Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Encephalitis with reversible splenial and deep cerebral white matter lesions associated with Epstein–Barr virus infection in adults
Authors Guo YJ, Wang SH, Jiang B, Li JL, Liu L, Wang J, Zhao WQ, Jia J
Received 24 February 2017
Accepted for publication 5 May 2017
Published 3 August 2017 Volume 2017:13 Pages 2085—2092
DOI https://doi.org/10.2147/NDT.S135510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Yanjun Guo,1 Shuhui Wang,1 Bin Jiang,1 Jianle Li,1 Lei Liu,2 Jiawei Wang,2 Weiqin Zhao,1 Jianping Jia1
1Department of Neurology, Beijing Friendship Hospital, Capital Medical University. Beijing, China; 2Department of Neurology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
Background: Approximately 200 cases of mild encephalitis with reversible splenial (MERS) and deep cerebral white matter lesions have been reported since MERS was first defined in 2004. MERS occurs more frequently in children; in adults, only ~60 cases have been reported. Until now, only four cases of MERS in adults have been associated with Epstein–Barr virus (EBV).
Case presentation: We report three adult cases of MERS associated with EBV infection in China. For all three patients, cranial magnetic resonance imaging (MRI) indicated solitary reversible splenial and/or perilateral ventricle white matter lesions with reduced diffusion. In the present report, all patients were adults presenting with high fever, headache, apathy, and confusion, as well as significant signs of meningeal inflammation. These symptoms peaked 10–14 days after disease onset, with serious hyponatremia (112–129 mmol/L), an elevated cerebrospinal fluid white blood cell count (80–380/mm3), and significantly increased protein levels (1,010–1,650 mg/dL). Cranial MRI indicated abnormal signal intensity in the splenium of corpus callosum and symmetrically reversible lesions scattered in the thalamus and deep cerebral white matter. The clinical symptoms tended to improve after ~10–14 days of antiviral treatment. However, these patients recovered more slowly than patients with viral meningitis.
Conclusion: MERS associated with EBV infection in adults occurs less frequently but with more severe symptoms than in children. EBV infection should be considered for patients with MERS symptoms. MERS has a good prognosis.
Keywords: mild encephalitis with reversible splenial lesions, Epstein-Barr virus, apathy, hyponatremia, corpus fluid
Background
Clinically mild encephalitis/encephalopathy with a reversible splenial (MERS) lesion is a new clinico-radiological syndrome.1 MERS has a benign clinical course and is characterized on magnetic resonance imaging (MRI) by reversible splenial lesions with reduced diffusion. MERS occurs more commonly in children in Japan and East Asia.1–6 Epstein–Barr virus (EBV) has been associated on rare occasions with a variety of central nervous system complications,1,7 usually in pediatric patients8–10 and almost exclusively during acute primary infections.7 Here, we report three cases of adult MERS induced by EBV infection.
Case presentation
Case 1
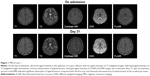
In May 2007, a 46-year-old man was admitted with high fever (up to 39°C), nausea, and projectile vomiting persisting for 10 days, which were accompanied with general fatigue, neck pain, and temporal bilateral blunt headache. He was somnolent, apathetic, and showed slow response. The patient showed strong signs of meningeal inflammation. The chin–sternum distance was four fingers. No paresis or sensibility deficit was detected. Laboratory results revealed hyponatremia (sodium, 112 mmol/L) and hypochloridemia (chloride, 81 mmol/L). Serum EBV IgM was negative. A lumbar puncture indicated an elevated cerebrospinal fluid (CSF) pressure of 260 mmH2O, white blood cell (WBC) count of 250×106/L (75% monocytes, 25% neutrophils), RBC count of 0, and severe elevation of total proteins (1,418 mg/L). Glucose was normal. CSF EBV IgM antibody screens were positive. Cranial MRI (3.0 T; General Electric, Boston, MA, USA) showed meningeal enhancement without edema or swelling. Diffusion-weighted imaging (DWI) revealed a single abnormal lesion of the splenium of corpus callosum (SCC), signal hyperintensity in fluid-attenuated inversion recovery (FLAIR), and T2 without contrast enhancement (Figure 1).
Beginning on the day of admission, the patient received 750 mg of acyclovir intravenous drop infusion (IV) 3 times/day for 2 weeks. By day 10, symptoms were under control. Persistent hyponatremia (sodium, 116–125 mmol/L) did not respond to sodium supplementation. Inappropriate antidiuretic hormone secretion due to meningitis was diagnosed. Daily water intake was restricted to <1 L/day, and a 7-day treatment regimen with hydrocortisone (100 mg/day, IV, twice/day) was administered beginning on day 12.
Lumbar puncture performed on day 14 showed CSF cell count was unremarkable but demonstrated elevated proteins (833 mg/L). CSF EBV IgM was negative. A subsequent cranial MRI on day 21 indicated a decrease in the area of enhancement of the cerebral pia mater and significant absorption of splenial lesions (Figure 1). The patient was discharged without any clinical symptoms on day 25.
Case 2
In February 2011, a 33-year-old male was admitted for tracheitis and febrile infection with headache, nausea, and vomiting. Initial examination revealed confusion, apathy, and neck stiffness. Neck rigidity and a chin–sternum distance of four fingers were observed. Kernig’s sign was positive. Blood sodium was 128.2 mmol/L, and chloride was 91 mmol/L. Serological EBV IgM was positive. Serum EBV antibodies showed IgM/VCA positive (1:10); IgG/VCA 1:20; IgA/VCA (−); IgA/EA (−).
On day 1, a lumbar puncture revealed high CSF pressure (220 mmH2O) and WBC count of 80×106/L (60% monocytes, 40% neutrophils). The CSF protein content was 1,050 mg/L and Pandy’s test was positive; glucose was normal. CSF EBV antibodies test showed IgM/VCA positive (1:5); EBV IgG/VCA 1:10; EBV IgA/VCA (−); EBV IgA/EA (−).
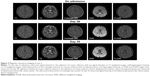
Cranial MRI showed T2, FLAIR, and DWI signal hyperintensities in the SCC on day 2 (Figure 1).
From the date of diagnosis, the patient received 750 mg of acyclovir every 8 hours for 3 weeks. By day 10, the patient regained full consciousness in the absence of headache and vomiting. Temperature returned to normal on day 11. Neck rigidity had gradually disappeared by day 14.
Hyponatremia was persistent (sodium, 125–128 mmol/L) although sodium supplementation (10% sodium chloride, 20–40 mL, intravenous glucose tolerance test, once daily) was administered from the day of admission. Beginning on day 10, daily water intake was restricted to no more than 1 L, and a 7-day treatment regimen of corticosteroids (hydrocortisone 100 mg/day, IV over 2 hours) was administered.
The patient underwent a lumbar puncture on day 14. The initial CSF pressure was 120 mmH2O. CSF WBC count was 100.0×106/L, CSF total protein level was 123.00 mg/dL (normal range, 15–45 mg/dL), CSF chloride was 117 mmol/L (normal range, 120–132 mmol/L), and glucose was 2.16 mmol/L (normal range, 2.24–3.92 mmol/L). EBV antibody in CSF showed IgM/VCA positive (1:10); IgG/VCA 1:10; IgA/VCA (−); IgA/EA (−); while serum EBV antibodies showed IgM/VCA positive (1:10); IgG/VCA 1:20; IgA/VCA (−); IgA/EA (−).
Lumbar puncture was repeated on day 30: intracranial pressure was 90 mmH2O, WBC count was 30.0×106/L, total protein level was 84.00 mg/dL, glucose was 2.08 mmol/L, and CSF chloride was 113 mmol/L. EBV antibody in CSF showed IgM/VCA negative (−); IgG/VCA 1:10; IgA/VCA (−); IgA/EA (−); while serum EBV antibodies showed IgM/VCA positive (1:5); IgG/VCA 1:20; IgA/VCA (−); IgA/EA (−).
The initial MRI on day 2 showed abnormal signals in the SCC only. T1 showed equal signal intensity. T2 and FLAIR showed high signal intensity. DWI showed high signal intensity of the corpus callosum lesions without enhancement and the leptomeninges showed slight enhancement (Figure 2, row 1). The MRI on day 26 showed that the splenium corpus callosum lesions had almost disappeared, but showed symmetric interspersed lesions in bilateral periventricular white matter (Figure 2, row 2). Cranial MRI performed on day 35 showed absence of abnormal meningeal enhancement, and absence of hyperintensity in the splenial lesions on the T2-weighted and DWI images; most of the abnormal signal in bilateral periventricular white matter disappeared, while a small lesion in the white matter in right basal ganglia did not diminish (Figure 2, row 3). The patient was discharged on day 42 because of clinical recovery. Cranial MRI follow-up was carried out on day 105, with similar abnormal MRI results as on day 35 (data not shown). With a clinical follow-up for 3 years, the patient remained healthy.
Case 3
In September 2012, a 23-year-old man was admitted due to headache with high fever (39°C), nausea, and vomiting persisting for 8 days, hiccups for 3 days, and somnolence for 2 days. On admission, his eyes could not be entirely abducted, and he showed symptoms of confusion, apathy, neck stiffness, and nystagmus. The finger-to-nose test revealed bilateral instability and inaccuracies. The neck was rigid with neck resistance and a chin–sternum distance of 4 fingers width and the Kernig’s sign was bilaterally positive. Brudzinski’s sign was bilaterally negative.
On day 1, blood WBC count was 8.31×109/L (68.1% neutrophils), and platelet level was 319×109/L. Sodium was 128 mmol/L, chloride was 90 mmol/L, lactate dehydrogenase (LDH) was 251 U/L, and creatine kinase (CK) was 314 U/L. On day 2, CSF analysis showed intracranial pressure at 170 mmH2O, WBC count of 380×106/L (80% monocytes, 20% neutrophils), positive Pandy test, total proteins of 113 mg/dL, glucose of 2.64 mmol/L, and chloride of 108 mmol/L. CSF EBV IgM/VCA was positive (1:10), IgG/VCA was also 1:10 screens for IgA/VCA, and IgM/EA was negative. Blood EBV IgM/VCA was positive (1:5), IgG/VCA was also 1:20, and IgA/VCA and IgM/EA were also negative. Polymerase chain reaction (PCR) assay revealed an EBV viral load of 3.2×103 copies/L.
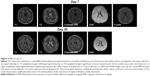
A cranial MRI on day 7 showed abnormal signal intensities in the SCC and white matter periventricle (Figure 3). Significant enhancements of the cerebral pia mater and right tentorium of the cerebellum were observed, indicating possible meningitis.
Antiviral treatment began on day 2 consisting of acyclovir (0.85 mg every 8 hours), mannitol (250 mL every 8 hours), and restricted water intake and sodium supplementation. Consciousness improved on day 9, and a blood sodium concentration of 135 mmol/L was achieved on day 10. Signs of meningeal inflammation and bilateral lower extremity pain gradually disappeared by day 12. Bilateral nystagmus had resolved on physical examination on day 13. On day 21, blood CK and LDH were normal, CSF pressure was 90 mmH2O, WBC count was 38×106/L, monocyte content was 90%, total proteins was 95 mg/dL, and chloride was 117 mmol/L. The patient was discharged on day 30. On day 30, cranial MRI showed that lesions in the SCC almost disappeared. Abnormal signal intensity in bilateral periventricle white matter reduced. Contrast enhancement of the cerebral pia mater significantly decreased. The enhancement of the tentorium of right cerebellum mildly decreased (Figure 3). However, FLAIR MRI was not carried out, because the patient refused.
Characteristics of the three adult cases of MERS associated with EBV infection are outlined in Table 1.
Discussion
MERS typically presents as clinically mild encephalitis or encephalopathy, characterized by reversible splenial lesions with reduced diffusion on MRI, and a clinically benign course.2,8 MERS accounts for ~16% of all cases of encephalitis in children in Japan; 34% of these cases are associated with influenza virus infection9 and the remaining cases are associated with rotavirus infection, mumps vaccination, and other viruses.4,11,12 One pediatric case was associated with EBV, but with a normal CSF cell count.13 In a series of 54 children with MERS, the prodromal syndromes were fever, vomiting, diarrhea, and cough.7 The neurological symptoms occurred 1–3 days after prodromal syndromes in 71% of these patients. In another series of 30 children with MERS, hyponatremia occurred in 25 children.2 In a series of 22 children with MERS, decreased serum sodium was observed in 18 patients, with normal CSF.8
Compared with the literature, the present case reports suggest that MERS could be more severe in adults than in children. Among the rare adult case reports, we found two cases of EBV encephalitis,9,10 one of tick-borne encephalitis (CSF WBC 33/μL),13 one of anti-Yo rhombencephalitis,14 one of viral encephalitis of unknown etiology (CSF WBC 408/μL),15 one of influenza virus A encephalitis, one of HIV encephalitis, and some cases of MERS associated with systemic infections.11,16,17 Among these cases, CSF WBC count and protein level were both unremarkable. Alleviation of clinical symptoms and imaging changes were achieved within a short period of time.
Only four cases were reported as encephalitis with reversible splenial and deep cerebral white matter lesions associated with EBV. In the first case, a 21-year-old man presented with fever, somnolence, and disorientation.15 Laboratory tests showed CSF WBC count at 18/μL and protein levels at 1,500 mg/L. Cranial MRI showed focal splenial and temporal and occipital lesions. His medical condition improved with antiviral treatment. On day 21, MRI indicated disappearance of the lesions.15 In the second case, a 20-year-old man presented with fever, headache, consciousness, neck rigidity, CSF WBC count of 14/μL, and protein levels of 1,607 mg/L. The abnormal signal intensity in the SCC resolved after 16 days of treatment.16 An 8-year-old Chinese girl was admitted with generalized tonic–clonic seizures and mental deterioration following 1 day of prodromal symptoms consisting of severe headache with vomiting and high fever (40.0°C).17 CSF showed an abnormal cell count (125×106 cells/L, 75% lymphocytes) and protein level of 11 mg/dL. DWI revealed high signal intensities and widespread cortical and splenial lesions. She completely recovered with complete disappearance of the MRI lesions by day 28. Another 6-year-old patient with MERV was associated with EBV infection, but with a normal CSF cell count.13
In the present report, all patients were adults presenting with high fever, headache, apathy, and confusion, as well as significant signs of meningeal inflammation, but without epileptic seizure. These symptoms peaked 10–14 days after disease onset, with serious hyponatremia (112–129 mmol/L), an elevated CSF WBC count (80–380/mm3) (predominantly monocytes), and significantly increased protein levels (1,010–1,650 mg/dL). Cranial MRI indicated abnormal signal intensity in the SCC and symmetrically reversible lesions scattered in the thalamus and deep cerebral white matter. The clinical symptoms tended to improve after ~10–14 days of antiviral treatment. Clinical cure was achieved after ~3 weeks, and resolution of the lesions was observed within 3–14 weeks.
The altered states of consciousness and signs of meningeal inflammation noted in EBV-associated MERS in the present report and in the cases presented above seem more severe than in patients with non-herpes simplex virus encephalitis-associated MERS.9 In addition, these symptoms and signs observed in adults seem to be more severe and with prolonged duration compared with those of children with MERS.12
Among the three cases presented here, MRI of case 1 showed an isolated SCC lesion (type 1), while MRI of the two other cases showed extensive lesions in the deep cerebral white matter and entire corpus callosum with homogenously reduced diffusion (type 2). Deep cerebral white matter lesions were also observed in two other cases of EBV-associated MERS reported in the literature.15,16 Case 2 showed a type 2 lesion (symmetrical lesions in deep cerebral white matter) that appeared after the complete resolution of type 1 lesion (isolated SCC), which showed a different time course compared with a case reported by Takanashi et al.18
Conclusion
Encephalitis with reversible splenial and/or deep cerebral white matter lesions in adults is characterized by severe clinical conditions and altered states of consciousness, usually complicated by hyponatremia and urine retention. MERS can be associated with EBV infection and has a good prognosis with antiviral treatment. Complete resolution of clinical symptoms and features can be achieved, as revealed by MRI. When patients present with symptoms of MERS, special attention should be given to serological or PCR testing of possible pathogens, in particular EBV.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81301032) and China Scholarship Council, Liujinfa (grant number [2016]3035: 201608110052). The funding sources had no role in the study design, data collection, and analysis.
Written informed consent for publication of this case series was obtained from patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63:1854–1858. | ||
Takanashi J, Tada H, Maeda M, Suzuki M, Terada H, Barkovich AJ. Encephalopathy with a reversible splenial lesion is associated with hyponatremia. Brain Dev. 2009;31:217–220. | ||
Kosami K, Kenzaka T, Sagara Y, Minami K, Matsumura M. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion caused by methicillin-sensitive Staphylococcus aureus bacteremia with toxic shock syndrome: a case report. BMC Infect Dis. 2016;16:160. | ||
Takanashi J, Shiihara T, Hasegawa T, et al. Clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. J Neurol Sci. 2015;349:226–228. | ||
Pan JJ, Zhao YY, Lu C, Hu YH, Yang Y. Mild encephalitis/encephalopathy with a reversible splenial lesion: five cases and a literature review. Neurol Sci. 2015;36:2043–2051. | ||
Ka A, Britton P, Troedson C, et al. Mild encephalopathy with reversible splenial lesion: an important differential of encephalitis. Eur J Paediatr Neurol. 2015;19:377–382. | ||
Takanashi J. Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev. 2009;31:521–528. | ||
Kashiwagi M, Tanabe T, Shimakawa S, et al. Clinico-radiological spectrum of reversible splenial lesions in children. Brain Dev. 2014;36:330–336. | ||
Lin FY, Yang CY. Reversible splenial lesion of the corpus callosum in migraine with aura. Neurologist. 2011;17:157–159. | ||
Chacko J, Pramod K, Sinha S, et al. Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: insights into possible pathogenesis. Neurol India. 2011;59:743–747. | ||
Ko SY, Kim BK, Kim DW, et al. Reversible splenial lesion on the corpus callosum in nonfulminant hepatitis A presenting as encephalopathy. Clin Mol Hepatol. 2014;20:398–401. | ||
Hoshino A, Saitoh M, Oka A, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 2012;34:337–343. | ||
Hakyemez B, Erdogan C, Yildirim N, Gokalp G, Parlak M. Transient splenial lesion of corpus callosum associated with antiepileptic drug: conventional and diffusion-weighted magnetic resonance images. Acta Radiol. 2005;46:734–736. | ||
Garcia-Monco JC, Martinez A, Brochado AP, Saralegui I, Cabrera A, Beldarrain MG. Isolated and reversible lesions of the corpus callosum: a distinct entity. J Neuroimaging. 2010;20:1–2. | ||
Hagemann G, Mentzel HJ, Weisser H, Kunze A, Terborg C. Multiple reversible MR signal changes caused by Epstein–Barr virus encephalitis. AJNR Am J Neuroradiol. 2006;27:1447–1449. | ||
Takeuchi S, Takasato Y, Masaoka H. Epstein–Barr virus encephalitis with a reversible splenial lesion. Intern Med. 2012;51:341–342. | ||
Zhang S, Feng J, Shi Y. Transient widespread cortical and splenial lesions in acute encephalitis/encephalopathy associated with primary Epstein–Barr virus infection. Int J Infect Dis. 2016;42:7–10. | ||
Takanashi J, Imamura A, Hayakawa F, Terada H. Differences in the time course of splenial and white matter lesions in clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). J Neurol Sci. 2010;292:24–27. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.