Back to Archived Journals » Journal of Vascular Diagnostics and Interventions » Volume 8
Endomyocardial Fibrosis: Diagnosis and Management
Authors Khalil SI
Received 9 August 2019
Accepted for publication 18 January 2020
Published 12 February 2020 Volume 2020:8 Pages 1—9
DOI https://doi.org/10.2147/JVD.S196348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deipolyi
Siddiq Ibrahim Khalil
University of Medical Sciences and Technology, Khartoum, Sudan; The Heart Clinic, Khartoum 11111, Sudan
Correspondence: Siddiq Ibrahim Khalil 21 Courts Drive, Little Rock, AR 72223, USA
Email [email protected]
Introduction: Endomyocardial fibrosis (EMF) is a form of restrictive cardiomyopathy of unknown etiology, characterized by endocardial fibrosis of the apices and inflow tracts of the right ventricle, left ventricle or both. The majority of people who suffer high morbidity and mortality are children and young females of the poor and deprived communities of tropical Africa. EMF has been reported from other subtropical countries; Egypt, Nigeria, Brazil, Kerala in India, and Sudan. It is exceedingly rare in Europe and North America; however few cases have been reported from China and Japan. In Uganda, it accounted for 25% of cases who reported for echocardiography and 20% in a random population sample in Mozambique. Although ethnicity, diet, poverty, eosinophilia, infection, and malaria have been shown to be associated with EMF, the etiology of the disease still remains undetermined.
Diagnosis: Echocardiography is now the gold standard tool for the diagnosis of EMF. There are five prime diagnostic echocardiographic features of EMF; apical fibrosis, ventricular wall fibrosis, huge atrium, atrioventricular valve regurgitation and obliteration of ventricular cavity. The presence of pericardial effusion, endocardium fibrous shelf, and layering of the posterior wall lend further diagnostic support.
Treatment: Most patients are seen at a late stage of the disease with heart failure, consequently, medical care is directed accordingly and includes diuretics, angiotensin enzyme inhibitors, and beta-blockers. Surgical care includes pericardectomy, a relatively safe procedure that leads to some improvement in heart failure symptoms. However, endocardial decortication seems to be more definitive and beneficial for many patients with advanced disease. Successful surgery has a clear benefit in terms of symptoms and seems to affect survival favorably. The prognosis is poor and the duration of illness from the time of presentation to death was less than one year in 43.5% of patients, three years in 39.1% of patients, and three to twelve years in 17.4% of patients.
Conclusion: EMF is a mysterious disease whose etiology is still unraveling. The afflicted individuals are mostly young females and children who succumb to high rates of morbidity, and mortality from heart failure. Medical and surgical treatment are both practiced with results varying from temporary relief of symptoms to surgery with high mortality rates.
Keywords: endomyocardial fibrosis, apical fibrosis, ventricular outflow tract fibrosis, ascites, pericardiectomy, endocardial decortication
Introduction
Endomyocardial fibrosis (EMF), also referred to as Loffler and Davies disease, is a form of restrictive cardiomyopathy of unknown etiology characterized by endocardial fibrosis of the apices and inflow tracts of the right ventricle, left ventricle, or both, consequently leading to ventricular obliteration and atrioventricular valves regurgitation. EMF is a neglected, underdiagnosed disease and frequently mislabeled as rheumatic valvular disease or hypertrophic cardiomyopathy. Regional occurrence, unclear etiology, and lack of standard diagnostic criteria have contributed to the misperception of this condition.
EMF is known to afflict the poor and deprived communities of tropical Africa, especially those regions least equipped with diagnostic aides such as echocardiography and where medical and cardiovascular surgery is rare or non-existant. The majority of people who suffer high morbidity and mortality are children and young females of the same family leading to the disruption of families and communities frame. Dietary, environmental, and infectious factors seem to combine in susceptible individuals to give rise to an inflammatory process that leads to endomyocardial damage and scar formation.1 This article was aimed at shedding light on the pathophysiology, etiology, diagnosis, and treatment of EMF with the intention of improving doctors’ awareness, especially those working in the tropics, to the recognition of EMF and its management.
Pathophysiology
EMF is characterized by the replacement of the endocardium and myocardium by fibrous tissue, leading to a restrictive functional state. Olsen recognized three phases of EMF. The first phase duration may reach five months and is characterized by eosinophilic infiltration of the myocardium with necrosis of the subendocardium; a picture consistent with acute myocarditis. Myocardial involvement was considered a manifestation of an acute necrotic stage of the eosinophilic endomyocardial disease.2 The second stage, which starts after ten months, is the thrombotic stage, which is associated with thrombus formation and continues for several years until the fibrotic phase is reached. During the fibrotic phase, the endocardium is replaced by collagenous fibrous tissue which is the main feature of EMF. Fibrosis involves the apex, ventricular walls, papillary muscles especially the posterior papillary muscle and chordae tendinae, and spreads to engulf the posterior mitral valve leaflet in left ventricular EMF. The extent of fibrosis and its details were described by Davies who added that the posterior mitral cusp was wholly immobilized by adherence to the endocardium of the posterior wall of the ventricle. The result was a fibrous surface running straight down from the ventricle to the atrium where the cusp had become embedded and formed an endocardial fibrous shelf.3 The anterior mitral valve leaflet, though affected by the endocardial thickening, remains mobile making the whole valve a single leaflet valve.
The combination of environmental, infectious and other unknown factors in susceptible individuals leads to endomyocardial damage and scar formation.1 This progression from inflammation to the fibrotic stage takes different pathological shapes and indeed passes through acute myocarditis, necrotic and thrombotic phases ending eventually in the fibrotic phase. It is this fibrotic phase that transforms into the clinical EMF that reaches us in hospitals.
Epidemiology
In 1936 Loffler described what was considered the first case of EMF, in a patient who had pronounced eosinophilia.4 A decade later (1946) Bedford and Konstam described a form of heart disease in 40 West African soldiers, serving in the Middle East during the Second World War; a post-mortem revealed subendocardial fibrosis with features consistent with EMF.5 However, the clinicopathological features of the classical disease were first recognized by Davies in Uganda in 1948.6 Although sporadic cases with similar clinical and pathological features have since been reported from other parts of the world, the majority of reported cases have come from West and Central Africa. In Uganda, it accounted for 25% of cases who reported for echocardiography and 20% in a random population sample in Mozambique.7,8 Results of SubSaharan Africa Study of Heart Failure (THESUS-HF 2012), which included nine SubSaharan Africa countries, showed that EMF accounted for only 1.3 of acute heart failure admissions.9 While the THESUS-HF Survey was of small size and represented patients with acute heart failure, the high Ugandan rate was due to the fact that patients represented a sample attending the echocardiography laboratory for evaluation of heart disease. The Mozambique survey was a population-based study and may represent what could be found in endemic areas of EMF in other parts of the world. The worldwide prevalence of EMF has been estimated as 10–12 per million.10
EMF has been reported from other subtropical countries; Egypt,11 Nigeria,9,12 Brazil, Kerala in India,13,14 and Sudan.15,16 It is exceedingly rare in Europe and North America; however few cases have been reported in China17 and Japan.18 It is a disease of the low disadvantaged families in tropical and subtropical Africa where, like rheumatic heart disease (RHD), it afflicts children and young females with a female preponderance of 2:1.19 The disparity in gender ratios has been mentioned before but, a preponderance of females has been reported. In Uganda, women of childbearing age present a 2-fold higher prevalence compared with men.20 In contrast, other studies have not observed a specific sex difference in adults.9
Etiology
The etiology of EMF remains obscure, and research work in this field has been scarce and of limited outcomes. One possible reason for the lack of a breakthrough is the fact that most cases are not seen in the acute stage, and arrive at hospitals when the initial features of the disease had disappeared. In Africa, several associations with the disease have been implicated, among them were ethnicity, diet, poverty, eosinophilia, infection, and malaria. The issue of ethnicity seemed a relevant etiological factor as most of the cases described were from tropical and subtropical African countries, especially Uganda, Rwanda, Kenya, Nigeria, Sudan, and Mozambique.21 In addition to a shared ethnic background, other factors included tuber rich (cassava) low protein diet, poverty, and infection.7 Some of those additional factors prevail in other non-African tropical and subtropical countries like Brazil and India where EMF has been reported.9,10 In Africa, patients in endemic areas suffer from malnutrition, additionally, they consume cassava, which contains the earth element Cerium. It is reported that Cerium and hypomagnesemia in combination may induce EMF-like disease in experimental animals. However, no similar finding has been reported in humans. Valiathan reported that when measuring the level of Cerium in patients with EMF and a control group, the level of Cerium in the serum of EMF patients were significantly elevated compared to control subjects (p < 0.05).22 Despite this study, the issue of involvement of Cerium remains unconfirmed, and more population-based studies are needed to verify it.
The disease is reported to be rare in Europe and North America, but Brockington reported a case of EMF in a European resident of 15 years in Eastern Nigeria. He also reviewed an additional twenty-three European patients from other West African countries and concluded that EMF cannot be caused by a genetic characteristic nor by malnutrition and that there seems little reason to associate it with plantain eating. The distribution and pattern of the disease favor an infective agent as the cause.23
Eosinophilia has been closely related to EMF since the first mention by Loffler, back in 1936, and is presently considered a significant risk factor in Africa. It is commonly seen in the African population, related to microfilaria and helminthic infestations.24 Eosinophils' degranulation releases toxic cationic proteins and protein X that are released in the endocardium and myocardium and may result in thrombosis, fibrosis, and eventually EMF.25–27 In keeping with that observation, eosinophilia has already been recognized as a feature of the early stages of the disease and disappears when chronicity is reached.
Andrzej and et al reported more data on the genetics and biochemistry of Eotaxin and its receptor – CCR3 as well as molecular mechanisms and their impact on eosinophils.28 Eotaxin is the most effective in vivo chemoattractant for eosinophils and regulates eosinophil migration to peripheral tissue against its gradient. Further, it seems to delay eosinophil apoptosis outside blood vessels, at the same time activating the cells by binding to cell surface receptors and eventually leading to eosinophil degranulation. Based on its properties and functions, Eotaxin or its receptor may become a reasonable future target for the treatment of EMF with tissue eosinophilia.26
On the epidemiological basis, EMF behaves like a vector-transmitted disease and many parasites have been incriminated as associates, among them was microfilaria infection characterized by hypereosinophilia. In Egypt, schistosomiasis is reported to cause periportal fibrosis, pulmonary hypertension, and EMF.6 Malaria assumed a stronger association with EMF, as both are prevalent in sub-Saharan Africa. Early research has shown that patients with EMF have a high titer of cardiac myosin antibodies (IgM) that correlates well with an increasing titer of malaria antibody, thus lending support to the role of malaria in the pathogenesis of EMF.29
As a conclusive remark, on the etiology of EMF, it appears that the actual cause has not been defined and that more research is needed to unravel the cause of this mysterious disease.
Diagnosis of EMF
Most of the earlier researchers depended on postmortem findings and lately the use of angiography in the diagnosis of EMF.30 Recently progress has been made in the diagnosis of EMF by the introduction of echocardiography and cardiac magnetic resonance (CMR).31,32
Mocumbi et al adopted a scoring system for the diagnosis and assessment of the severity of EMF.8 The use of a scoring system has certain limitations, among them, is the fact that in the hospital practice, cases that present early are missed due to non-specific symptoms and signs. Only those who come with a full-blown picture of late presentation are labeled as EMF. Consequently, the scoring system works well in population-based surveys where early stages of the disease may be spotted.
Clinical Presentation
Early symptoms of EMF are nonspecific and may point to febrile illness with lymphadenopathy and sometimes splenomegaly. Most patients are provisionally diagnosed with malaria, which is equally prevalent in the same region as EMF. Unfortunately, the disease becomes more recognizable at the late stage of heart failure. Alderman described the initial clinical presentation and stages of the eosinophilic endomyocardial disease as follows.33
The necrotic stage starts when the patient presents in the first five weeks with fever, lymphadenopathy, sweating, and splenomegaly. Carditis is seen in 20–50% of patients with features of anorexia, weight loss, atrioventricular valves regurgitation, and biventricular failure. The thrombotic stage is characterized by thrombotic emboli (10–20%) and present with cerebral, splenic, renal, and coronary infarction. Splinter hemorrhages are commonly seen during this stage. The fibrotic stage, characterized by restrictive myopathy, is seen in 10% of patients, who present with atrioventricular valvular regurgitation, and right or left heart failure.
Most of the patients with EMF present late, with an advanced form of heart failure with gross ascites, anorexia, weight loss, and occasional chest pain. Tense ascites is commonly seen in EMF and accounted for 30% in a series reported from Sudan.34 It is associated with mild or no edema in some cases. Freers et al reported exudative content, unlike passive ascites seen in congestive failure, and the appearance of peritoneal fibrosis in some cases.35 This finding reflects a systemic connection of the disease.
Electrocardiography and Chest X-Ray
ECG findings were nonspecific; sinus tachycardia was found in 22% of patients, atrial abnormality in 43%, first-degree heart block in 39%, and atrial fibrillation in 13% of patients. Chest X-ray findings were also nonspecific and showed cardiomegaly in 92% and pericardial effusion in 87% of patients.34
Laboratory Findings
Hematological findings included iron deficiency anemia with hemoglobin reaching as low as 5g/dL and depleted iron stores.36 In all cases, absolute eosinophilia was not a feature in patients who presented late with heart failure, and early researchers have indicated that the presence of high eosinophil count characterized the early phases of the disease.23 Leukocytosis was not a feature of this disease.
Hypomagnesemia has been reported in several patients and was incriminated as a risk factor when combined with Cerium, found in tuber rich diet.20
Echocardiography of EMF
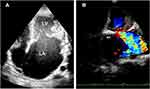
Echocardiography is now the gold standard tool for the diagnosis of EMF. The heart is examined using five standard echocardiographic views: parasternal long axis (PLX), short axis (SX), apical four chambers (AP4), apical long-axis (APLX) and apical two chambers (AP2). All images are acquired according to guidelines of the American Society of Echocardiography.37 There are five diagnostic echocardiographic features of EMF; apical fibrosis, ventricular wall fibrosis, huge atrium, atrioventricular valve regurgitation and obliteration of ventricular cavity (Figure 1A and B).34
These features are commonly seen together in the same patient and are considered the prime diagnostic findings.
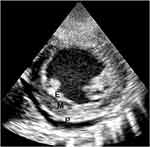
There is a second group of features that are not seen in all patients but if present lend confirmation to the diagnosis, among them are: pericardial effusion, endocardium fibrous shelf (EFS) formation. EFS was described by Davies back in 1948 in the pathological specimens. He described the fibrous endocardium extending into the posterior left ventricular wall recess engulfing the posterior mitral valve leaflet. Figure 2 depicts the echocardiographic features that correlate with what Davies had described, namely the EFS that extends into the posterior wall recess and covers the posterior mitral valve leaflet.
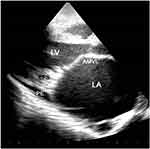 |
Figure 2 LAP view showing huge left atrium (LA), endomyocardial fibrous shelf (EFS), anterior mitral valve leaflet (AMVL), pericardial effusion (PE), and left ventricle (LV). |
Figure 3 is an M-Mode image of the posterior left ventricular wall, the interventricular septum, and left ventricular cavity. The posterior wall shows the typical “layering” where the thick fibrosed endocardium is seen distinctly separated from the myocardium below by a line of cleavage.
Figure 4 shows pericardial calcification where the pericardium is seen thickened and densely calcified. The presence of pericardial calcification creates a form of pericardial constriction with clinicopathological features of constrictive pericarditis.
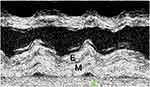 |
Figure 3 M-Mode view showing the layering of the posterior LV wall with a cleavage line between the endocardium (E) and the myocardium (M). |
This feature merits the term endomyocardiopericarial fibrosis (EMPF) and is considered a new additional echocardiographic finding.34
Three echocardiographic types of EMF are recognized; left ventricular which constituted 53% of cases (Figure 1), right ventricular seen in 18% of cases (Figure 5A), and biventricular which was seen in 29% of cases (Figure 5B).
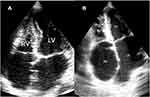 |
Figure 5 AP4 view: (A) view of a case of right ventricular (RV) EMF. (B) Shows a case of biventricular EMF of both RV and LV. Abbreviations: RA, right atrium; LA, left atrium. |
Echocardiographic findings correlate well with intraoperative and postmortem findings. Davies was the first to identify the three layers (layering), during autopsy, and how the endocardium was densely fibrosed with the formation of a line of cleavage between the two layers, this line of cleavage is utilized by cardiac surgeons during endocardial decortication surgery. The huge atria are not seen in other conditions such as mitral stenosis and are due to mitral regurgitation and high left ventricular filling pressure brought about by the obliterated ventricular cavity. A further noticeable finding is the spread of the thick endocardium of the posterior left ventricular wall to engulf the posterior mitral valve leaflet with the formation of an endocardial fibrous shelf (EFS).34 This autopsy finding was noted by Davies and is shown echocardiographically in Figure 2. Pericardial effusion is a common finding and is seen in 87% of patients who present with heart failure. Recently we reported the presence of pericardial calcification leading to a form of constrictive pericarditis in one case.34
Cardiac Magnetic Resonance (CMR)
Cardiac magnetic resonance has now replaced cardiac catheterization as a diagnostic tool for EMF. The use of cine imaging, first-pass contrast-enhanced perfusion, and Late Gadolinium Enhancement (LGE) can identify the presence of endomyocardial fibrosis. Post-enhanced imaging can also show obliterated ventricle, apical dimple, thrombi, grossly dilated atria and regurgitant atrioventricular valves. Pericardial effusion and ascites are common in CMR images of EMF.32
Endomyocardial Biopsy
Cardiac catheterization is still utilized, especially for excision biopsy of the endocardium. The histologic findings of EMF show reactive fibrosis and type-1 collagen deposition. Subendocardial infarction, eosinophil infiltration of the upper myocardium and thrombus formation are commonly seen.38 Mild inflammatory infiltrates, predominantly with lymphocytes, are frequent but intense eosinophilic infiltrates and small vessel disease are unusual.39
Differential Diagnosis
The diagnosis of EMF needs a high index of suspicion as few cases have been missed and are often mislabeled as rheumatic heart disease or Hypertrophic Obstructive Cardiomyopathy (HOCM). The differentiation of EMF from HOCM, especially the apical type, can be difficult. Apical fibrosis of HOCM is often recognized by the normal size or slightly enlarged left atrium as opposed to the huge left atrium of EMF. Additionally, EMF apical obliteration appears during both systole and diastole, in contrast to HOCM, which occurs only in systole.40
Rheumatic mitral valve disease may be excluded by the appearance of the huge left atrium and obliterated ventricle; the finding of EFS lends further support to the diagnosis of EMF. Other conditions that may show apical and endocardial fibrosis and pose diagnostic difficulty are carcinoid heart disease, drug-induced cardiotoxicity as in methysergide.41
Treatment of EMF
Medical Care
Most patients present late with a picture of full-blown heart failure. At this stage, fluid overload, ascites, and infections are the main difficulties. First attention should go to nutritional corrections and diuretics to reduce fluid overload. The use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and beta-blockers may confer further benefit.42 In another study by Subair et al, it was found that ACE inhibitors could not prolong survival. In fact, those on ACE inhibitors died earlier, but on subgroup analysis, the 5-year survival of left ventricular EMF on ACE inhibitors was 86.67%, 10-year survival was 75.83%, and 15-year survival was 75.83%. Digoxin and Spironolactone did not confer any improvement in survival.43 This may be attributed to the fact that most of these drugs are used during the advanced stage of the disease. Anticoagulation may be reserved for patients with atrial fibrillation and those who have thrombus on echocardiography. Where anticoagulants are difficult to monitor or not available in remote areas, the use of aspirin and clopidogrel may be considered as an alternative. Recently a trial of prednisolone in the prevention of re-accumulation of ascites showed that prednisolone did not prevent the accumulation of ascites.44 Finally, medical treatment provides more of a bridging approach to buy time before surgery or heart transplant is available.
Recently exercise rehabilitation was reported to improve cardiac volumes and functional capacity in patients with EMF. Exercise rehabilitation is a nonpharmacological intervention that improves functional capacity, cardiac volumes, and quality of life in EMF patients after endocardial resection surgery. In addition, exercise rehabilitation should be prescribed to EMF patients to improve their clinical condition.45
Surgical Care
These patients constitute a high-risk group for open-heart surgery, because of high operative mortality (15–20%). Pericardectomy or pericardiectomy is performed to relieve symptoms in patients with advanced disease, especially those with pericardial effusion.21,46 However, endocardial decortication seems to be more definitive and beneficial for many patients with advanced disease. Successful surgery has a clear benefit in terms of symptoms and seems to affect survival favorably.
Endocardiectomy or stripping of the endocardium combined with mitral valve repair or replacement in left ventricular EMF or tricuspid repair or replacement in cases of right ventricular disease, under cardiopulmonary bypass, is a feasible and easy way to accomplish surgery, as endocardium fibrosis creates a line of cleavage between the endocardium and myocardium layers and thus makes stripping easy. In this case, the myocardium is less affected and the outcome of surgery is often good with improvement in cardiac output and relief of edema and pulmonary congestion. A new procedure, cavopulmonary shunting, has been added to deal with right ventricular obliteration and has shown success in some patients. Immediate complications included ventricular arrhythmia and heart block.47,48
The operative mortality within 30 days of the procedure and late mortality during the first two years post-operation were 21.7% and 13%, respectively. Age under 15 years was a significant correlate of operative mortality (p = 0.05). An estimate of survival inclusive of operative mortality at two years was 67%.49
Prognosis
Most patients with EMF present at a late stage of the disease and suffer a high rate of in-hospital mortality. The duration of illness from the time of presentation to death was less than one year in 43.5% of patients, three years in 39.1% of patients, and three to twelve years in 17.4% of patients. Most patients had extensive disease at the time of presentation; and death usually occurs as a result of progressive heart failure, sudden deterioration and collapse possibly due to arrhythmia, pulmonary events such as pulmonary edema or bronchopneumonia, and pulmonary embolism.50
Research and Future Prospects of EMF
Knowledge of EMF has been advanced by the introduction of echocardiography, MRI, and endocardial biopsy. But, despite those advances, more research is needed to unveil the cause and inner details of the disease. Bukhman et al51 stated that the fusion protein FIP1L1-PDGFRα, a constitutively activated tyrosine kinase found in as many as half of those with the idiopathic hypereosinophilic syndrome, has emerged as a therapeutic target for imatinib. The prevalence of FIP1L1-PDGFRα among those with EMF could give another important clue about the etiology and treatment of this disease.52 Echocardiographic studies of patients with hyper-reactive malarial splenomegaly could shed light on the prevalence of early endocardial disorders in this population.
The recent finding that serotonin acts as a chemotactic factor for eosinophils may reignite inquiries into the role of this pathway in EMF.53 Studies that measure levels of markers, such as C-reactive peptide or inflammatory cytokines such as tumor necrosis factor α, could help explore the role of inflammation in EMF and suggest therapeutic strategies in early forms of the disease.54
Future research needs to focus on the early phases of the disease and design studies of follow-up for at least five years. This will enable the detection of etiologies hitherto undiscovered, and shed more light on the progress of the disease.
Conclusion
EMF is a mysterious disease whose etiology is unraveling. The afflicted individuals are mostly young females and children who succumb to high rates of morbidity, and mortality from heart failure. Medical and surgical treatment are both practiced with results varying from temporary relief of symptoms to surgery with high mortality rates. Ongoing research is constrained by the financial strains in endemic areas and consequently, the outcome is limited. EMF is prevalent in low-income regions of the world where it is not considered a health priority. It will, therefore, need a lot of advocacy and commitment to bring it forward to the attention of health authorities. One target should be to incorporate EMF in the Primary Health Care Program which advocates that primary care services are available, affordable, and provided equally to all individuals irrespective of their gender, age, ethnicity or location.
It is anticipated that budgetary support for research work on EMF will be nil or meager and, therefore, resorting to international sponsorship or grants might be a possible alternative.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Mbanze J, Cumbane B, Jive R, Mocumbi A. Challenges in addressing the knowledge gap on endomyocardial fibrosis through community-based studies. Cardiovasc Diagn Ther. 2019. doi:10.21037/cdt.2019.08.07
2. Olsen EG, Spry CJ. The relation between esinophilia and endomyocardial disease. Prog Cardiovasc Dis. 1985;27(4):241–254. doi:10.1016/0033-0620(85)90008-8
3. Davies JNP, Ball JD. The pathology of endomyocardial fibrosis in Uganda. Br Heart J. 1955;17:337–359. doi:10.1136/hrt.17.3.337
4. Loffler W. Endocarditis parietalis fibroplastica mit Bluteosinophilie: ein eingenartiges Krankheitsbild. Schweiz Med Wochenschr. 1936;17:817.
5. Bedford DE, Konstam GLS. Heart failure of unknown etiology in Africans. Br Heart J. 1946;8:236.
6. Davies JNP. Endomyocardial fibrosis in Africans. East Afr Med J. 1948;25:10–17.
7. Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005;112(23):3577–3583. doi:10.1161/CIRCULATIONAHA.105.542894
8. Mocumbi AO, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med. 2008;359:43–49. doi:10.1056/NEJMoa0708629
9. Damasceno A, Mayosi BM, Sani M, et al. The Sub-Saharan Africa survey of heart failure (THESUS-HF). Arch Intern Med. 2012;172(18):1386–1394. doi:10.1001/archinternmed.2012.3310
10. Beaton A, Mocumbi AO. Diagnosis and management of endomyocardial fibrosis. Cardiol Clinic. 2017;35:87–98. doi:10.1016/j.ccl.2016.08.005
11. Rashwan MA, Ayman M, Ashour S, Hassanin MM, Ziena AA. Endomyocardial fibrosis in Egypt. Br Heart J. 1995;73(3):284–289. doi:10.1136/hrt.73.3.284
12. Mocumbi AOH, Falase AO. Recent advances in the epidemiology, diagnosis and treatment of endomyocardial fibrosis in Africa. Heart. 2013;99:1481–1487. doi:10.1136/heartjnl-2012-303193
13. Guimarães AC, Esteves JP, Filho AS, Macedo V. Clinical aspects of endomyocardial fibrosis in Bahia, Brazil. Am Heart J. 1971;81(1):7–19. doi:10.1016/0002-8703(71)90049-4
14. Vijayaraghavan G, Sivasankaran S. Tropical endomyocardial fibrosis in India: a vanishing disease. Indian J Med Res. 2012;136(5):729–738.
15. O’Brien W. Endocardial fibrosis in Sudan. Br Med J. 1954;2:899–903. doi:10.1136/bmj.2.4893.899
16. El Hassan AM, Wasfi AI. Cardiovascular disease in Khartoum: post- mortem and clinical evidence. Trop Geogr Med. 1972;24:118–123.
17. Yin R. Endomyocardial fibrosis in China. Chin Med Sci J. 2000;15(1):55–60.
18. Niino T, Shiono M, Yamamoto T, et al. A case of left ventricular endomyocardial fibrosis. Ann Thorac Cardiovasc Surg. 2002;8:173–176.
19. Connor DH, Somer K, Hutt MSR, Manion WC, D’Arbela PG. Endomyocardial fibrosis in Uganda. Am Heart J. 1967;74:687. doi:10.1016/0002-8703(67)90509-1
20. Freers J, Mayanja-Kizza H, Ziegler JL, Rutakingirwa M. Echocardiographic diagnosis of heart disease in Uganda. Trop Doct. 1996;26:125–128. doi:10.1177/004947559602600310
21. Falase AO. Endomyocardial fibrosis in Africa. Postgrad Med J. 1983;59:170–177. doi:10.1136/pgmj.59.689.170
22. Valiathan MS, Kartha CC. Endomyocardial fibrosis: a possible connection with the myocardial level of magnesium and Cerium. Int J Cardiol. 1989;28:1–5. doi:10.1016/0167-5273(90)90002-M
23. Brockington IF, Olsen EGJ, Goodwin JF. Endomyocardial fibrosis in Europeans resident in tropical Africa. Lancet. 1967;289:583–588. doi:10.1016/S0140-6736(67)90440-0
24. Rutakingirwa M, Ziegler JL, Newton R, Freers J. Poverty, and esinophilia are risk factors for endomyocardial fibrosis (EMF) in Uganda. Trop Med Int Health. 1999;4:229–235. doi:10.1046/j.1365-3156.1999.43376.x
25. Freers J, Amandua J, Mugerwa R, Sezi C. Endomyocardial fibrosis and eosinophilia. Lancet. 1993;342:1233–1234. doi:10.1016/0140-6736(93)92211-B
26. Andy JJ, Ogunowo PO, Akpan NA, Odigwe CO, Ekanem IA, Esin RA. Helminth associated hypereosinophilia and tropical endomyocardial fibrosis in Nigeria. Acta Trop. 1998;69:127–140. doi:10.1016/S0001-706X(97)00125-3
27. Tai PC, Ackerman SJ, Spry CJ, Dunnette S, Olsen EG, Gleich GJ. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial fibrosis. Lancet. 1987;1:643–647. doi:10.1016/S0140-6736(87)90412-0
28. Andrzej M, Rosiek M, Biegus J, Malolepszy J. Eotoxin and its role in the pathophysiology of eosinophilic inflammation. Alergia Astma Immunologia. 2003;8:19–24.
29. Shaper AG. Endomyocardial Fibrosis In: Shaper AG, Hunt MSR, and Fejfar Z, editors. Cardiovascular Disease in the Tropics. London: British Medical Association; 1968.
30. Shaper AG, Hutt MSR, Coles RM. Necropsy study of endomyocardial fibrosis and rheumatic heart disease in Uganda. Br Heart J. 1968;30:391. doi:10.1136/hrt.30.3.391
31. Acquatella H, Nelson B, Schiller M. Value of two-dimensional echocardiography in endomyocardial disease with and without eosinophilia; a clinical and pathologic study. Circulation. 1983;67:1219–1226. doi:10.1161/01.CIR.67.6.1219
32. Syed IS, Martinez MW, Feng DL, Glockner JF. Cardiac magnetic resonance imaging of eosinophilic endomyocardial disease. Int J Cardiol. 2008;126:e50–e52. doi:10.1016/j.ijcard.2007.01.019
33. Alderman EL. Non-infective endocardial disease. Cardiovasc Dis. 1999. 1–7. doi:10.1016/s0021-9673(99)00837-7
34. Khalil SI, Khalil SS, El Tigani S, Saad HA. Endomyocardial fibrosis in Sudan: clinical and echocardiographic features. Cardiovasc J Afr. 2017;28:222–228. doi:10.5830/CVJA-2016-079
35. Freers J, Mayanja-Kizza H, Rutakingirwa M, Gerwing E. Endomyocardial fibrosis: why is there striking ascites with little or no peripheral edema? Lancet. 1996;347:197. doi:10.1016/S0140-6736(96)90383-9
36. Makubi A, Hage C, Lwakatare J, et al. Prevalence and prognostic implications of anaemia and iron deficiency in Tanzanian patients with heart failure. Heart. 2015;101:592–599. doi:10.1136/heartjnl-2014-306890
37. Lang RM, Luigi P, Badano LP, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi:10.1016/j.echo.2014.10.003
38. Connor DH, Somers K, Hutt MS, Manion WC, D’Arbela PG. Endomyocardial fibrosis in Uganda (Davies’ disease). II. An epidemiologic, clinical, and pathologic study. Am Heart J. 1968;75:107–124. doi:10.1016/0002-8703(68)90122-1
39. Mocumbi AO. Endomyocardial fibrosis: a form of endemic restrictive cardiomyopathy. Glob Cardiol Sci Pract. 2012;2012:11–23. doi:10.5339/gcsp.2012.11
40. Fawzy ME, Ziady G, Halim NM, et al. Endomyocardial fibrosis: report of eight cases. J Am Coll Cardiol. 1985;5:983–988. doi:10.1016/S0735-1097(85)80444-7
41. Harbin AD, Gerson MC, O’Connell JB. Simulation of acute myopericarditis by constrictive pericardial disease with endomyocardial fibrosis due to methysergide therapy. J Am Coll Cardiol. 1984l;4:196–199. doi:10.1016/S0735-1097(84)80342-3
42. Loseva MI, Purtova LA, Gavalova RF. Angiotensin converting enzyme inhibitor enalapril in the treatment of endomyocardial fibrosis in patients with lymphogranulomatosis subjected to radio- and chemotherapy. Kardiologiia. 2002;42:48–51.
43. Subair KM, Gupta PN, Suresh K, et al. The medical treatment of endomyocardial fibrosis in 2009. Heart Asia. 2011;120e123. doi:10.1136/ha.2010.003525
44. Nabunnya YB, Kayima J, Longenecker CT, Josephson RA. The safety and efficacy of prednisolone in preventing reaccumulation of ascites among endomyocardial fibrosis patients in Uganda: a randomized clinical trial. BMC Res Notes. 2015;8:783. doi:10.1186/s13104-015-1761-0
45. Sayegh ALC, Santos D, Marcelo R, et al. Exercise rehabilitation improves cardiac volumes and functional capacity in patients with endomyocardial fibrosis. J Cardiopulm Rehabil Prev. 2019;39:373–380. doi:10.1097/HCR.0000000000000445
46. Alvarez DS, Rodríguez CA, Chino-Mendoza JM, Quispe-Villca YA, Arias-Godínez JA. Endomyocardial fibrosis of the right ventricle. Imaging J Clin Medical Sci. 2019;6(1):094–096. doi:10.17352/2455-8702.000228
47. Bertrand E, Chauvet J, Assamoi MO, et al. Results, indications and contra-indications of surgery in restrictive endomyocardial fibrosis: comparative study on 31 operated and 30 non-operated patients. East Afr Med J. 1985;62:151–160.
48. Uva MS, Jebara VA, Acar C, et al. Mitral valve repair in patients with endomyocardial fibrosis. Ann Thorac Surg. 1992;54:89–92. doi:10.1016/0003-4975(92)91146-Z
49. Valiathan MS, Balakrishnan KG, Sankarkumar MS, Kartha CC. Surgical treatment of endomyocardial fibrosis. Ann Thorac Med. 1987;43:68–73. doi:10.1016/S0003-4975(10)60169-5
50. D’Arbela PG, Mutazindwa T, Patel AK, Somers K. Survival after the first presentation with endomyocardial fibrosis. Br Heart J. 1972;34:403–407. doi:10.1136/hrt.34.4.403
51. Bukhman G, Ziegler J, Parry E. Endomyocardial sears. PLoS Negl Trop Dis. 2008;2(2):e97. doi:10.1371/journal.pntd.0000097
52. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by the fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–1214. doi:10.1056/NEJMoa025217
53. Boehme SA, Lio FM, Sikora L, et al. Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J Immunol. 2004;173(6):3599–3603. doi:10.4049/jimmunol.173.6.3599
54. Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368(9536):687–693. doi:10.1016/S0140-6736(06)69253-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.