Berdahl JP, Chan CC, et al. The corneal endothelium: clinical review of endothelial cell health and function. J Cataract Refract Surg. 2021;47(9):1218–1226. doi:10.1097/j.jcrs.0000000000000650
2. Edelhauser HF. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea. 2000;19(3):263–273. doi:10.1097/00003226-200005000-00002
3. Bourne WM. Biology of the corneal endothelium in health and disease. Eye. 2003;17(8):912–918. doi:10.1038/sj.eye.6700559
4. Acar BT, Utine CA, Acar S, Ciftci F. Endothelial cell loss after phacoemulsification in eyes with previous penetrating keratoplasty, previous deep anterior lamellar keratoplasty, or no previous surgery. J Cataract Refract Surg. 2011;37(11):2013–2017. doi:10.1016/j.jcrs.2011.05.033
5. Hwang HB, Lyu B, Yim HB, Lee NY. Endothelial cell loss after phacoemulsification according to different anterior chamber depths. J Ophthalmol. 2015;2015:210716. doi:10.1155/2015/210716
6. Fea AM, Consolandi G, Pignata G, et al. A comparison of endothelial cell loss in combined cataract and MIGS (Hydrus) procedure to phacoemulsification alone: 6-month results. J Ophthalmol. 2015;2015:769289. doi:10.1155/2015/769289
7. Choi JY, Han YK. Long-term (>/=10 years) results of corneal endothelial cell loss after cataract surgery. Can J Ophthalmol. 2019;54(4):438–444. doi:10.1016/j.jcjo.2018.08.005
8. Dewan T, Malik PK, Kumari R. Comparison of effective phacoemulsification time and corneal endothelial cell loss using 2 ultrasound frequencies. J Cataract Refract Surg. 2019;45(9):1285–1293. doi:10.1016/j.jcrs.2019.04.015
9. Teoh LS, Foo SW, Mansurali VN, Ang EL, Md Noh UK, Bastion MC. Evaluation of corneal endothelial cell loss after uncomplicated phacoemulsification cataract surgery with intracameral phenylephrine. Asia Pac J Ophthalmol. 2017;6(4):318–325.
10. Kim EC, Kim MS. A comparison of endothelial cell loss after phacoemulsification in penetrating keratoplasty patients and normal patients. Cornea. 2010;29(5):510–515. doi:10.1097/ICO.0b013e3181c11e0e
11. Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass micro-stent. Am J Ophthalmol. 2019;208:211–218. doi:10.1016/j.ajo.2019.07.016
12. Walkow T, Anders N, Klebe S. Endothelial cell loss after phacoemulsification: relation to preoperative and intraoperative parameters. J Cataract Refract Surg. 2000;26(5):727–732. doi:10.1016/S0886-3350(99)00462-9
13. Mahdy MA, Eid MZ, Mohammed MA, Hafez A, Bhatia J. Relationship between endothelial cell loss and microcoaxial phacoemulsification parameters in noncomplicated cataract surgery. Clin Ophthalmol. 2012;6:503–510. doi:10.2147/OPTH.S29865
14. Reuschel A, Bogatsch H, Barth T, Wiedemann R. Comparison of endothelial changes and power settings between torsional and longitudinal phacoemulsification. J Cataract Refract Surg. 2010;36(11):1855–1861. doi:10.1016/j.jcrs.2010.06.060
15. Tsuneoka H, Shiba T, Takahashi Y. Ultrasonic phacoemulsification using a 1.4 mm incision: clinical results. J Cataract Refract Surg. 2002;28(1):81–86. doi:10.1016/S0886-3350(01)01235-4
16. Gogate P, Ambardekar P, Kulkarni S, Deshpande R, Joshi S, Deshpande M. Comparison of endothelial cell loss after cataract surgery: phacoemulsification versus manual small-incision cataract surgery: six-week results of a randomized control trial. J Cataract Refract Surg. 2010;36(2):247–253. doi:10.1016/j.jcrs.2009.09.023
17. Rosado-Adames N, Afshari NA. The changing fate of the corneal endothelium in cataract surgery. Curr Opin Ophthalmol. 2012;23(1):3–6. doi:10.1097/ICU.0b013e32834e4b5f
18. Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22(8):1079–1084. doi:10.1016/S0886-3350(96)80121-0
19. Richard J, Hoffart L, Chavane F, Ridings B, Conrath J. Corneal endothelial cell loss after cataract extraction by using ultrasound phacoemulsification versus a fluid-based system. Cornea. 2008;27(1):17–21. doi:10.1097/ICO.0b013e3181583115
20. Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16(1):31–37. doi:10.1016/S0886-3350(13)80870-X
21. Gimbel HV, Neuhann T. Continuous curvilinear capsulorhexis. J Cataract Refract Surg. 1991;17(1):110–111. doi:10.1016/S0886-3350(13)81001-2
22. Friedman NJ, Palanker DV, Schuele G, et al. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37(7):1189–1198. doi:10.1016/j.jcrs.2011.04.022
23. Grewal DS, Schultz T, Basti S, Dick HB. Femtosecond laser-assisted cataract surgery--current status and future directions. Surv Ophthalmol. 2016;61(2):103–131. doi:10.1016/j.survophthal.2015.09.002
24. Kranitz K, Takacs A, Mihaltz K, Kovacs I, Knorz MC, Nagy ZZ. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011;27(8):558–563. doi:10.3928/1081597X-20110623-03
25. Packer M, Teuma EV, Glasser A, Bott S. Defining the ideal femtosecond laser capsulotomy. Br J Ophthalmol. 2015;99(8):1137–1142. doi:10.1136/bjophthalmol-2014-306065
26. Lin CC, Rose-Nussbaumer JR, Al-Mohtaseb ZN, et al. Femtosecond laser-assisted cataract surgery: a report by the American Academy of ophthalmology. Ophthalmology. 2022;129(8):946–954. doi:10.1016/j.ophtha.2022.04.003
27. Stodulka P, Packard R, Mordaunt D. Efficacy and safety of a new selective laser device to create anterior capsulotomies in cataract patients. J Cataract Refract Surg. 2019;45(5):601–607. doi:10.1016/j.jcrs.2018.12.012
28. Chang DF, Mamalis N, Werner L. Precision pulse capsulotomy: preclinical safety and performance of a new capsulotomy technology. Ophthalmology. 2016;123(2):255–264. doi:10.1016/j.ophtha.2015.10.008
29. Thompson VM, Berdahl JP, Solano JM, Chang DF. Comparison of manual, femtosecond laser, and precision pulse capsulotomy edge tear strength in paired human cadaver eyes. Ophthalmology. 2016;123(2):265–274. doi:10.1016/j.ophtha.2015.10.019
30. Waltz K, Thompson VM, Quesada G. Precision pulse capsulotomy: initial clinical experience in simple and challenging cataract surgery cases. J Cataract Refract Surg. 2017;43(5):606–614. doi:10.1016/j.jcrs.2017.01.023
31. Ifantides C, Lee J, Rouweyha R, Vital M, Sretavan D. Precision pulse capsulotomy: performance metrics and utility in routine and complex cases. J Cataract Refract Surg. 2020;46(11):1522–1529. doi:10.1097/j.jcrs.0000000000000318
32. Gundersen KG, Potvin R. Clinical results after precision pulse capsulotomy. Clin Ophthalmol. 2020;14:4533–4540. doi:10.2147/OPTH.S293819
33. Chougule P, Warkad V, Badakere A, Kekunnaya R. Precision pulse capsulotomy: an automated alternative to manual capsulorhexis in paediatric cataract. BMJ Open Ophthalmol. 2019;4(1):e000255. doi:10.1136/bmjophth-2018-000255
34. Park MJ, Bang CW, Han SY. Precision pulse capsulotomy in challenging cataract surgery cases. Clin Ophthalmol. 2019;13:1361–1368. doi:10.2147/OPTH.S217919
35. Singh B, Sharma S, Bharti N, Bharti S. Precision pulse capsulotomy during combined penetrating keratoplasty with cataract surgery and intraocular lens in small nondilating pupil. Eye Contact Lens. 2021;47(4):219–222. doi:10.1097/ICL.0000000000000734
36. Bang SP, Jun JH. Comparison of postoperative axial stability of intraocular lens and capsulotomy parameters between precision pulse capsulotomy and continuous curvilinear capsulotomy: a prospective cohort study. Medicine. 2019;98(48):e18224. doi:10.1097/MD.0000000000018224
37. Ifantides C, Sretavan D. Automated precision pulse capsulotomy vs manual capsulorhexis in white cataracts: reduction in procedural time and resource utilization. JCRS. 2023;49(4):392–399.
38. Doughty MJ. Prevalence of ‘non-hexagonal’ cells in the corneal endothelium of young caucasian adults, and their inter-relationships. Ophthalmic Physiol Opt. 1998;18(5):415–422. doi:10.1046/j.1475-1313.1998.00376.x
39. Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an Ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. doi:10.1016/j.ophtha.2019.03.006
40. Benetz BA, Gal RL, Ruedy KJ, et al. Specular microscopy ancillary study methods for donor endothelial cell density determination of cornea donor study images. Curr Eye Res. 2006;31(4):319–327. doi:10.1080/02713680500536738
41. Lass JH, Gal RL; Cornea Donor Study Investigator G. Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. Specular microscopy ancillary study results. Ophthalmology. 2008;115(4):627–632 e628.
42. Lass JH, Benetz BA, Verdier DD, et al. Corneal endothelial cell loss 3 years after successful descemet stripping automated endothelial keratoplasty in the cornea preservation time study: a randomized clinical trial. JAMA Ophthalmol. 2017;135(12):1394–1400. doi:10.1001/jamaophthalmol.2017.4970
43. Kelkar J, Kelkar A, Pandit A, Kelkar S. A prospective comparative study on endothelial cell loss and morphology after femtolaser-assisted cataract surgery and phacoemulsification. Int Ophthalmol. 2020;40(5):1299–1305. doi:10.1007/s10792-020-01297-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
