Back to Journals » Journal of Pain Research » Volume 14
Endoscopic and Microscopic Interlaminar Discectomy for the Treatment of Far-Migrated Lumbar Disc Herniation: A Retrospective Study with a 24-Month Follow-Up
Authors Yang F , Ren L, Ye Q, Qi J , Xu K, Chen R, Fan X
Received 23 January 2021
Accepted for publication 19 May 2021
Published 4 June 2021 Volume 2021:14 Pages 1593—1600
DOI https://doi.org/10.2147/JPR.S302717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Erika Petersen
Fei Yang,1,* Liangjuan Ren,1,* Qingqing Ye,2,* Jianhua Qi,1 Kai Xu,1 Rigao Chen,1 Xiaohong Fan1
1Department of Spine Surgery, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 2Department of Spine Surgery, Yibin Hospital of Traditional Chinese Medicine, Yibin, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rigao Chen; Xiaohong Fan
Department of Spine Surgery, Hospital of Chengdu University of Traditional Chinese Medicine, 39 Shierqiao Road, Jinniu District, Chengdu, Sichuan, People’s Republic of China
Tel +86 13880450622
; +86 18981883960
Fax +86 28-87765483
Email [email protected]; [email protected]
Purpose: Percutaneous endoscopic lumbar discectomy for the treatment of far-migrated lumbar disc herniation (LDH) is clinically challenging. The aim of this study was to compare the efficacy and safety of interlaminar endoscopic lumbar discectomy (IELD) and interlaminar microscopic lumbar discectomy (IMLD) for the treatment of far-migrated LDH.
Materials and Methods: We retrospectively analyzed 50 consecutive cases of far-migrated LDH treated by IELD or IMLD. Clinical data and outcomes were assessed before the operation and 1 day and 3, 12, and 24 months after the surgery using the visual analog scale (VAS) and Oswestry disability index (ODI). Modified MacNab criteria were used to evaluate patient satisfaction at the 24-month follow-up.
Results: A significant reduction in leg pain and improvement in ODI (P< 0.01) were observed in both groups after surgery. Lower back pain (LBP) was reduced at 24 months postsurgery in the IELD group (P< 0.05) but not in the IMLD group (P> 0.05). There were significant intergroup differences in VAS LBP score at 1 day and 24 months postsurgery (p=0.01 and 0.02, respectively) and in ODI at 24 months (p=0.03). The rate of excellent or good outcome was 90.32% with IELD and 78.95% with IMLD (p=0.55). Hospital stay and time to ambulation were shorter in the IELD group than in the IMLD group, but the former had a longer operative time (p< 0.01). Low and comparable complication rates were reported in the IELD (16.13%) and IMLD (10.53%) groups (p=0.70).
Conclusion: Both IELD and IMLD achieve favorable clinical results in the treatment of far-migrated LDH, with only minor complications. Compared to IMLD, LBP was significantly reduced with IELD presumably because it involved less trauma.
Keywords: minimally invasive spinal surgery, highly migrated lumbar disc herniation, interlaminar approach, downward migration, upward migration
Introduction
Lumbar disc herniation (LDH) is one of the most common disorders of the lumbar spine,1 with disc fragment migration occurring in 35%–72% of cases.2,3 Far-migrated disc herniation (DH) is accompanied by back pain and severe sciatica and can lead to sensory changes and motor weakness.4 Such cases often respond poorly to conservative treatment and require surgical intervention.5
Interlaminar microscopic lumbar discectomy (IMLD), which is considered as the gold standard procedure for the treatment of LDH, involves removal of part of the lamina, ligamentum flavum, and medial facet joints.6–9 When there is herniation of a migrated disc, additional laminae or medial facet joints must be removed.10 In 10%–20% of cases, iatrogenic lumbar instability may occur after open and microscopic fenestrated discectomy, leading to failed back surgery syndrome or reoperation.11–13 With improvements in instrumentation and advances in minimally invasive surgical techniques, percutaneous endoscopic lumbar discectomy (PELD) has become a popular approach that has several advantages over conventional discectomy including less paravertebral muscle injury, lower risk of iatrogenic instability, and rapid recovery.14–17 Meanwhile, percutaneous endoscopic transforaminal lumbar discectomy (PETLD) and percutaneous endoscopic interlaminar lumbar discectomy are alternatives to microscopic discectomy for the treatment of various types of LDH, including severe and extremely difficult cases.18 However, because of anatomic barriers and disc fragmentation, the treatment of far-migrated DH remains clinically challenging.19–21 Although previous studies have demonstrated the feasibility of PETLD for the treatment of far-migrated DH, the failure rate is very high (5%–22%).4,21,22
Interlaminar endoscopic lumbar discectomy (IELD) is similar to conventional open discectomy and can expand the scope of endoscopic approaches through limited removal of the lamina. The objective of this study was to evaluate the efficacy and safety of IELD compared to IMLD for the treatment of far-migrated LDH.
Materials and Methods
Patient Population
This retrospective study included 53 consecutive patients with symptomatic far-migrated LDH who underwent IELD or IMLD from December 2016 to November 2018. Patients’ data were retrieved from an electronic medical records system for analysis. A single surgeon with experience in minimally invasive spine surgery performed IELD (n=33) and IMLD (n=20). Ease of performance or feasibility did not play a role in the selection of the surgical approach. All participants provided written, informed consent to participate in the study. The study protocol was approved by the Ethics Committee of the Hospital of Chengdu University of Traditional Chinese Medicine and was in accordance with the Declaration of Helsinki.
The inclusion criteria were as follows: (1) patients with typical radiculopathy, radiating pain in the lower limbs, sensory changes, motor weakness, and abnormal pain radiation; (2) magnetic resonance imaging (MRI) and computed tomography (CT) findings corresponding to symptoms and signs; (3) failure of conservative treatment for at least 4 weeks; and (4) preoperative MRI showing far-upward or -downward migrated DH,10,21,23 defined as extending beyond 3 mm below the inferior margin of the upper pedicle and beyond the center of the lower pedicle, respectively (Figure 1).21,24 Exclusion criteria were as follows: (1) central stenosis confirmed by MRI and CT; (2) extreme lateral DH; (3) previous surgical treatment at the same disc level; (4) confirmed flexion/extension segmental instability; and (5) other diseases or injuries affecting the spine such as infections, tumors, or fractures.
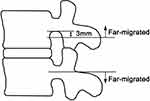 |
Figure 1 Definition of far-migrated LDH used in this study.Note: Data adapted from Lee et al21 and Ahn et al.24 |
Surgical Methods
IMLD
After general anesthesia, the patient lay prone on the C-arm fluororadiolucent table in a flexed hip position. Taking the lesion segment as the center, a standard median incision about 3 cm in length was made to cut through the lumbodorsal fascia while avoiding damage to the supraspinous and interspinous ligaments. Medial subperiosteal dissection of paravertebral muscles was performed along the spinous process to expose the interlaminar space and facet process. The laminar retractor was placed on the outer edge of the facet process and the microscope was placed in an appropriate position. According to the location of the migrated disc, part of the lamina, medial facet joints, and ligamentum flavum were resected using a Kerrison rongeur to enter the epidural space. The nerve root, dural sac, and migrated disc were identified and the disc was completely removed. The annulus fibrosus perforation was located and loose fragments were removed using discectomy forceps. Once decompression was completed and hemostasis was confirmed, the fascia and skin were sutured layer by layer without drainage.
IELD
IELD was performed with patients under general anesthesia and laying in the prone position over a Wilson frame with appropriate flexion to widen the interlaminar window. All surgical segments were selected for the interlaminar window at the same level as the herniated disc, and the skin entry point was confirmed using anteroposterior radiographs. After making an incision about 8 mm long beside the spinous process, a series of dilators was advanced to the surface of the ligamentum flavum and the operative sheath was introduced along the dilators. The endoscope (Joimax, Karlsruhe, Germany; model FX6342208O, 30°, 6.3 mm) was then inserted and the operation was performed under continuous saline irrigation. According to the position of the migrated disc, the cranial or caudal portion of the lamina was resected with a drill and rongeur, with removal of the medial facet joint in some cases. After exposing the interlaminar window, part of the ligamentum flavum was removed using scissors to enter the epidural space. By adjusting the angle of the endoscope, the dural sac, nerve root, and distal margin of the migrated disc were fully exposed on the monitor. After removing the migrated disc, loose fragments inside the annulus were removed through the annular defect. Once decompression was achieved, hemostasis was confirmed, and the skin was sutured without drainage.
Postoperative Care
Both groups of patients received intravenous injection of nonsteroidal anti-inflammatory analgesics postoperatively and were asked to perform straight leg raises while in the bed. All patients were given a lumbar brace to get out of the bed after the operation, which they were required to wear for 8 weeks.
Clinical Evaluation and Follow-Up
We reviewed pre- and postoperative clinical data for each patient including age, sex, lesion location, direction of disc migration, operative time, and length of hospital stay. The visual analog scale (VAS)25 and Oswestry disability index (ODI)26 were recorded before and 1 day and 3, 12, and 24 months after the operation to evaluate clinical efficacy. The modified MacNab criteria27 were used to assess patients’ satisfaction at the last follow-up. All patients underwent MRI on the second day after surgery to determine whether the migrated disc was completely removed.
Statistical Analysis
All statistical analyses were performed with SPSS v20.0 software (SPSS Inc, Chicago, IL, USA). Distributions of measurement data were assessed with the Kolmogorov–Smirnov test or Shapiro–Wilk test to assess the normality assumption, and intergroup differences were evaluated with the t test or Mann–Whitney U-test. Data are expressed as mean±SD or median (interquartile range). Continuous measurement data were analyzed by 2-way repeated measures analysis of variance. Count data were analyzed with the chi-squared test or Fisher’s exact test, and Ridit analysis was used for ranked data. The test level was α=0.05, and p<0.05 was the threshold for statistical significance.
Results
Characteristics of the Study Population
As 3 patients were lost to follow-up, the analysis included 31 patients treated by IELD and 19 treated by IMLD. There were no significant differences in baseline demographic and clinical characteristics including age, sex, body mass index, lesion location, or direction of disc migration between the 2 groups (Table 1).
 |
Table 1 Baseline and Clinical Characteristics of the Study Population |
Clinical Outcomes with IELD and IMLD
Perioperative data are shown in Table 2. The mean VAS score (±SD) for lower back pain (LBP) improved from 3.87±1.23 to 2.71±1.49 in the IELD group (p=0.03) and from 4.11±1.20 to 3.63±1.34 in the IMLD group (p>0.05; Figure 2A). Compared to before the operation, there was no difference in VAS LBP score 1 day after surgery in the IELD group, but significant differences were observed at 3 months (p<0.001), 12 months (p=0.002), and 24 months (p=0.03) post surgery. In the IMLD group, a significant difference in VAS LBP score was observed at 3 months after surgery (p=0.003) but not at 1 day, 12 months, or 24 months (all p>0.05). There were no significant differences in LBP scores between the 2 groups at 3 and 12 months after surgery (p=0.68 and 0.25, respectively); however, intergroup differences in LBP score were significant at 1 day and 24 months post surgery (p=0.01 and 0.03, respectively).
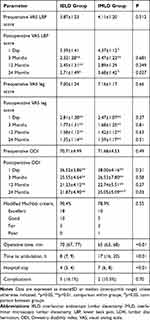 |
Table 2 Clinical Outcomes of IELD and IMLD for Far-Migrated LDH |
The mean VAS leg pain score improved from 7.00±1.24 to 1.35±1.14 in the IELD group (p<0.001) and from 7.16±1.17 to 1.59±1.17 in the IMLD group (p<0.001) (Figure 2B). Intergroup differences in leg VAS score over the 24-month follow-up were nonsignificant (p<0.05). Mean ODI improved from 70.71±4.99 to 21.87±4.90 in the IELD group (p<0.001) and from 71.68±4.53 to 25.05±5.09 in the IMLD group (p<0.001) (Figure 2C). Intergroup differences in mean ODI were significant at 24 months after surgery (p=0.04). According to the modified MacNab criteria, a surgical satisfaction was excellent or good for 90.4% of patients in the IELD group and 78.9%of those in the IMLD group at the 24-month review (p=0.55). In the IELD group, 18 patients were excellent, 10 patients were good, and 3 patients were fair; and in the IMLD group, 10 patients were excellent, 5 patients were good, 3 patients were fair, and 1 patient was poor. At the 24-month follow-up, there was recurrence in 2 cases (6%, 1 case per group).
Time to ambulation and length of hospital stay were shorter in the IELD group than in the IMLD group. However, the mean operative time was shorter for IMLD than for IELD (65 vs 70 min, p<0.01). Although the incidence of complications was higher with IELD than with IMLD (16.13% vs 10.53%; p=0.695), the difference was nonsignificant and no major complications such as nerve injury and infection occurred. In the IMLD group, there was 1 case of a dural tear that was repaired during surgery, with no cerebrospinal fluid leakage or infection occurring after surgery. Five cases in the IELD group and 1 case in the IMLD group had postoperative paresthesia, but the symptom disappeared within 4 weeks after treatment with oral mecobalamin.
Case Illustration
A 49-year-old female patient presented with LBP and severe radiating pain in the right leg that had lasted for 1 month. Hypoesthesia was reported on the right L5 dermatome. Right great-toe dorsiflexion was grade III in a manual muscle test; right straight leg raise was positive at 30°; VAS LBP score was 3; VAS leg score was 8; and ODI was 76%. The pain did not improve with conservative treatments such as acupuncture and medicines. The MRI showed far down-migrated disc material from L4/L5 to the lower edge of L5 (Figure 3A and B). By limited resection of the lamina and ligamentum flavum, a working channel was created under the pedicle of L5 (Figure 3C and D). Decompression was achieved after removing the migrated disc and loose fragments inside of the annulus (Figure 3E and F). The postoperative MRI showed that the migrated disc fragments were completely removed (Figure 3G and H). Although the patient’s great-toe dorsiflexor strength was grade IV at the 2-year follow-up, there was clinical improvement overall (VAS LBP score=2, VAS leg score=1, and ODI=16%). The case illustration has obtained the patient’s informed consent, and her case details and accompanying pictures can be published.
Discussion
Although far-migrated LDH was previously considered difficult to treat by PELD,19,21,28 in the present 24-month retrospective study, we found no difference in efficacy between IELD and IMLD for the treatment of sciatica. However, the intensity of LBP was lower while the rate of functional recovery was higher with IELD. Although the incidence of complications was higher with the latter, no major complications occurred.
Modified PELD techniques have been used to treat far-migrated LDH, with good clinical results;20,22,29–33 However, these methods have various shortcomings and limitations. Several studies have reported good clinical results in more than 90% of patients with far-migrated DH treated by PETLD, but it is difficult to directly view the distal free nucleus pulposus due to obstruction of the facet joint and pedicle, especially as the far-migrated disc is often separated into many fragments.4,20,22,34 In a study of 53 patients who underwent foraminoplasty for far down-migrated disc, the remnant disc material rate was 13% and the same proportion of patients complained of transient postoperative dysesthesia.22 In order to ensure complete decompression, the herniated disc must be removed under visual control. Therefore, some surgeons use a transpedicular or translaminar approach by creating a hole in the pedicle or lamina to treat far-migrated LDH.29,31,33 It should be noted that the target location, limited possibility of endoscopic adjustment, and risks of dural tears and incomplete removal of the herniated disc may limit the application of these methods.29,31,33 Two-level PELD techniques such as the transforaminal approach alone or in combination with an interlaminar approach reduce the incidence of postoperative nucleus pulposus residue,30,32 but these procedures are cumbersome, have a long operative time, and cannot be used for L5–S1 far-migrated LDH.29,32 The results of our study show that sciatica in both the IELD and IMLD groups significantly improved and remained satisfactory at the 24-month follow-up, indicating that adequate decompression was achieved, which is in accordance with other published reports.15,16,35–37 An interlaminar approach was used in the IELD group as the anatomic structure was easier to identify under endoscopy; this is in line with the operating habits of most spine surgeons. With the aid of 30° optics and endoscopic tilt, the surgeon can directly view far-migrated fragments in the spinal canal with limited removal of lamina or facet joints.23 In this study, the far-migrated discs were removed under direct visualization in the IELD group, which was critical for ensuring complete decompression.
IELD combines the advantages of conventional fenestration discectomy and an endoscopic technique that preserves the paraspinal muscles and causes less damage to bone structure, which is important for the stability of the motion segment. At the last follow-up, the VAS LBP score and ODI were less in the IELD group than in the IMLD group. Although both groups showed significant improvement in sciatica, the difference between pre- and postoperative VAS LBP score was greater with IMLD than with IELD. This may be attributable to the fact that there was less tissue trauma with the former approach, which was reported in several studies.16,35,37 As with conventional fenestration discectomy, IMLD often requires dissection of more paravertebral muscles, ligaments, laminae, and facets for far-migrated LDH, which could aggravate segmental instability and cause LBP.38,39 A study of 111 patients who underwent single-level discectomy for radiculopathy found that 23% had moderate back pain and 9% had severe back pain that required subsequent fusion surgery at the site of the primary discectomy.40 Another study conducted over a 10-year period found that clinical outcomes tended to deteriorate over time after standard open discectomy for LDH; additionally, increased LBP worsened clinical outcomes and was associated with radiologic degeneration.41 A recent study found that 22.2% of patients with highly migrated intracanal DH treated by conventional microdiscectomy had severe LBP, and surgical satisfaction ratings of excellent and good declined over time.10 This may be related to the fact that the ODI was higher with IELD at the last follow-up.
Postoperative dysesthesia is one of the most common complications after PELD, with an incidence ranging from 0% to 17.88%.42–48 The rate in our study was within this range, and was higher with IELD (5 cases,16.12%) than with IMLD (1 case, 5.26%). Postoperative paresthesia may be related to irritation of the spinal ganglion or nerve root during surgery.37,49,50 Additionally, repeated hemostasis by bipolar coagulation and compression of the working channel may contribute to postoperative dysesthesia. In our study population, postoperative dysesthesia disappeared within 4 weeks with conservative treatment and did not affect the daily life of patients.
There were several limitations to the current study. Firstly, this was a single-center retrospective analysis of a small sample size, as far-migrated LDH is relatively rare. Secondly, because the lamina and isthmus of the upper lumbar spine are narrow, IMLD or IELD is difficult to perform for migrated upper LDH; in the past, we have used PETLD to treat this condition. Thirdly, we did not perform a correlation analysis between postoperative flexion/extension and LBP. A comprehensive assessment of the efficacy of IELD and IMLD will require prospective, randomized studies.
Conclusion
Both endoscopic and microscopic interlaminar discectomy have achieved favorable clinical results in the treatment of far-migrated DH, with only minor complications. Compared to IMLD, IELD—which has the advantages of less trauma and reduced LBP—showed better clinical outcomes at the 2-year follow-up.
Abbreviations
CT, computed tomography; DH, disc herniation; IELD, interlaminar endoscopic lumbar discectomy; IMLD, interlaminar microscopic lumbar discectomy; LBP, lower back pain; LDH, lumbar disc herniation; MRI, magnetic resonance imaging; ODI, Oswestry disability index; PELD, percutaneous endoscopic lumbar discectomy; PETLD, percutaneous endoscopic transforaminal lumbar discectomy; VAS, visual analog scale.
Acknowledgments
We thank the hospital staff for guidance, assistance, support, and collaboration.
Disclosure
The authors report no conflicts of interest for this work nor concerning the materials or methods related to the findings described in this paper.
References
1. Anderson G. Epidemiology of spinal disorders. In: Frymoyer JW, Ducker TB, Hadler NM, editors. The Adult Spine: Principles and Practice. New York: Raven Press; 1997:93–141.
2. Ebeling U, Reulen HJ. Are there typical localisations of lumbar disc herniations? A prospective study. Acta Neurochir. 1992;117(3–4):143–148. doi:10.1007/BF01400611
3. Fries JW, Abodeely DA, Vijungco JG, Yeager VL, Gaffey WR. Computed tomography of herniated and extruded nucleus pulposus. J Comput Assist Tomogr. 1982;6(5):874–887. doi:10.1097/00004728-198210000-00003
4. Ahn Y, Jang IT, Kim WK. Transforaminal percutaneous endoscopic lumbar discectomy for very high-grade migrated disc herniation. Clin Neurol Neurosurg. 2016;147:11–17. doi:10.1016/j.clineuro.2016.05.016
5. Jordan J, Konstantinou K, O’Dowd J. Herniated lumbar disc. BMJ Clin Evid. 2011;2011.
6. Dowling TJ, Dowling TJ Microdiscectomy. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555984/.
7. Goffin J. Microdiscectomy for lumbar disc herniation. Clin Neurol Neurosurg. 1994;96(2):130–134. doi:10.1016/0303-8467(94)90046-9
8. Garg B, Nagraja UB, Jayaswal A. Microendoscopic versus open discectomy for lumbar disc herniation: a prospective randomised study. J Orthop Surg. 2011;19(1):30–34. doi:10.1177/230949901101900107
9. Hamawandi SA, Sulaiman II, Al-Humairi AK. Open fenestration discectomy versus microscopic fenestration discectomy for lumbar disc herniation: a randomized controlled trial. BMC Musculoskelet Disord. 2020;21(1):384. doi:10.1186/s12891-020-03396-x
10. Minimal Incision HM. Multifidus-sparing microendoscopic diskectomy versus conventional microdiskectomy for highly migrated intracanal lumbar disk herniations. J Am Acad Orthop Surg. 2016;24(11):805–813. doi:10.5435/JAAOS-D-15-00588
11. Mascarenhas AA, Thomas I, Sharma G, Cherian JJ. Clinical and radiological instability following standard fenestration discectomy. Indian J Orthop. 2009;43(4):347–351. doi:10.4103/0019-5413.55465
12. Kotilainen E, Valtonen S. Clinical instability of the lumbar spine after microdiscectomy. Acta Neurochir. 1993;125(1–4):120–126. doi:10.1007/BF01401838
13. Ebeling U, Reichenberg W, Reulen HJ. Results of microsurgical lumbar discectomy. Review on 485 patients. Acta Neurochir. 1986;81(1–2):45–52. doi:10.1007/BF01456264
14. Lee SH, Chung SE, Ahn Y, Kim TH, Park JY, Shin SW. Comparative radiologic evaluation of percutaneous endoscopic lumbar discectomy and open microdiscectomy: a matched cohort analysis. Mt Sinai J Med. 2006;73(5):795–801.
15. Li Q, Zhou Y. Comparison of conventional fenestration discectomy with Transforaminal endoscopic lumbar discectomy for treating lumbar disc herniation: minimum 2-year long-term follow-up in 1100 patients. BMC Musculoskelet Disord. 2020;21(1):628. doi:10.1186/s12891-020-03652-0
16. Choi KC, Kim JS, Park CK. Percutaneous endoscopic lumbar discectomy as an alternative to open lumbar microdiscectomy for large lumbar disc herniation. Pain Physician. 2016;19(2):E291–E300. doi:10.36076/ppj/2016.19.E291
17. Akcakaya MO, Yorukoglu AG, Aydoseli A, et al. Serum creatine phosphokinase levels as an indicator of muscle injury following lumbar disc surgery: comparison of fully endoscopic discectomy and microdiscectomy. Clin Neurol Neurosurg. 2016;145:74–78. doi:10.1016/j.clineuro.2016.04.004
18. Kim HS, Paudel B, Jang JS, Lee K, Oh SH, Jang IT. Percutaneous endoscopic lumbar discectomy for all types of lumbar disc herniations (LDH) including severely difficult and extremely difficult LDH cases. Pain Physician. 2018;21(4):E401–E408.
19. Lee SH, Kang BU, Ahn Y, et al. Operative failure of percutaneous endoscopic lumbar discectomy: a radiologic analysis of 55 cases. Spine. 2006;31(10):E285–E290. doi:10.1097/01
20. Choi G, Lee SH, Lokhande P, et al. Percutaneous endoscopic approach for highly migrated intracanal disc herniations by foraminoplastic technique using rigid working channel endoscope. Spine. 2008;33(15):E508–E515. doi:10.1097/BRS.0b013e31817bfa1a
21. Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J. 2007;16(3):431–437. doi:10.1007/s00586-006-0219-4
22. Kim HS, Ju CI, Kim SW, Kim JG. Endoscopic transforaminal suprapedicular approach in high grade inferior migrated lumbar disc herniation. J Korean Neurosurg Soc. 2009;45(2):67–73. doi:10.3340/jkns.2009.45.2.67
23. Kim CH, Chung CK, Woo JW. Surgical outcome of percutaneous endoscopic interlaminar lumbar discectomy for highly migrated disk herniation. Clin Spine Surg. 2016;29(5):E259–E266. doi:10.1097/BSD.0b013e31827649ea
24. Ahn Y, Jeong TS, Lim T, Jeon JY. Grading system for migrated lumbar disc herniation on sagittal magnetic resonance imaging: an agreement study. Neuroradiology. 2018;60(1):101–107. doi:10.1007/s00234-017-1943-7
25. Chapman CR, Casey KL, Dubner R. Pain measurement: an overview. Pain. 1985;22(1):1–31. doi:10.1016/0304-3959(85)90145-9
26. Lue YJ, Hsieh CL, Huang MH, Lin GT, Lu YM. Development of a Chinese version of the Oswestry Disability Index version 2.1. Spine. 2008;33(21):2354–2360. doi:10.1097/BRS.0b013e31818018d8
27. Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53(5):891–903. doi:10.2106/00004623-197153050-00004
28. Schaffer JL, Kambin P. Percutaneous posterolateral lumbar discectomy and decompression with a 6.9-millimeter cannula. Analysis of operative failures and complications. J Bone Joint Surg Am. 1991;73(6):822–831. doi:10.2106/00004623-199173060-00005
29. Xin Z, Liao W, Ao J, et al. A modified translaminar osseous channel-assisted percutaneous endoscopic lumbar discectomy for highly migrated and sequestrated disc herniations of the upper lumbar: clinical outcomes, surgical indications, and technical considerations. Biomed Res Int. 2017;2017:3069575. doi:10.1155/2017/3069575
30. Wu X, Fan G, He S, Gu X, Yang Y. Comparison of clinical outcomes of two-level PELD and foraminoplasty PELD for highly migrated disc herniations: a comparative study. Biomed Res Int. 2019;2019:9681424. doi:10.1155/2019/9681424
31. Du J, Tang X, Jing X, Li N, Wang Y, Zhang X. Outcomes of percutaneous endoscopic lumbar discectomy via a translaminar approach, especially for soft, highly down-migrated lumbar disc herniation. Int Orthop. 2016;40(6):1247–1252. doi:10.1007/s00264-016-3177-4
32. Zhao Y, Fan Y, Yang L, et al. Percutaneous endoscopic lumbar discectomy (PELD) via a transforaminal and interlaminar combined approach for very highly migrated lumbar disc herniation (LDH) between L4/5 and L5/S1 level. Med Sci Monit. 2020;26:e922777. doi:10.12659/MSM.922777
33. Krzok G, Telfeian AE, Wagner R, Iprenburg M. Transpedicular lumbar endoscopic surgery for highly migrated disk extrusions: preliminary series and surgical technique. World Neurosurg. 2016;95:299–303. doi:10.1016/j.wneu.2016.08.018
34. Kim HS, Yudoyono F, Paudel B, et al. Suprapedicular circumferential opening technique of percutaneous endoscopic transforaminal lumbar discectomy for high grade inferiorly migrated lumbar disc herniation. Biomed Res Int. 2018;2018:5349680. doi:10.1155/2018/5349680
35. Jarebi M, Awaf A, Lefranc M, Peltier J. A matched comparison of outcomes between percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for the treatment of lumbar disc herniation: a 2-year retrospective cohort study. Spine J. 2021;21(1):114–121. doi:10.1016/j.spinee.2020.07.005
36. Liu X, Yuan S, Tian Y, et al. Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: minimum 2-year follow-up results. J Neurosurg Spine. 2018;28(3):317–325. doi:10.3171/2017.6
37. Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine. 2008;33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
38. Hu ZJ, Fang XQ, Zhou ZJ, Wang JY, Zhao FD, Fan SW. Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J Bone Joint Surg Am. 2013;95(24):e191–e192. doi:10.2106/JBJS.L.01607
39. Hanley EJ, Shapiro DE. The development of low-back pain after excision of a lumbar disc. J Bone Joint Surg Am. 1989;71(5):719–721. doi:10.2106/00004623-198971050-00013
40. Parker SL, Xu R, McGirt MJ, Witham TF, Long DM, Bydon A. Long-term back pain after a single-level discectomy for radiculopathy: incidence and health care cost analysis. J Neurosurg Spine. 2010;12(2):178–182. doi:10.3171/2009.9
41. Son IN, Kim YH, Ha KY. Long-term clinical outcomes and radiological findings and their correlation with each other after standard open discectomy for lumbar disc herniation. J Neurosurg Spine. 2015;22(2):179–184. doi:10.3171/2014.10
42. Xie TH, Zeng JC, Li ZH, et al. Complications of lumbar disc herniation following full-endoscopic interlaminar lumbar discectomy: a large, single-center, retrospective study. Pain Physician. 2017;20(3):E379–E387.
43. Tonosu J, Oshima Y, Shiboi R, et al. Consideration of proper operative route for interlaminar approach for percutaneous endoscopic lumbar discectomy. J Spine Surg. 2016;2(4):281–288. doi:10.21037/jss.2016.11.05
44. Chen HT, Tsai CH, Chao SC, et al. Endoscopic discectomy of L5–S1 disc herniation via an interlaminar approach: prospective controlled study under local and general anesthesia. Surg Neurol Int. 2011;2:93. doi:10.4103/2152-7806.82570
45. Passacantilli E, Lenzi J, Caporlingua F, et al. Endoscopic interlaminar approach for intracanal L5–S1 disc herniation: classification of disc prolapse in relation to learning curve and surgical outcome. Asian J Endosc Surg. 2015;8(4):445–453. doi:10.1111/ases.12214
46. Choi G, Lee SH, Raiturker PP, Lee S, Chae YS. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5–S1 using a rigid working channel endoscope. Neurosurgery. 2006;58(1Suppl):S59–S68. doi:10.1227/01.neu.0000192713.95921.4a
47. Ahn Y, Lee SH, Park WM, Lee HY, Shin SW, Kang HY. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine. 2004;29(16):E326–E332. doi:10.1097/01.brs.0000134591.32462.98
48. Li ZZ, Hou SX, Shang WL, Song KR, Zhao HL. The strategy and early clinical outcome of full-endoscopic L5/S1 discectomy through interlaminar approach. Clin Neurol Neurosurg. 2015;133:40–45. doi:10.1016/j.clineuro.2015.03.003
49. Cho JY, Lee SH, Lee HY. Prevention of development of postoperative dysesthesia in transforaminal percutaneous endoscopic lumbar discectomy for intracanalicular lumbar disc herniation: floating retraction technique. Minim Invasive Neurosurg. 2011;54(5–6):214–218. doi:10.1055/s-0031-1287774
50. Silav G, Arslan M, Comert A, et al. Relationship of dorsal root ganglion to intervertebral foramen in lumbar region: an anatomical study and review of literature. J Neurosurg Sci. 2016;60(3):339–344.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.