Back to Journals » International Medical Case Reports Journal » Volume 16
Endoscopic Diagnosis of Hookworm Disease in a Patient with Severe Iron Deficiency Anemia: A Case Report
Authors Tiremo SN, Shibeshi MS
Received 8 October 2023
Accepted for publication 10 December 2023
Published 15 December 2023 Volume 2023:16 Pages 841—845
DOI https://doi.org/10.2147/IMCRJ.S443625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Shamil Nuri Tiremo,1,* Mulugeta Sitot Shibeshi2,*
1Division of Gastroenterology, Department of Internal Medicine, Hawassa University, Hawassa, Ethiopia; 2Department of Pediatrics and Child Health, Hawassa University, Hawassa, Ethiopia
*These authors contributed equally to this work
Correspondence: Mulugeta Sitot Shibeshi, Email [email protected]
Abstract: Hookworm infection is an important cause of iron deficiency anemia, especially in heavily infected high-risk populations residing in underdeveloped tropical countries. Stool examination is the main method used for hookworm diagnosis; however, its sensitivity is relatively low. Hookworm infections have been incidentally diagnosed during routine upper gastrointestinal endoscopies. We present the case of a 60-year-old Ethiopian farmer who had severe iron deficiency anemia and positive occult blood in the stool. Repeated stool examinations revealed no ova or parasites. Esophagogastroduodenoscopy revealed the presence of hookworms in the duodenum. The patient was treated with albendazole and ferrous sulfate. Careful endoscopic examination of the duodenum is crucial for identifying unsuspected hookworm infection responsible for chronic gastrointestinal bleeding and iron deficiency anemia.
Keywords: hookworm, iron-deficiency anemia, endoscopy
Introduction
Hookworm infection is one of the most prevalent infectious diseases in humans and a leading cause of anemia and undernutrition.1 Hookworm infection in humans is mainly caused by the helminth nematode parasites Necator americanus and Ancylostoma duodenale. The zoonotic hookworm Ancylostoma ceylanicum, which primarily infects dogs and cats, can also infect, mature, mate and produce eggs in humans.2 Earlier studies have described A. ceylanicum to be present as mostly light or non-patent, single-sex infections;3 however, it has recently been found to be highly prevalent in humans in Southeast Asia and the Pacific.4,5 Similarly, zoonotic hookworms Ancylostoma caninum and Ancylostoma braziliense continue to sporadically infect humans in different parts of the world.6
An estimated 740 million people in developing tropical and subtropical countries are infected with hookworm.7 Hookworm infection is more prevalent in poor communities, and several factors have been implicated, such as inadequate sanitation and deposition of human feces on soil, poor housing construction with dirt floors, and lack of access to essential medicines.1 In Ethiopia, an estimated 11 million people are infected with hookworm.8
Humans acquire hookworm infection when the infective larval stages living in the soil penetrate the skin (N. americanus, A. duodenale and A. ceylanicum) or when they are ingested (Ancylostoma species).9 In the body, adult hookworms parasitize the proximal small intestine and can reside for many years, causing chronic intestinal hemorrhage and iron deficiency anemia (IDA). The buccal capsules of N. americanus and A. duodenale contain cutting plates and sharp teeth, respectively, which allow the adult parasites to feed on intestinal mucosa, submucosa, and blood. The female hookworm is larger than the male one; and depending on the species and sex of the worm, the length of the adult worm ranges from 5 to 13 mm.7,10
The major morbidity of human hookworm infection is a direct result of intestinal blood loss (mostly from leakage around the attachment site of the worm and, to a lesser extent, from actively feeding adult worms).4 Each adult hookworm causes loss of an estimated 0.3 mL to 0.5mL of blood each day.11 Hence, there is a direct correlation between the number of adult hookworms in the gut and the volume of fecal blood loss. Occasionally, hookworms can cause overt intestinal bleeding.12,13 Depending on the dietary reserves of the host, heavy hookworm infections can also cause hypoproteinemia which can lead to anasarca and ascites.4
Stool microscopic examination is the main diagnostic method for hookworm infection, although the yield depends on the worm burden and the diagnostic technique.14 There have been reports of incidental diagnosis of hookworm infection during routine upper gastrointestinal endoscopy15–18 and capsule endoscopy.11,19 Here, we report the case of an elderly man who underwent endoscopic examination for unexplained severe iron-deficiency anemia, which revealed hookworm infection in the duodenum. The infection was successfully treated using albendazole.
Case Presentation
A 60-year-old Ethiopian man presented with a one-year history of fatigability, shortness of breath on mild exertion, marked loss of appetite, tinnitus, and dizziness. He also had a history of significant weight loss and intermittent non-bloody diarrhea that occurred at an interval of 2–3 months that lasted for approximately 1 week and improved without treatment. Over the preceding 3 months, his symptoms worsened, and he started to spend most of the days in bed. The patient had no history of cough, orthopnea, or swelling. He had no fever, night sweats, bone pain, or epistaxis. The patient denied abdominal pain, vomiting, dyspepsia, hematemesis, melena, or rectal bleeding. He is a farmer who performs his farming activities barefoot but cannot recollect having developed dermatitis of any kind during the past years.
He visited a nearby hospital 2 months prior to presentation to our hospital, and severe iron deficiency anemia was diagnosed (hemoglobin = 3.4g/dL, haematocrit = 10.1%, mean corpuscular volume = 69.4fl, peripheral morphology revealed hypochromic microcytic red blood cells and anisopoikilocytosis), although a definite etiology was not identified. A complete blood count did not reveal eosinophilia. The erythrocyte sedimentation rate was elevated (140 mm/hr). Stool examination using direct wet-mount microscopy revealed no ova or parasites. Renal and liver function test results were within normal limits. He was transfused with blood and given ferrous sulfate tablets for 2 months, which resulted in a significant improvement in the hemoglobin to 10.1 g/dL. The treatment also resulted in the improvement of most of his symptoms, although the loss of appetite and fatigue persisted. The patient was referred to our hospital for endoscopic evaluation under the impression of IDA secondary to chronic gastrointestinal blood loss.
At presentation to our hospital, the physical examination was unremarkable except for low body mass index (BMI = 16.98Kg/m2) and conjunctival pallor. The stool examination was positive for occult blood, but no ova or parasites were identified. Abdominal ultrasound examination was unremarkable. Lower gastrointestinal endoscopy (colonoscopy) was normal. Esophagogastroduodenoscopy revealed multiple small translucent, blood-filled hookworms in the first part of the duodenum (as shown in Figure 1). The patient was treated with oral albendazole 400 mg once, provided with oral iron supplementation, and returned to the referring hospital for follow-up.
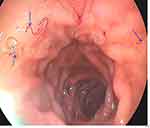 |
Figure 1 Endoscopic view of the first part of the duodenum revealing multiple translucent red worms (blue arrows). |
Ethical Review
The patient provided written informed consent for the publication of this case report after obtaining permission from the Institutional Review Board of Hawassa University College of Medicine and Health Sciences.
Discussion
We report an incidental finding of hookworm infection in an elderly Ethiopian farmer who underwent endoscopic examination to identify a gastrointestinal cause of iron-deficiency anemia. Hookworm infection is the most common soil-transmitted helminth (STH) and the most neglected tropical disease in sub-Saharan Africa.8 Studies from different regions of Ethiopia have revealed a variable prevalence of hookworm depending on the study design; a hookworm prevalence of 14.7% was reported in a health institution-based study,20 while a school-based study involving school children showed a prevalence of 20.6%.21 Although the hookworm prevalence in adults may not be affected, the prevalence of hookworm infection in children is expected to decrease following the commencement of the National Deworming Program of Ethiopia in 2015, as evidenced by a study conducted in northern Ethiopia.22
Most infected individuals are asymptomatic; however, individuals with heavy chronic infections (or moderate infections in patients with underlying iron and protein nutritional deficiencies) suffer from intestinal blood loss that results in iron deficiency anemia as well as protein malnutrition.9 The under nutrition (low BMI) and severe iron-deficiency anemia in our patient indicate that the patient has been chronically and heavily infected. The fact that the patient walked barefoot for almost his entire life and his farming activities might have predisposed him to repeated hookworm infections. Hookworm infections are common among individuals involved in agricultural activities.9
Different laboratory methods, including direct wet mount microscopy of stool, concentration, flotation, Kato-Katz, and molecular analysis, have been used to diagnose hookworm infections.4,14,23 However, due to its low cost and the ease of procedure, wet mount microscopy is the most commonly used method to diagnose intestinal parasites in almost all healthcare facilities in Ethiopia.14 Although a higher hookworm burden is expected to result in a higher fecal egg count, repeated stool examinations did not reveal hookworm ova in our patient. This may be explained by the poor sensitivity of the direct wet-mount microscopy method.24 The negative stool examination result could as well be explained by the possibility of A. ceylanicum infection in our patient due to the relatively lower egg-shedding intensity of people infected with this zoonosis than those infected with N. americanus.5 A. ceylanicum is known to be endemic in dogs and cats in African countries such as Madagascar, Sierra Leone, South Africa and Tanzania; however, there have not been reports of human infection with this zoonotic hookworm in Africa3,5 Moreover, a single-sex, non-patent infection may also be the reason for the negative stool examination result.3
Hookworm infection is associated with eosinophilia, which is usually most pronounced during the tissue invasion stage, and then slowly decreases over time.25 Our patient did not have eosinophilia, which can be explained by the down regulation of host responses observed in chronic hookworm infection.4
Hookworms have been incidentally detected during the endoscopic evaluation of some patients with gastrointestinal bleeding. In a one-year review of endoscopically diagnosed hookworm infections, Zein Ahmad et al17 found duodenitis in all study subjects, bleeding manifestations in three-fourths of them, and the need for endoscopic hemostasis in one-third of the participants.
In our case, endoscopic evaluation was performed to identify the cause of gastrointestinal bleeding, which revealed multiple blood-filled hookworms in the first part of the duodenum; however, no active bleeding was observed. Because we did not perform histological speciation of the worms, we did not identify the species of the hookworm in our patient. Nevertheless, speciation is not necessary for treatment purposes.
Currently, a single dose of either albendazole (400 mg) or mebendazole (500 mg) is the treatment of choice for hookworm infection.7 However, three consecutive daily doses of either drug were found to improve both cure and egg reduction rates.4 Although less effective than albendazole, pyrantel pamoate and levamisole are alternative drugs for hookworm treatment.4 In addition to antihelminthic treatment, iron supplementation and nutritional support for those with malnutrition, and health education on how to prevent re-infection should be provided for better long-term outcome.
The current WHO recommendations for hookworm prevention include periodic deworming to eliminate infecting worms, health education to prevent re-infection, and improved sanitation to reduce soil contamination with infective eggs. However, control programs for hookworm often do not consider the role of zoonotic reservoirs of infection. Control programs that focus solely on human mass drug administration in the absence of concurrent animal health programs may be a causal factor for the high prevalence of A. ceylanicum infection in some parts of the world.26 Thus, for sustainable control of hookworm infection, there is a need to integrate interventions targeting human, animal and environmental reservoirs through inter-sectoral coordination and collaboration.3,5,26
Conclusion
Hookworm infection should always be considered as a cause of IDA, particularly in farmers living in endemic areas with a history of walking barefoot, even if stool examination is negative for hookworm ova. In the evaluation of patients with IDA due to gastrointestinal bleeding, careful endoscopic examination of the small intestinal mucosa can help identify unsuspected parasitic infections, such as hookworms. Periodic deworming of at-risk adults should be incorporated as part of the preventive strategy for hookworm infection.
Acknowledgment
We thank everyone who helped with the diagnosis, treatment, and follow-up of the patient. The authors acknowledge the patient for providing consent for the publication of this case.
Author Contributions
Both authors contributed equally to the work reported, whether in the conception, study design, execution, data acquisition, analysis, and interpretation, or in all of these areas; participated in the drafting, revising, or critical review of the article; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and agreed to be responsible for all aspects of the work.
Disclosure
The authors have no conflicts of interest in this case report.
References
1. Hotez P. Hookworm and poverty. Ann N Y Acad Sci. 2008;1136(1):38–44. doi:10.1196/annals.1425.000
2. Betson M, Alonte AJI, Ancog RC, et al. Zoonotic Transmission of Intestinal Helminths in Southeast Asia: Implications for Control and Elimination.
3. Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43(12–13):1009–1015. doi:10.1016/j.ijpara.2013.07.006
4. Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Prim. 2016;2. doi:10.1038/nrdp.2016.88
5. Stracke K, Jex AR, Traub RJ. Zoonotic ancylostomiasis: an update of a continually neglected zoonosis. Am J Trop Med Hyg. 2020;103(1):64–68. doi:10.4269/ajtmh.20-0060
6. Clements ACA, Addis Alene K. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3(1):e72–e79. doi:10.1016/S2666-5247(21)00181-6
7. Hotez PJ, Brooker S, Bethony JM, Elena Bottazzi M, Alex Loukas SX. Hookworm infection. N Engl J Med. 2004;351(8):799–807. doi:10.1056/NEJMra032492
8. Hotez PJ, Kamath A. Neglected Tropical Diseases in Sub-Saharan Africa: review of Their Prevalence, Distribution, and Disease Burden. PLoS Neglected Trop Dis. 2009;3:2–11.
9. Brooker S, Bethony J, Hotez PJ. Human Hookworm Infection in the 21 st Century. Adv Parasitol. 2008;58:197–288.
10. Velikkakam T, Fiuza JA, Gaze ST. Overview of Hookworm Infection in Humans. Neglected Tropical Diseases-South Asia. 2017;121–135.
11. Seidelman J, Zuo R, Udayakumar K, et al. Caught on Capsule: iron-deficiency Anemia Due to Hookworm Infection. Am J Med. 2016;129(2):167–169. doi:10.1016/j.amjmed.2015.08.004
12. Tan X, Cheng M, Zhang J, et al. Hookworm infection caused acute intestinal bleeding diagnosed by capsule: a case report and literature review. Korean J Parasitol. 2017;55(4):417–420. doi:10.3347/kjp.2017.55.4.417
13. Wei KY, Yan Q, Tang B, et al. Hookworm infection: a neglected cause of overt obscure gastrointestinal bleeding. Korean J Parasitol. 2017;55(4):391–398. doi:10.3347/kjp.2017.55.4.391
14. Zeleke AJ, Addisu A, Derso A, et al. Evaluation of Hookworm Diagnosis Techniques from Patients in Debre Elias and Sanja Districts of the Amhara Region, Ethiopia. J Parasitology Res. 2021:548.
15. Wu KL, Chuah SK, Hsu CC, et al. Endoscopic diagnosis of hookworm disease of the duodenum: a case report. J Intern Med Taiwan. 2002;13:27–30.
16. Mahadeva S, Qua CS, Yusoff W, et al. Repeat endoscopy for recurrent iron deficiency anemia: an (un)expected finding from Southeast Asia. Dig Dis Sci. 2007;52(2):523–525. doi:10.1007/s10620-006-9387-7
17. Z A, Amanah A, Fariz Malvi Zamzam Zein A. Adult Patients with an Endoscopic Diagnosis of Hookworm Infection: one-year Experience in a Primary Referral Hospital in Indonesia. Trop Gastroenterol 135 Trop Gastroenterol. 2020;41:135–139.
18. Kuo YC, Chang CW, Chen CJ, et al. Endoscopic diagnosis of hookworm infection that caused anemia in an elderly person. Int J Gerontol. 2010;4(4):199–201. doi:10.1016/j.ijge.2010.11.008
19. Kalli T, Karamanolis G, Triantafyllou K. Hookworm Infection Detected by Capsule Endoscopy in a Young Man With Iron Deficiency. Clin Gastroenterol Hepatol. 2011;9(4):e33. doi:10.1016/j.cgh.2010.10.026
20. Hailu T, Mulu W, Abera B. Prevalence and determinant factors of hookworm infection among school age children in Jawe district. NorthWest Ethiopia. 2019;19:2439–2445.
21. Sitotaw B, Shiferaw W. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors among the First-Cycle Primary Schoolchildren in Sasiga District, Southwest Ethiopia. J Parasitol Res. 2020;2020:1–13. doi:10.1155/2020/8681247
22. Zvi B, Jemal A, Allison P, et al. Deworming school children in Ethiopia: the importance of a comprehensive approach. Open J Trop Med. 2019;3(1):001–006. doi:10.17352/ojtm.000008
23. Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge university press; 2005.
24. Yimer M, Hailu T, Mulu W, et al. Evaluation performance of diagnostic methods of intestinal parasitosis in school age children in Ethiopia. BMC Res Notes. 2015;8(1):1–5. doi:10.1186/s13104-015-1822-4
25. Connell EMO, Nutman TB, Section HI, et al. Eosinophilia in Infectious Diseases. Immunol Allergy Clin North Am. 2016;35:1–29.
26. Inpankaew T, Schär F, Dalsgaard A, et al. High Prevalence of Ancylostoma ceylanicum Hookworm Infections in Humans, Cambodia, 2012. Emerg Infect Dis. 2014;20(6):976–982. doi:10.3201/eid2006.131770
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
