Back to Journals » International Journal of Nanomedicine » Volume 18
Emulsion Technology in Nuclear Medicine: Targeted Radionuclide Therapies, Radiosensitizers, and Imaging Agents
Authors Winuprasith T , Koirala P , McClements DJ, Khomein P
Received 11 April 2023
Accepted for publication 19 July 2023
Published 3 August 2023 Volume 2023:18 Pages 4449—4470
DOI https://doi.org/10.2147/IJN.S416737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Thunnalin Winuprasith,1 Pankaj Koirala,1 David J McClements,2 Piyachai Khomein3
1Institute of Nutrition, Mahidol University, Nakhon Pathom, 73170, Thailand; 2Department of Food Science, University of Massachusetts Amherst, Amherst, MA, 01003, USA; 3Division of Nuclear Medicine, Department of Radiology, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
Correspondence: Piyachai Khomein, Division of Nuclear Medicine, Department of Radiology, Faculty of Medicine, Chulalongkorn University, 1873 Rama 4 Road, Pathumwan, Bangkok, 10330, Thailand, Email [email protected]
Abstract: Radiopharmaceuticals serve as a major part of nuclear medicine contributing to both diagnosis and treatment of several diseases, especially cancers. Currently, most radiopharmaceuticals are based on small molecules with targeting ability. However, some concerns over their stability or non-specific interactions leading to off-target localization are among the major challenges that need to be overcome. Emulsion technology has great potential for the fabrication of carrier systems for radiopharmaceuticals. It can be used to create particles with different compositions, structures, sizes, and surface characteristics from a wide range of generally recognized as safe (GRAS) materials, which allows their functionality to be tuned for specific applications. In particular, it is possible to carry out surface modifications to introduce targeting and stealth properties, as well as to control the particle dimensions to manipulate diffusion and penetration properties. Moreover, emulsion preparation methods are usually simple, economic, robust, and scalable, which makes them suitable for medical applications. In this review, we highlight the potential of emulsion technology in nuclear medicine for developing targeted radionuclide therapies, for use as radiosensitizers, and for application in radiotracer delivery in gamma imaging techniques.
Keywords: emulsions, nanoemulsions, nanoparticles, radiosensitizers, radiopharmaceuticals
Graphical Abstract:
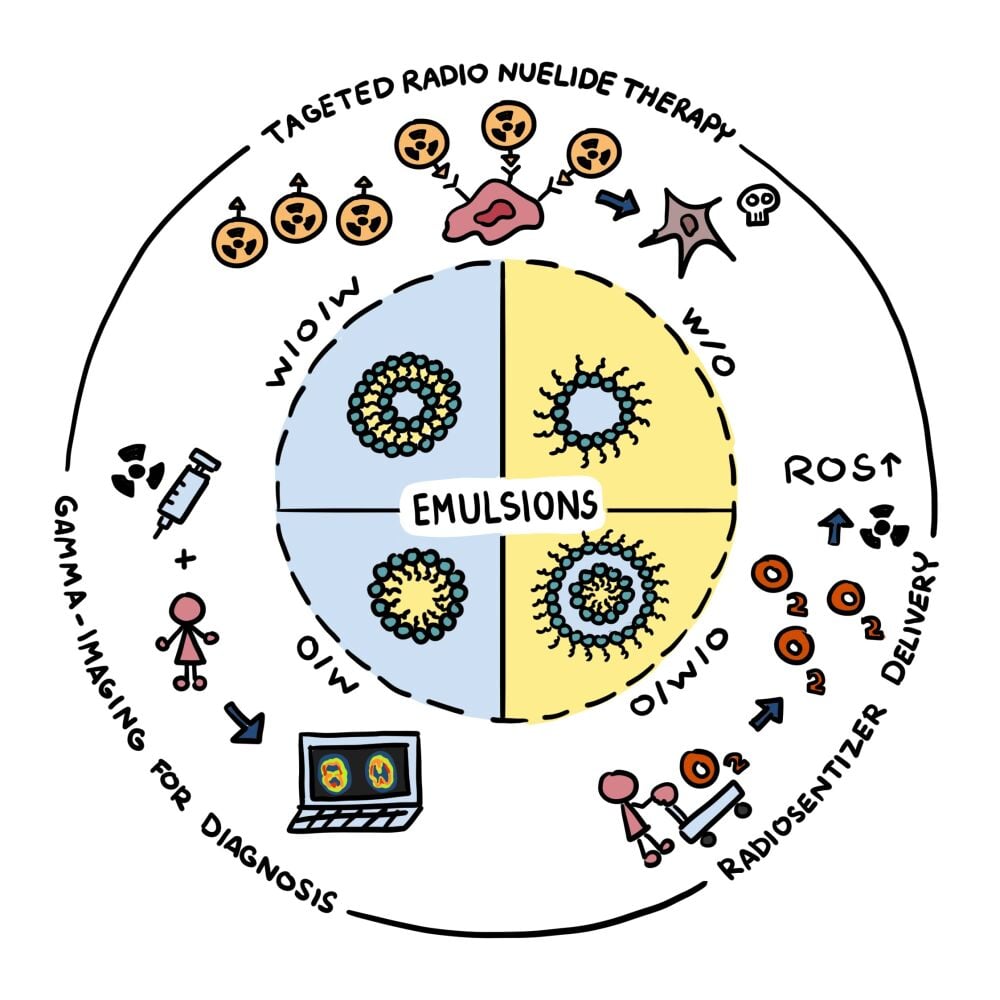
Introduction
Traditionally, nuclear imaging, nuclear therapy, and radiopharmaceutical technologies were mainly used in research laboratories. However, they are now routinely used in clinical practice for diagnosis, prognosis, and prediction of various diseases, especially in oncology,1,2 cardiology,3,4 neurology,5 and infectious and inflammatory disorders.6 This change can be attributed to advances in nuclear imaging technologies for improving image quality and spatial resolution,7 as well as the development of more economically feasible ways of preparing and measuring radiopharmaceuticals.8 Furthermore, traditional radionuclides such as technetium-99m (Tc-99m), iodine-131 (I-131), and fluorine-18 (F-18) have now been complemented with newly approved ones, ranging from alpha (α), beta (β-), or Auger electron-emitting radionuclides such as actinium-225 (Ac-225), lutetium-177 (Lu-177), copper-67 (Cu-67), to positron (β+) or gamma (γ)-emitting radionuclides such as Cu-64, zirconium-89 (Zr-89), and I-123. These rapid advancements in nuclear medicine have brought about new opportunities for the next generation of radiopharmaceuticals, particularly in targeted radionuclide therapy (TRT).9,10
TRT is a type of radiotherapy in addition to the more common ones: external beam radiation therapy (teletherapy) and internal radiation therapy (brachytherapy). TRT is similar to brachytherapy in the way that the radiation is from a source located inside the body, but the way that the radiation source is introduced to the target site is different. Brachytherapy requires a special instrument to insert the radioactive source in or near the target tissue. On the other hand, TRT utilizes radioactive sources linked to targeting-molecules to guide them to the target tissue. These therapeutic agents are called radiopharmaceuticals and can be taken orally or parentally with no requirement for delivery devices with needles, catheters, or applicators. TRT is therefore less invasive than brachytherapy. Moreover, TRT also has several other advantages when it comes to metastases, including simultaneous treatment of multiple metastatic foci, repeatability, and the ability to be coupled with other treatments.11 Many radiopharmaceuticals are already being used in nuclear medicine to treat tumors such as thyroid, lymphomas, or bone metastases, but these substances are also being investigated for treating other types of tumors.12
In general, ionizing radiation (such as α particle, β- particle, and Auger electrons) is capable of killing any type of cell. However, in radionuclide therapy it is beneficial to target specific cells (eg, cancer cells) and avoid other cells (eg, healthy cells). For example, I-131 has been used to specifically treat hyperthyroidism and thyroid cancer since this radionuclide is preferentially taken up by thyroid tissue and I-131 distributed in other non-target tissues is removed mainly through the bladder. Thus, drinking lots of fluid and urinating frequently will help minimize the side effects of this treatment. Unfortunately, most radionuclides do not exhibit active uptake like I-131 and cannot selectively accumulate in tumor sites. Consequently, there has been great interest in the development of delivery systems for TRT.
A common strategy for improving target localization is to form a complex between a radionuclide metal ion and a targeting ligand. This method has been widely used due to the simple protocol involved in forming these complexes. Indeed, in some cases, the radioactive metal and desired ligand can simply be mixed together to form a complex. Currently, there are four radionuclide complexes that have been approved by the US Food and Drug Administration (FDA) for treating cancers: samarium-153 (Sm-153) lexidronam (Quadramet®) for pain relief in patients with confirmed osteoblastic metastatic bone cancer, lutetium-177 (Lu-177) Dotatate (Lutathera®) for somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors, Lu-177 PSMA for prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer, and yttrium-90 (Y-90) ibritumomab tiuxetan (Zevalin®) for treatment of relapsed or refractory, low-grade or follicular B-cell non-Hodgkin’s lymphoma (NHL). Some of the main challenges with the radionuclide-ligand complex system are its stability, non-specific interactions leading to off-target localization, and its versatility since the system is designed for a certain kind of radionuclide and may not be adapted to other radionuclide systems.
The utilization of carrier-based targeted drug delivery is well established in the field of nanomedicine including for radiopharmaceuticals.13,14 Carriers consisting of nanoemulsions, microemulsions, liposomes, micelles, polymersomes, inorganic nanoparticles, dendrimers, and hydrogels have all been explored for this purpose. Most of the particles in these carrier systems can be modified with specific functional groups to improve their specificity toward targets, weaken their non-specific interactions with off-targets, and prolong their blood circulation times for improving cellular uptake efficiency.15,16 As an example, liposome-based delivery systems have been used to protect mRNA from enzyme degradation and to deliver the payload to cells via endocytosis, which have been used globally in COVID vaccines. In nuclear medicine, liposomes have also been applied to deliver rhenium-188 (Re-188) for the treatment of primary solid tumors in advanced or metastatic stages. A preclinical study using this technology demonstrated a higher intratumoral retention compared to normal organs.17 The enhanced permeability and retention (EPR) effect which is a unique feature of primary solid tumors18 improves the accumulation of macromolecules and nanoparticles, like liposomes. However, this system failed in a clinical study due to concerns about the accumulation of radioactivity in the liver and spleen (NCT02271516). This off-target accumulation is a major issue for the development of therapeutic radiopharmaceuticals. The high accumulation in both liver and spleen is due to the presence of the mononuclear phagocytic system (MPS), which was previously known as the reticuloendothelial system (RES). These organs have similar features to solid tumors like dense blood vessels and fenestrated capillaries. Phagocytes are present, which usually help clearing foreign particles such as bacteria or dust, but in this case, they also capture drug-loaded particles. Recently, a novel approach for evading MPS has been developed,19 which involves incorporating esterase-labile molecule-containing radionuclide (I-124/131) into polyethylene glycol (PEG)-containing liposomes. Since esterases are normally present in healthy cells but absent in tumors, the liposomes taken up in the liver and spleen will no longer have radionuclides attached. The results of this study demonstrated highly specific tumor uptake and with only low levels of radioactivity being detected in the bladder and none in the liver and spleen. Other kinds of delivery systems have also been used for this purpose. For example, radium-224 (Ra-224) has been encapsulated within calcium carbonate microparticles (Radspherin®) for targeting metastases in body cavities. Currently, this drug is under clinical trial (NCT03732768) for the investigation of recurrent ovarian cancer patients with peritoneal carcinomatosis. The potential of using nanoparticle-based systems for the delivery of radionuclides has recently been reviewed.13 Moreover, the use of polymeric nanoparticles for this purpose has also been reviewed.14 However, no reviews have previously focused on the utilization of emulsion technology to create colloidal delivery systems for this purpose.
Emulsion-based delivery systems have a number of potential advantages over many other colloidal delivery systems. For instance, they tend to be easier and cheaper to prepare in large volumes than liposome-or polymersome-based systems, as well as being more resistant to changes in environmental conditions than liposome. Non-toxic and non-irritant components can be utilized to formulate emulsions, such as oils and emulsifiers. Moreover, they can be designed to increase the dispersibility, stability, bioavailability, and bioactivity of drugs, as well as to include both hydrophilic and lipophilic drugs in the same formulation.20 Emulsion technology has been widely studied for fluorescence, magnetic resonance, ultrasound, and nuclear imaging applications.21–23 In cancer therapy, emulsion technology has also been utilized for targeted drug delivery applications, since the surfaces of the emulsifier-coated oil droplets can be decorated with specific ligands.24 Studies have shown that emulsions are safe and can deliver drugs to target tissues. For instance, encapsulating docetaxel, an anticancer drug for treating breast, lung, prostate, head, neck, and gastric cancers, in emulsions was shown to increase its efficacy and reduce its toxicity.25 The promising results found in other areas of medicine suggest that emulsion-based systems may also be suitable in the field of nuclear medicine. Indeed, several of these systems have already shown promise in improving the efficacy, stability, and targeting of radiopharmaceuticals to specific tissues or organs. This high degree of specificity enhances the diagnostic and therapeutic efficacy of radiopharmaceuticals, as well as reducing the dose required for treatment, thereby reducing the exposure of patients to radioactivity. Moreover, combining radiosensitizer and TRT technologies using emulsion-based carriers may have synergistic effects, leading to better therapeutic outcomes.26
The aim of this review was therefore to highlight recent advances in emulsion technology in nuclear medicine, with a focus on their applications as TRT, radiosensitizer, and imaging tools. In addition, the introduction of emulsion concepts will be briefly described prior to those sections providing the fundamental understanding of this emerging technology in nanomedicine applications.
Emulsions as Drug Delivery Systems
Emulsions are colloidal systems that consist of two or more immiscible liquids, with at least one of them being present as small droplets dispersed in the other. Emulsion-based delivery systems have the potential to improve the dispersibility, stability, bioavailability, pharmacokinetic profile, and bioactive of medicines that normally have poor absorption profiles, thereby enhancing their therapeutic efficacy in pharmaceutical settings.27–29 Furthermore, emulsion-based delivery systems can reduce the adverse side effects experienced by some patients after drug administration.30 Encapsulation of drugs in emulsion-based delivery systems may also improve their stability during storage and passage through the gastrointestinal tract.31 This section provides an overview of the properties of emulsion-based delivery systems, their compositions, and formulation techniques that can be used for nanomedicine applications. The main factors impacting their application in drug and nanomedicine delivery are also discussed.
Types of Emulsions Used in Drug Delivery
Emulsions contain dispersed (internal) and continuous (external) phases, with the dispersed phase being the droplets and the continuous phase being the surrounding fluid (Figure 1). Typically, the immiscible liquids used to formulate emulsions are oils and water. Emulsions are typically classified according to the relative location of the different phases. Oil-in-water emulsions (O/W) consist of oil droplets dispersed in water, whereas water-in-oil emulsions (W/O) consist of water droplets dispersed in oil. More sophisticated emulsions can also be prepared for certain applications, such as water-in-oil-in-water (W1/O/W2) or oil-in-water-in-oil (O1/W/O2) double emulsions. Typically, the droplets in emulsions are spherical because the droplet dimensions are so small that the Laplace pressure (which aims to minimize the contact between oil and water) is relatively large. Different kinds of emulsions may be used to encapsulate drugs with different polarities. Lipophilic drugs or conjugates such as oleic acid-platinum II,32 Gd-labeled DTX,33 and so on are usually encapsulated inside the oil droplets in O/W emulsions. In contrast, hydrophilic drugs are usually encapsulated within the water droplets in W/O or W1/O/W2 emulsions.
Despite their long history, Pickering emulsions (PEs) have only gained considerable attention as drug delivery systems over the past two decades, which can be attributed to their potential advantages over conventional emulsions for certain applications. Rather than being stabilized by conventional molecular emulsifiers, PEs are normally stabilized by solid or semi-solid particles. The accumulation of these particles at the oil-water interface provides strong protection against droplet coalescence, while their aggregation in the continuous phase can lead to the formation of a particle network that provides elastic-like properties and inhibits gravitational separation. Many kinds of inorganic and organic particles have been utilized as Pickering emulsifiers, including food-grade polysaccharides and proteins. One of the main advantages of using particle-based emulsifiers in emulsions is that they can be assembled from sustainable resources that are biodegradable and low in toxicity, and their properties are tunable properties, such as their wettability, charge, porosity, and responsiveness.34 Pickering emulsifiers are less prone to diffusion and migration from one interface to another than small-molecule surfactants, which may help to increase the stability of double emulsions by forming a robust and rigid interfacial film.35,36
Nanoemulsions are emulsions that are characterized by having droplet diameters below a few hundred nanometers. The small size of the droplets in nanoemulsions makes them an attractive platform for some drug delivery applications because of their good stability, penetration, and rapid release properties.37,38 Moreover, the surfaces of the droplets in nanoemulsions can be decorated with specific ligands, which can enhance the targeted delivery and efficacy of chemotherapeutic treatments, such as platinum-related therapies.37 For example, Natesan et al38 reported that chitosan-stabilized camptothecin nanoemulsions had uniform particle size distributions, prolonged drug release characteristics, significant cytotoxicity against MCF-7 cancer cells, and low DNA damage to lymphocytes, highlighting their potential for application in chemotherapy. Nevertheless, it is important to optimize the formulation and formation of the nanoemulsions to ensure they are safe and efficacious.
Emulsion Composition
The composition of emulsion-based delivery systems should be optimized for each application. The main functional components used to assemble emulsions are oil, water, and emulsifier, but other ingredients may also be required, such as thickeners, stabilizers, and preservatives. For food and drug applications, all ingredients need to be biocompatible with minimal or no toxicity. The selection of an appropriate oil phase depends on the solubility and loading capacity of the drug, as well as the digestibility and chemical stability of the oil. Several kinds of oils can be used to formulate emulsions, including triglycerides, free fatty acids, mineral oils, essential oils, and pharmaceutical grade oils.39 Commonly, soybean oils are used in the preparation of pharmaceutical emulsions, although other triglyceride oils may also be used including sesame, sunflower, cottonseed, rice bran,40 olive oil,41 and flaxseed oil.37 The oils may be used individually or in combination.
The types and proportions of oils used impact the formation, stability, and functionality of emulsions.42 Oils with high polarities, such as essential oils, often lead to the formation of unstable emulsions because they are prone to Ostwald ripening due to diffusion of the oil molecules from small to large oil droplets. This process can be inhibited by adding a ripening inhibitor, which is usually a strongly hydrophobic substance with a very low water solubility, like a long-chain triglyceride. Indeed, some studies have shown that oil polarity has a significant effect on the stability and rheology of emulsions stabilized by polymeric surfactants.43 Oils with higher viscosities tend to produce large oil droplets during homogenization. For instance, one study reported that the degree of unsaturation and chain length of triglyceride oils influenced the droplet size of drug-loaded nanoemulsions, with long chain triglycerides (LCTs) giving larger droplets than medium chain triglycerides (MCT), which was mainly attributed to the higher viscosity of the LCTs.44,45
The presence of other components in an emulsion, especially emulsifiers, also influence the interfacial tension between the oil and water phases. The ability of emulsifiers to reduce the interfacial tension is critical for the formation of emulsions, as it facilitates the disruption of droplets during homogenization, thereby leading to smaller droplets being formed. Moreover, the emulsifier coatings around droplets improves the stability of emulsions by inhibiting droplet aggregation. They typically do this by generating strong steric and/or electrostatic repulsive interactions between the droplets, which depends on the thickness and charge of the adsorbed interfacial layer. When choosing an appropriate emulsifier, it is important to consider its impact on the formation, stability, functionality, pharmacokinetics, and toxicity of the delivery system. Emulsifiers such as egg yolk or soybean lecithin are commonly used in nanoemulsions for drug delivery.37 According to Singh et al40 and Gubbiyappa,46 other kinds of emulsifiers commonly used in these kinds of emulsions, include: hydrophilic surfactants (sodium deoxycholate, Cremophor EL, Lauroglycol, Labrasol, Tween, Solutol HS-15, Kolliphor®); hydrophobic surfactants (Span 20, 40, 60, and 80); amphiphilic proteins (β-lactoglobulin, whey protein, and casein); amphiphilic polysaccharides (gum Arabic and starch derivatives), and amphiphilic synthetic polymers (polyethylene glycol or PEG).
Nanoemulsions that are optically clear can be formed by using combinations of emulsifiers and homogenization conditions that produce sufficiently small droplets (d < 50 nm). Typically, an emulsifier that rapidly adsorbs to oil-water interfaces and greatly reduces the interfacial tension is suitable for forming this type of nanoemulsion. Small molecule surfactants are often the most suitable for this purpose. In some cases, nanoemulsions containing small droplets are produced by combining surfactants with co-surfactants or co-solvents, as together they are more effective at reducing the interfacial tension and producing small droplets during homogenization. Some of most commonly used co-surfactants and co-solvents for the formation of nanoemulsions are propylene glycol, polyethylene glycol (PEG), diethylene glycol monoethyl ether (transcutol-HP), ethylene glycol, ethanol, glycerin, Pluronic F-127, and propanol.40,47 The selection of an appropriate surfactant and co-surfactant/co-solvent combination requires an understanding of their polarity and solubility characteristics. For instance, in a pine oil-based emulsion, pine oil was immiscible with PEG 200 or 300 because of insufficient hydroxyl (OH) groups on the PEG moiety. PEG 400, on the other hand, was miscible under the same conditions due to the presence of sufficient OH groups.48 Consequently, it was more suitable for formulating nanoemulsions with pine oil.
The shelf-life and safety of emulsion-based formulations are often enhanced by adding preservatives. For instance, protein-derived ingredients within an emulsion formulation are susceptible to microbial decay. Consequently, antimicrobial preservatives are required to prevent their degradation during storage. Some of the most common preservatives used in emulsion formulations are benzoic acid, potassium sorbate, sorbic acid, propionic acid, dehydroacetic acid, and chlorobutanol. Ideally, these preservatives should exhibit a broad spectrum of antibacterial activity, low toxicity, good heat resilience, good matrix compatibility, low cost, high consistency, and good ease of use.
Formulation Techniques
In general, emulsions and nanoemulsions can be fabricated using either high-energy or low-energy methods.
High Energy Methods
Typically, high-energy homogenization methods use mechanical devices capable of generating intense disruptive forces, such as shear, cavitation, and/or turbulent forces. The most commonly used mechanical devices for producing nanoemulsions are high-pressure valve homogenizers, microfluidizers, and sonicators. The main advantages of these high-energy methods are that they can economically produce large quantities of nanoemulsions, and that they can be used to produce nanoemulsions from a broad range of oils and emulsifiers. The size of the droplets produced depends on the type and amount of oil and emulsifier used, the physicochemical properties of the oil and water phases (such as their viscosity and interfacial tension), the type of homogenizer used, and the homogenization conditions. For example, for high-pressure valve homogenizers and microfluidizers, the droplet size typically decreases with increasing operating pressure and number passes,49 whereas for sonicators, it typically decreases with increasing sonication time and intensity.50 However, the droplet size also depends on the type and amount of ingredients used to formulate a nanoemulsion. For instance, the droplet size tends to decrease with increasing emulsifier concentration, increasing emulsifier adsorption rate, decreasing interfacial tension, and decreasing viscosity ratio between the oil and water phases. The main drawbacks of high-energy homogenization methods are the need to purchase and maintain the mechanical devices to generate the disruptive forces, which can be relatively costly.
Low Energy Methods
Low-energy homogenization methods rely on the spontaneous formation of small droplets when system conditions such as temperature or composition are changed in a controlled fashion. Typically, this approach can only be used with a limited number of synthetic surfactants, and so it is much less versatile than high-energy homogenization methods. On the other hand, there is no need to purchase and maintain a mechanical homogenizer. Low-energy homogenization methods can be conveniently categorized as isothermal or thermal methods. Spontaneous emulsification is an example of an isothermal methods because it can be carried out at a fixed temperature, whereas the Phase Inversion temperature approach is an example of a thermal method because it requires that the temperature of the sample to be changed. Isothermal methods are particularly suitable for encapsulating heat sensitive bioactive components, whereas thermal methods are suitable for forming solid lipid nanoparticles (SLNs) or nanostructured lipid carriers (NLCs). SLNs and NLCs are formed by creating a nanoemulsion at a high temperature and then cooling to fully or partially crystallize the lipid phase, respectively. Low-energy methods provide several advantages over high-energy ones, including lower energy consumption, greater efficiency, and the absence of the need for sophisticated mechanical homogenizers.
Formulation Optimization
Numerous physicochemical and structural parameters impact the in vivo behavior of emulsions, which must be optimized to ensure good functional performance. In this section, we therefore briefly discuss some of the most important factors that must be considered when formulating emulsion-based nanomedicines designed for diagnostic or therapeutic purposes.
Size
In addition to the structural organization of emulsions (O/W, W/O, or W/O/W), the size of the droplets in emulsions is also an important characteristic for drug delivery applications. The size of the droplets in nanoemulsions influences their physical stability and biological fate, as well as the chemical stability and release kinetics of any active agents encapsulated inside them. Typically, smaller droplets are more resistant to aggregation and gravitational separation, exhibit greater penetration into tissues, and are digested more rapidly. However, small droplets may lead to a rapid release profile due to their small internal dimensions. Consequently, it is important to optimize the droplet size for specific applications.
The droplet size of nanoemulsions can be manipulated by controlling various factors, including homogenizer type and operating conditions (intensity and duration), system composition (oil, surfactant, and co-surfactant type and concentration), and oil and water properties (viscosities and interfacial tensions).42,51 Controlling the droplet size is also important because it can influence the cellular uptake of drugs. For instance, Jiang, et al52 reported that 50 nm oil droplets had a higher cellular absorption than 60 or 20 nm ones, which suggests that there is an intermediate size range for optimum uptake. This may be because smaller or larger particles are removed by renal excretion or reticuloendothelial systems. The mechanism of cellular absorption also depends on droplet size.53 For example, particles smaller than about 200 nm tend to be taken up via pinocytosis pathways, whereas particles larger than 250 nm tend to be taken up by phagocytosis. The cellular uptake of emulsion droplets with diameters close to a micron is also determined by non-receptor-mediated micropinocytosis.54 Compared to polydisperse emulsions, monodisperse ones tend to have more reproducible and predictable biological behaviors.55 Consequently, it is often important to control both the mean size and polydispersity of the droplets in emulsions.
Surface Properties
The surface properties of the droplets in emulsions influence their stability and functional performance, as well as their interactions with biological tissues. After an emulsion containing drug-loaded droplets is administered, amphiphilic substances in the biological fluids (especially proteins) tend to adsorb to the droplet surfaces and form a new coating, which is referred to as the biocorona. These amphiphilic substances may fully or partially displace the original emulsifiers from the droplet surfaces, or they may adsorb on top of them. The tendency for a biocorona to form is largely determined by the surface characteristics of the emulsion droplets, such as surface chemistry, hydrophobic, and charge. The formation of a biocorona influences the stability, transportation mechanism, circulation half-life, and intracellular distribution of drug-loaded emulsions.30 The potential importance of this effect has been demonstrated for poly(ε-caprolactone) nanoparticles.56 The nature of the electrical characteristics on these nanoparticles influenced their electrostatic interactions with proteins, thereby impacting their ability to inhibit tumor cells. It was found that the surface charge should be kept as low as possible to minimize opsonization, ie, the absorption of protein molecules from the biological fluids. Consequently, when developing emulsion-based drug delivery systems it is important to carefully control the surface characteristics of the droplets they contain.
Excipients
The functional performance of emulsion-based drug delivery systems can often be improved by including excipients, which are substances that are not bioactive themselves but increase the efficacy of the drug. To meet FDA guidelines, these excipients should be non-toxic, biocompatible, and readily solubilize the targeted drug. In emulsions, excipients may be oils, surfactants, co-surfactants, co-solvents, or other substances. Typically, the oil phases are obtained from plant-based oils (triacylglycerols) that contain fatty acids with different chain lengths and degrees of unsaturation. Numerous surfactants have been approved for use as excipients in the pharmaceutical industry, which may be natural (like egg or soy lecithin) or synthetic (like Spans and Tweens). The selection of an appropriate surfactant plays a crucial role on emulsion properties.
Administration Routes
Based on the nature of the drugs used and the disease type, different routes are available for the administration of drugs. Intravenous is commonly used for the administration of a wide range of drugs. The advantage over other administration routes is the rapid drug action due to direct drug entry into systemic circulation. However, some drugs given by injection are chemically unstable and promote vascular irritation, which is undesirable. For instance, intravenous injection of Clopidogrel, a drug used to treat acute thrombosis, causes irritation.57,58 However, oral administration of this drug led to delayed and limited absorption, which reduced its effectiveness. Such drawbacks can be overcome by identifying the most appropriate administration route and by utilizing emulsion technology. Abd-Elhakeem et al59 developed solid self-nanoemulsifying drug delivery systems (S-SNEDDS) for oral administration of Clopidogrel. They reported a nine-fold increase in Clopidogrel bioavailability for the emulsions compared to a conventional formulation. In another study, Chen et al58 developed injectable clopidogrel-loaded nanoemulsions to enhance antiplatelet aggregation effects and overcome the limitations of the current clopidogrel aqueous formulation. These examples demonstrated the versatility of emulsion-based delivery systems for the enhancement of drug delivery on different administration routes.
Emulsion Technology in Nuclear Medicine
Nuclear medicine is a sub-branch in radiology that specializes in the application of radionuclides for diagnoses, evaluation, and treatment of various diseases. In this section, we discuss advances in the utilization of emulsion technology in nuclear medicine, focusing on targeted radionuclide therapy, radiosensitizer, and imaging agents.
Emulsion-Based Targeted Radionuclide Therapies
Major advances in targeted radionuclide therapy (TRT) against cancer have been made over the past few decades.12,60 Indeed, seven types of radiopharmaceuticals have already been approved by the FDA. However, there are some limitations of these radioactive substances, especially their non-targeted accumulation. This can cause a decrease in their efficacy, as well as undesirable side effects because the radionuclides can harm healthy parts of the body. Improvements in targeted radionuclide delivery would lead to higher efficacy TRT. As mentioned earlier, nanotechnology is increasingly being utilized for targeted drug delivery,61 including the delivery of radionuclides. Many carrier systems have been utilized in nuclear medicine in an attempt to realize the high efficacy and specificity in TRT, including liposomes,62 dendrimers,63 polymeric nanoparticles,14 metal oxide-based nanoparticles,64 and metal-based nanoparticles.65 The small oil droplets in O/W emulsions or nanoemulsions can also be used for this purpose, which may have some advantages over other colloidal delivery systems as radionuclide carriers. For instance, emulsions can be created from biocompatible materials using rapid and simple preparation methods. Moreover, the composition, size, charge, and surface properties of the droplets can easily be controlled by varying the formulation and processing conditions used to prepare them. The previous section provided a brief background on various emulsion systems, as well as their application as drug carriers. Many studies have shown that emulsion-based delivery systems can enhance drug localization at a target site, and improving pharmacokinetic profiles.66 Similar systems and concepts can be directly adapted for radionuclide delivery. In the remainder of this section, some of the most recent developments of TRT using emulsion technology are discussed.
An oil-in-water emulsion loaded with the radionuclide I-131 is the first example of an emulsion-based delivery system for TRT, which was developed to fight against hepatocellular carcinoma (HCC), the second most deadly cancer globally.67 The lipid phase in this emulsion was Lipiodol®, which is the trademark name of poppy seed oil that has been iodinated and ethylated. This lipid was developed by a healthcare company (Guerbet) and has been used clinically in a bulk or emulsified form for diagnosis and therapy of HCC, such as radiopaque, chemoembolization, and radioembolization. Since Lipiodol® already has iodide in its structure, I-131 can be simply labelled on the lipid via isotropic exchange reaction.68 I-131- Lipiodol® is the first radiopharmaceutical employed for trans-arterial radioembolisation (TARE) due to its selective deposition on the nodules of hepatocellular carcinoma.69 It was shown to have treatment efficiency similar to chemoembolization but with less morbidity.70 Bio-distribution data showed that there was a high accumulation (>75%) of I-131 Lipiodol® after arterial administration to the liver, with the remainder mainly accumulating within the lung.71 Several cases of lung disease in patients treated with this radiopharmaceutical were reported after its use,72 which caused this drug to be discontinued in 2010.
Emulsion technology was employed in an attempt to reduce the accumulation of I-131- Lipiodol® in the lung by controlling the droplet size and type of emulsion used. Baere et al73 evaluated the distribution of I-125 Lipiodol® and its emulsified forms in healthy liver, liver tumor, and lung in rabbits. I-125 was used in this work rather than I-131 because it emits soft x-ray and gamma with a maximum energy of 35 KeV, which is much lower than I-131, thereby reducing radiation exposure during the study. Emulsions where the majority of the droplets were relatively small (>70% with 20–30 μm in diameter) or relatively large (>70% with 70–100 μm in diameter) were prepared by varying the homogenization method used. After 4 days of the hepatic artery injection, all three tissues were harvested, and their radioactivity was recorded. The accumulation in the lung was clearly decreased in all emulsified forms compared to pure I-125 Lipiodol®. Interestingly, water-in-oil emulsions displayed a significantly higher liver tumor uptake than oil-in-water emulsions, especially for the larger droplet sizes. The authors proposed that this phenomenon was because the oil in the water-in-oil emulsions was the continuous phase, thereby providing larger oily emboli than the emulsifier oil in the oil-in-water emulsions, which were trapped in the tumor vessels. However, if that was the case, then the pure I-125 Lipiodol® with a single oil phase should also have had similar or better accumulation in the tumor. In practice, however, the effect of the pure oil was similar to that of the oil-in-water emulsion and lower than that of the water-in-oil emulsion. Thus, these results suggest that water-in-oil emulsions do have potential to boost the efficacy of TARE using I-131 but the mechanism of action still needs to be elucidated. The physical stability of the emulsions used in this study is a major concern because no stabilizer was used in these formulations. As a result, the emulsions would have to be freshly prepared prior to each injection. This is inconvenient for clinical routine, especially for a high-energy radionuclide like I-131 due to radiation exposure concerns.
Garin et al74 developed emulsions stabilized using polyethylene glycol (PEG)-40 hydrogenated castor oil (Cremophor rh 40) as an emulsifier and studied their bio-distribution in HCC-induced rats. In this study, Tc-99m-SSS was used instead of I-131 to reduce radioactivity concerns. Also, it has similar properties to Re-188, another potential radionuclide for TRT. Tc-99m-SSS is a lipophilic compound that was dissolved in the oil phase, rather than being covalently attached to the Lipiodol®. The stability of the emulsion was successfully improved with no significant change in their morphology up to 24 hr. Unfortunately, the bio-distribution results showed a poor uptake ratio between the liver and the tumor for all emulsion systems, which indicated a decrease in the Lipiodol® selectivity toward liver tumors. The main explanation given for this phenomenon was that PEG-40 was present on the surfaces of the oil droplets in the emulsions, which reduced the biological activity of Lipiodol®. In addition, accumulation of Tc-99m-SSS Lipiodol® in the lung was significantly increased compared to the control. This effect was attributed to reduced accumulation in the liver, which increased the concentration of free Tc-99m-SSS Lipiodol® availability. In addition, the emulsions containing smaller droplets (< 20 μm) were found to enhance the pulmonary uptake, as suggested by the previous study.73
Although the results of the previous study demonstrated the potentially adverse effects of certain kinds of emulsifiers on the efficacy of radionuclides, they also showed that surface modification of emulsion droplets could alter their biological fate, which may be useful for designing delivery systems with targeted and/or controlled release properties. For instance, Luo et al75 developed Re-188 Lipiodol® emulsions with thermal responsive characteristics and evaluated their therapeutic efficacy in a HCC-induced rat model (Figure 2). Re-188 is an emerging and promising radionuclide for theranostic applications since it can emit both β and γ. The relatively low energy (155 keV) of γ emission improves the radiation safety of Re-188 over I-131, while still being sufficient for imaging and diagnostic purposes. Re-188 is available as perrhenate salts dispersed in aqueous media, which are incompatible with oily Lipiodol®. Thus, a lipophilic ligand is required to ensure good mixing of these two components. N,N’-1,2-ethanediylbis-L-cysteine diethyl-ester dihydrochloride (ECD) is a lipophilic ligand used with Tc-99m for clinical brain perfusion. Combining Re-188 ECD with Lipiodol® (Re-EL) has been demonstrated by the same research group.76 A high accumulation and effective treatment of xenotransplanted liver tumors in a rat model was observed, which demonstrated the promising potential of this radiopharmaceutical. To improve the emulsions stability, yet maintain its biological properties, the authors developed a Re-188 EL-loaded thermogelling emulsion using a synthetic triblock copolymer, polyethylene glycol-b-poly-DL-lactic acid-co-glycolic acid-b-polyethylene glycol (PEG-PLGA-PEG) as a thermal responsive emulsifier/stabilizer. PEG possesses a unique temperature response. Above a critical temperature, known as the lower critical solution temperature (LCST), the polymer changes its from hydrophilic to hydrophobic, thereby altering its water solubility. This property depends on the molecular weight of the polymer. The PEG-PLGA-PEG used in this study was designed so that the critical temperature was around human body temperature. Thus, the thermogelling emulsion is stable at ambient temperatures below the critical point but after injection, the emulsion becomes unstable and releases the encapsulated Re-188 EL for targeting HCC. The researchers showed that these emulsion gels remained stable for more than 52 hr when stored at room temperature but released the oil phase when the temperature was raised above 40°C. A study in Sprague Dawley rats with xenotransplanted liver tumors showed the significant enhancement of the therapeutic effects on survival and response rates of Re-188 EL thermogelling emulsions compared to the control group (2-months survival rate of 75% vs 13%). Although Re-188 EL exhibited a better therapeutic effect (2-months survival rate of 83%), the administered radioactivity was higher compared to an emulsion system (48.58 MBq vs 25.52 MBq). Moreover, a higher accumulation in the lung was observed for Re-188 EL at 24 hr.76 On the other hand, the Re-188 EL thermogelling emulsion slightly accumulated in the lungs after administration and a decrease in radioactivity to the background level was observed. The main concern was the relatively high accumulation in the kidney and bladder, which could be managed by frequent urination to reduce the radioactivity in the urinary system. Overall, this new radiopharmaceutical-based emulsion technology was shown to have potential as an alternative TARE for HCC treatment.
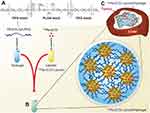 |
Figure 2 Re-188-ECD Lipiodol® emulsion with thermal responsive characteristic, (A) thermal responsive triblock copolymer emulsifier, (B) Illustration of the emulsion preparation, (C) Re-188-ECD Lipiodol® targeting N1-S1 hepatoma-bearing rats. Notes: Originally published by and used with permission from Dove Medical Press Ltd, Shih YH, Lin XZ, Yeh CH, et al Preparation and therapeutic evaluation of (188)Re-thermogelling emulsion in rat model of hepatocellular carcinoma. Int J Nanomedicine. 2014;9:4191–4201.75 |
An alternative strategy for the treatment of cancer is to use α-emitting radionuclides. Unlike β particles, α particles can travel much shorter distances (around 0.05 mm) inside the human body. This can offer more specific treatment location, thereby minimizing radiation exposure to healthy cells. In addition, its linear energy transfer (LET) is much greater than that of β particles providing high cytotoxicity. Thus, α-emitting radionuclides are more suitable for treating microscopic tumors. Examples of α-emitting radionuclides are Bismuth (Bi)-213, Astatine (At)-221, Radium (Ra)-223, and Actinium (Ac)-225. TRT using α-emitting radionuclides has been developed by employing several technologies, including antibody conjugation,77 ligand coordination,78 and encapsulation.79 Nedrow et al developed emulsified Lipiodol® systems containing Ac-225 for targeted alpha therapy of HCC.80 A lipophilic ligand, DOTA-TDA, was complexed with Ac-225 to ensure the radioactive compound could be dispersed within the oil phase. Ex-vivo bio-distribution was studied using a VX2 rabbit model. A relatively high concentration of Ac-225-DOTA-TDA emulsion was observed in the VX2 tumor with a percent of injection dose per gram (%ID/g) of 40.6% after 6 days of the injection. The liver was the second highest organ to have accumulated radioactivity with an %ID/g of 0.233%, corresponding to a 177:1 tumor-to-liver ratio. Notably, the accumulation of radioactivity in the lung was found to be very low, with values of 0.028%ID/g and 0.014%ID/g for 23.5 hours and 6 days after the injection, respectively. Unfortunately, the authors did not disclose the preparation, formulation, and characterization of the emulsion for such a high selectivity toward HCC. The therapeutic efficacy was evaluated in a mouse model. The survival rate was found to be almost 2-fold increase of the median survival for the treated group (receiving Ac-225-DOTA-TDA emulsion) compared to the control group (receiving saline or Lipiodol® alone). It should be noted that the decay of Ac-225 generates γ emission via its daughter radionuclides, such as Francium-221 and Bi-213, which have photons with energies of 218 keV and 441 keV, respectively. SPECT imaging can be used to monitor the distribution of the radiopharmaceutical and its daughters after administration. This study was the first to perform longitudinally SPECT/CT imaging of Ac-225-DOTA-TDA emulsion using its daughter’s detection windows in a VX2 rabbit model. Their imaging study exhibited the potential to be adapted with other Ac-225 labeled agents for accurately monitoring in vivo distribution over time and obtaining pharmacokinetic data to optimize therapeutic efficacy.
As well as being used as delivery systems themselves, emulsions can also be used as templates for fabricating nanoparticles or microparticles suitable for encapsulation of radiopharmaceuticals. The major advantages of emulsions for this purpose are the simplicity of their preparation and the ability to control their particle size. For example, Miyazaki et al81 developed yttrium (Y)-89 microcapsules using a water-in-oil emulsion as a template. Previously, these kinds of microcapsules had been prepared using an enzymatic precipitation method but their size was too large (around 500 μm).82 Y-89 microcapsules were fabricated using a sol-gel reaction in the internal aqueous phase of the water-in-oil emulsions, which resulted in microcapsules with diameters between about 20 and 30 μm. Y-89 microcapsules can be bombarded with neutrons to obtain Y-90 microcapsules, which emit beta particles. This type of Y-90 microcapsule has been clinically approved and widely used in TARE for HCC treatment.83 The microcapsules are delivered to the liver tumor via catheters connected to the hepatic arterial system (percutaneous transarterial technique). Since the hepatic artery is the main blood supply to the tumor,84 the microcapsules selectively accumulate at the cancer area through the embolization effect, thereby sparing the normal liver parenchyma. The preparation of Y-90 microsphere utilizing water-in-oil emulsions provided better control over the size of the microcapsules, thus, minimizing the potential incidence of postembolization syndrome, such as fatigue, nausea, vomiting, anorexia, fever, abdominal discomfort, and cachexia which is often found when large particles over 300 μm are applied.85
Overall, emulsion technology has several advantages for the development of TRT, including the utilization of biocompatible and biodegradable materials, surface functionalization capabilities, and facile preparation methods. Currently, however, TRT based emulsion systems have mainly been developed for the treatment of HCC. Some emulsions have been developed for targeting other locations for imaging purposes, which will be discussed later. It therefore seems likely that similar emulsion-based delivery systems could also be developed for TRT in the future, which could be as simple as switching a radionuclide.
Emulsion-Based Radiosensitizers
Radiotherapy (RT) treatments may involve direct or indirect mechanisms of cytotoxicity. In the direct mechanism, the ionizing radiation directly causes damage to biomolecules, especially deoxyribonucleic acid (DNA). Irreversible damage to DNA leads to apoptosis and cell death. In the indirect mechanism, the ionizing radiation interacts with water or oxygen and produces reactive oxygen species (ROS), such as hydroxide radicals (•OH) or superoxide anions (•O2−), which can break single-stranded and/or double-stranded DNA or lead to cross-linking between DNA and other biomolecules, which can also result in cell death.86 The ionization of oxygen is particularly important for radiation treatments because it induces double-stranded DNA breaks, which are highly toxic to cells.87 A low oxygen level in solid tumors (Hypoxia) has been reported to increase their resistance to radiation.88,89 Thus, larger radiation doses are required to treat tumors in a hypoxia condition, which can increase the adverse side effects associated with cancer treatment. In order to improve the radiosensitivity of hypoxia tumors, a radiosensitizer can be employed to enhance RT efficacy by suppressing radical-scavenger substances, creating cytotoxic substances from radiolysis, preventing repair mechanisms of biomolecules, acting like oxygen molecules, or transporting oxygen.90
Perfluorocarbon (PFC), a highly fluorinated organic compounds, has been demonstrated with several potential results as an oxygen carrier since it can dissolve large volumes of oxygen. For example, perfluorodecalin (PFD) and perfluorooctylbromide (PFOB) were reported with the oxygen carrying capacity of 42% and 50% v/v, respectively, compared to ~20% v/v of healthy human blood.91,92 However, PFC is immiscible with aqueous systems. Thus, to deliver PFC, it is prepared in an oil-in-water emulsion form. Oxygen is then perfused into the emulsion, and it is injected into the target tissue where the oxygen is released by diffusion. A number of PFC emulsions have been investigated as potential radiosensitizers such as PFD emulsion (Fluosol®)93 and PFOB emulsion (Oxygent®).94 The Fluosol® emulsion was studied in a clinical trial for the treatment of Glioblastoma.95 Unfortunately, a high dose was required to display significant effects for the treatment, which led to several adverse effects.96 Recently, a PFC emulsion with a higher oxygen carrying capacity than previous formulations has been developed, which can significantly reduce the dosage required in patients. This emulsion was prepared from 2% w/v dodecafluoropentane (DDFP) in buffer solution (pH ~7) containing sucrose and PEG Telomer B, a fluoro-surfactant.97 A mean droplet diameter of 250 nm was obtained after homogenization of this emulsion. The relatively high oxygen capacity of these emulsions is attributed to the fact that the boiling point of DDFP (29°C) is close to room temperature. As a result, the DDFP molecules start to expand and create more space for oxygen molecules to be dissolved in the oil phase.98,99 Although DDFP might be expected to turn into a gas at human body temperature, the internal blood pressure is sufficient to inhibit this process. This study showed that the half-life of DDFP emulsions in the blood was up to 2 minutes and the volatile DDFP could be cleared through the lungs within 2 hours.100 Emulsions based on this technology have been referred to as NVX-108 when used in cancer studies. A preclinical study of these emulsions in human pancreatic tumor implanted mice97 showed a greater than 400% increase in tumor oxygen levels when they received NVX-108 with carbogen breathing compared to carbogen breathing alone. RT was also applied to treat this cancer in mice. The survival rate was twice as high in the mice receiving NVX-108+cabogen+RT compared to the mice receiving treatment without NVX-108. Clinical trials (Phase Ib and II) of NVX-108 in association with chemoradiation of Glioblastoma have been performed in Australia.101 The trial in 11 patients with glioblastoma indicated safety and significant decrease in tumor volume. A Phase II double-blind, randomized, prospective, and placebo-controlled study is currently being evaluated for NVX-108 (NCT03862430).
The composition and structure of PFC emulsions can be manipulated to provide desirable functional attributes, such as sustained release, improved bioavailability, and reduced non-specific biological interactions. The current generation of PFC emulsions usually exhibit rapid and uncontrolled release of oxygen, which raises the risk of hyperoxia-induced cell death in non-targeted tissues.102 Cerruti et al103 utilized graphene oxide (GO) to improve the stability of PFC emulsions and provide more controlled oxygen delivery (Figure 3). GO consists of different kinds of oxygen rich-functional groups (such as -OH and -COOH) decorated on graphene sheets. The graphene itself is hydrophobic, whereas these functional groups are hydrophilic, which makes GO amphiphilic. As a result, it can adsorb to oil-water interfaces and impact the stability and release characteristics of oil droplets. These features make it suitable for modifying the functional performance of PFC emulsions. Cerruti et al successful demonstrated the sustained release behavior of GO-stabilized PFD emulsions. A burst release profile (100% release of oxygen within 5 min) was observed for PFC emulsions where either a non-ionic surfactant (Tween 20) or an anionic surfactant (sodium dodecyl sulfate, SDS) was used as stabilizers. In contrast, only 33% of the oxygen was released during the first 5 min and a sustained release profile was observed for over 90 min for PFC emulsions where GO was used as a stabilizer. The release kinetic profile could be tuned by varying the GO concentration. This was the first proof-of-concept for controlled oxygen delivery, which could reduce any side effects caused by hyperoxia toxicity.
 |
Figure 3 Perfluoro carbon (PFC) encapsulated emulsion with different emulsifiers (graphene oxide (GO), sodium dodecyl sulfate (SDS), and TWEEN20). Notes: Used with permission of Royal Society of Chemistry, from Jalani G, Jeyachandran D, Bertram Church R, Cerruti M. Graphene oxide-stabilized perfluorocarbon emulsions for controlled oxygen delivery. Nanoscale. 2017;9(29):10,161–10,166; permission conveyed through Copyright Clearance Center, Inc.103 |
The size and shape of the droplets in emulsion-based delivery systems are also important because they impact their stability, release characteristics, and biological fate. Ito et al have used a membrane homogenizer to produce PFC emulsions containing uniform-sized oil droplets.104 A series of emulsions containing monodisperse oil droplets with different diameters was prepared by using membranes with different pore sizes. In addition, the authors investigated the impact of surfactant molecular weight and hydrophilic-lipophilic balance (HLB) by using four different Pluronic copolymers (F127, F68, P85, and P103). The impact of temperature on the properties of the PFC emulsions produced was also examined to provide insights into their performance under different conditions. The stability of the PFC emulsions was tested at 4, 37, and 120°C to simulate refrigerated storage, human body, and autoclave sterilization temperature conditions, respectively. The emulsions formulated using the P85 and P103 surfactants, which had the lowest molecular weight and HLB numbers, were the least stable Both emulsions exhibited phase separation after storage at 4°C, and extensive droplet coalescence was observed in the emulsions formulated using P103 after treatment at 120°C. In contrast, the emulsions formulated using the F127 and F68 surfactants, which had the highest molecular weights and HLB numbers, were stable at all temperatures. Indeed, they exhibited good stability for up to 3 months when stored at 4 and 37°C, with little or no increase in their mean droplet size. The good thermal stability of these emulsions after being heated to 120°C is important for clinical applications since filtration cannot be used to sterilize emulsified systems (since it would remove the droplets). Consequently, autoclave sterilization is required.
The same research group further developed their PFC-based oxygen carrier systems by creating particles with a concave shape and deformable shell, which was inspired by human red blood cells (hRBCs).105 hRBCs have a biconcave disk shape with a dent in the center on both sides. This unique morphology and the ability to deform their shape allows hRBCs to flow through narrow vessel capillaries in the human body.106 Thus, a carrier with the same size and shape as hRBCs may provide several benefits, such as a reduction of the risk of endocytosis and improvement in blood retention time. The method used to prepare the hRBC-like oxygen carriers consisted of three steps: particle formation using membrane emulsification; evaporation-induced phase separation; and solvent-induced concave shape formation. The PFC-based oxygen carriers prepared using this method were shown to have sizes and shapes similar to hRBCs (Figure 4). Microchannel permeation tests confirmed the potential of these carriers to circulate through vascular capillaries and supply oxygen throughout the body.
 |
Figure 4 (a) preparation of bioinspired perfluorocarbon-based oxygen carriers with concave shape and deformable shell consisting of 1) Shirasu porous glass (SPG) emulsification to generate size-controlled emulsion, 2) evaporation-induced phase separation to form spherical and deformable PFC-based OCs (DFCs), and 3) solvent-induced shape change to obtain the “concave-shaped” DFCs (cDFCs) and (b) SEM images of DFCs, cDFCs, and human red blood cell (hRBC). Notes: Reprinted with permission from Fu X, Ohta S, Kawakatsu T, Kamihira M, Sakai Y, Ito T. Bioinspired Perfluorocarbon-Based Oxygen Carriers with Concave Shape and Deformable Shell. Adv Mater Technol. 2022;7(3):2,100,573. © 2021 Wiley-VCHGmbH.105 |
Although radiation can induce DNA damage leading to cell death, a study has shown that the same radiation can also promote multiple pro-survival signaling pathways, resulting in the induction of DNA repair and/or suppression of cell apoptosis.107 This effect enhances radiation resistance, especially in cancer cells with hypoxia conditions. Therefore, another radio-sensitizing strategy for enhancing RT is to utilize an inhibitor molecule that targets the pro-survival signaling pathways. Curcumin has been shown to exhibit inhibitory effects in cellular migration and growth of several tumors,108 including inhibition of endogenous TNF-α, which is involved in pro-survival signaling pathways. A study demonstrated the synergetic anticancer effects of radiation and curcumin using a human prostate cancer cell line (PC-3).109 However, the physicochemical characteristics of curcumin limit its usage due to its poor water-solubility and low chemical and biological stability. To overcome these challenges, delivery systems have been developed to enhance the dispersibility, stability, and activity of curcumin.110 Several emulsion-based delivery systems have been reported to improve the stability and bioavailability of curcumin,111 which may be adapted for RT applications. For example, curcumin-loaded solid lipid nanoparticles (SLNs) have been prepared using an emulsion-templating technique.112 These SLNs were then used as a radiosensitizer in breast cancer cells. Initially, the curcumin was dissolved in a high melting point oil with Pluronic F68 and a cationic surfactant as stabilizers. An emulsion was then formed by homogenization above the melting point of the oil phase. This emulsion was then cooled to room temperature, which caused the oil phase to solidify and form curcumin-loaded SLNs with a mean particle diameter around 300 nm. These particles were shown to exhibit a radio-sensitizing effect on two breast cancer cell lines (MCF7 and MDA-MB-231). However, the effect was also observed in a non-tumorigenic cell line (MCF10A), implying that it could cause toxicity in healthy tissues due to off-target radiation. Thus, improvement of targeted tissue localization of radiosensitizers is important. A brucea javanica oil emulsion (BJOE) has also been shown to exhibit a synergistic effect with RT at downregulating the expression of cyclin-dependent kinase (CDK) 4/6, thereby leading to cell apoptosis and migration suppression of esophageal cancer cells.26 Unfortunately, the formulation and preparation method of this emulsion were not disclosed.
The utilization of a combination strategy of radiosensitizers and TRT has gained considerable interest recently, and many preclinical results have confirmed that synergistic effects can lead to better therapeutic outcomes. The effect is partly due to an enhancement in cancerous cell killing ability, but also because some radiosensitizers have radioprotection effects that reduce off-target toxicity. For example, the use of the radiosensitizer Onalespib, a heat shock protein (HSP) 90 inhibitor, in neuroendocrine cancer bearing mice exhibited a strong radio-sensitizing effect when used with Lu-177 DOTATATE.113 It also induced a radioprotection effect in the kidney by upregulating HSP 70, resulting in the reduction in glomeruli contraction compared to a control with Lu-177 DOTATATE alone.
All these results suggest that emulsions and other colloidal delivery systems can improve the delivery of radiosensitizers to target tissues, which may be due to several mechanisms, including their ability to improve the stability and bioavailability of the active agents. In addition, their ability to enable multimodality therapy using the same treatment at the same time is a feature that can provide an important tool in fighting highly resistant cancers.
Emulsion-Based Imaging Agents
Advancements in imaging technologies have led to significant improvements in medical science, including in understanding biological mechanisms and pathologic processes, detecting early stages of diseases, and providing highly sensitive and accurate diagnostic tools.114,115 PET and SPECT are imaging technologies that are widely used because of their high tissue penetration and ability for simple quantitative analysis. In addition, they only require a low concentration of imaging agents (radiopharmaceuticals) requirement, thereby minimally interfering with biological activities, allowing for high accuracy diagnosis.116,117 Commonly, radiopharmaceuticals are radionuclides chelated or covalently attached to ligands that can target cell activities or bind to specific biological substrates, such as Tc-99m sestamibi for myocardial perfusion imaging, Piflufolastat F-18 for targeting prostate-specific membrane antigen (PSMA) in prostate cancer diagnosis, and Ga-68 DOTATE for binding to overexpressed somatostatin receptors to detect neuroendocrine tumors. Although the current generation of radiopharmaceuticals are routinely used in clinical practice, some limitations with these substances require improvement. For instance, the instability of some radiopharmaceuticals (especially chelated ones), where the ligand may detach or be exchanged with biological molecules in the environment, can induce false target detection, low target accumulation, and high uptake in non-target organs such as liver, spleen or renal. In addition, some radiopharmaceuticals themselves exhibit high liver adsorption such as Tc-99m sestamibi and Tc-99m tetrofosmin exposing unnecessary radiation dose to patients.118 Recently, nanotechnology has been realized as a promising technique to overcome several limitations for radionuclide imaging. Nano-carrier systems can be used to encapsulate radionuclides, increase their dispersion and distribution, and protect them from non-specific interactions, thereby leading to a reduction in off-target imaging. Also, the ability to carry out surface functionalization of nanoparticles provides great flexibility in controlling their biological fate, such as stealth properties, specific tissue/organ targeting functions, and optimization of their stability and physicochemical properties. Moreover, since many nano-carriers have the ability to carry more than one type of imaging agent, multimodal imaging is possible to distinguish different components.119
Emulsion technology can be used to develop carrier-based radionuclide imaging agents. For instance, de la Fuente et al developed Ga-67 and 68-radiolabeled sphingomyelin nanoemulsions (SN) and investigated their bio-distribution in healthy mice (Figure 5).21 Sphingomyelin was the main lipid phase used in the formulation of these nanoemulsions, which is reported to have several advantages for this purpose, including good biocompatibility, long-term stability, and high therapeutic payload.120,121 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (DTPA-PE) and octadecylamine conjugated with NOTA were used as surfactants/co-surfactants bearing chelating functional groups for radiolabeling with Ga-67 and 68. The nanoemulsions were prepared by simply injecting the oil phase into an aqueous phase with constant magnetic stirring. The average diameter of the droplets in these nanoemulsions was 125 nm. The radiolabeling efficiency, as expressed by the radiochemical yield (RCY), was higher for NOTA-SN with Ga-68 (92% RCY) than with DTPA-SN (82% RCY). With no surface modification, the Ga-68 NOTA-SN in healthy mice was mainly located in the reticuloendothelial system (RES) organs and the heart, which was attributed to the interactions of proteins with their surfaces (opsonization). In contrast, after surface modification with PEG (Mw of 2000), the Ga-68 NOTA-PEG-SN exhibited a significant increase in circulation and elimination pharmacokinetic profiles, implying low RES uptake. In addition, the authors also prepared hyaluronic acid (HA) coated Ga-68 NOTA-SN, which could also be a candidate system for improved accumulation in tumors.122
 |
Figure 5 Ga-67 and 68-radiolabeled sphingomyelin nanoemulsions using DTPA-PE and NOTA-SA as chelating ligand for PET and SPECT imaging and the demonstration of nanoemulsions surface modification by polyethylene glycol (PEG) and hyaluronic acid. Notes: Originally published by and used with permission from Dove Medical PressLtd, Díez-Villares S, Pellico J, Gómez-Lado et al Biodistribution of (68/67)Ga-Radiolabeled Sphingolipid Nanoemulsions by PET and SPECT Imaging. Int J Nanomedicine. 2021;16:5923–5935.21 |
The size and surface characteristics of the oil droplets in nanoemulsions impact endocytosis by phagocytic immune cells, thereby influencing their tendency to accumulate in RES organs. Thus, droplet characteristics can be adapted for use as an imaging probe to target inflammatory cells and biomarkers in various inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, and some cancers. F-18 FDG is used for detecting numerous inflammatory diseases. However, the strong uptake of FDG in high metabolic rate organs, such as the heart and kidney, can interfere image interpretation. In the case of solid tumors and metastasis, cancer-induced inflammatory biomarkers and cancer cells are difficult to distinguish. In order to improve diagnostic outcomes, Ahrens and coworkers123 developed multimodal imaging probes based on nanoemulsions for inflammatory macrophage detection using PET and F-19 MRI. Tetrahydroxamic acid linked to perfluorocarbon (PFC) chains was prepared as a ligand for Zr-89. Zr-89 is a positron emitting radionuclide with a half-life of around 3.3 days. Radionuclides with a long half-life are suitable for PET imaging of monoclonal antibody-targeted diseases due to their slow pharmacokinetics, as well as other in vivo processes such as inflammatory macrophage detection. After formation, the ligand was dissolved in another PFC and the solution was used as an oil phase. Nanoemulsions were then prepared using a microfluidizer, which had mean droplet diameters ranging from around 150 to 160 nm. Zr-89 radiolabeling was performed prior to the studies by simply mixing the radiometal solution and the ligand-encapsulated nanoemulsions. Blood pharmacokinetic analysis confirmed the co-complexation Zr-89 and F-19 during the RES uptake period, thereby exhibiting high in vivo stability. The detection of macrophage-associated inflammation in acute infection, inflammatory bowel disease (IBD), and breast cancer was studied using this technology in murine models. Other than RES organs, the imaging agent was only observed in inflamed lesion foci in both PET and F-19 MRI images, indicating a high macrophage specificity. The largely overlapped signals of both techniques could potentially provide a complementary representation of the true macrophage distribution. These preliminary results suggest the potential of this technology for broad range detection of inflammatory diseases. However, due to the high accumulation in the RES organs, safety assessment is still required.
In general, emulsion technology has been used in imaging methods to enhance the specificity, stability, and/or reduce off-target localization.22,23 Although, this technology has only recently been applied in nuclear imaging, there are already examples demonstrating its potential for creating radionuclide carriers for the development of novel-imaging tools with multimodal imaging capabilities. In future, more studies using other emulsion-based imaging tools need to be performed to confirm its usefulness for diagnosis. In addition, emphasis on the physicochemical and structure properties of emulsion-based delivery systems have been the main focus of many previous studies and more research is required on the biological fate and potential toxicity of these systems on living organisms. Thus, future studies should focus on long-term biocompatibility, bio-distribution, and clearance routes of those materials to validate the safety and efficacy of emulsion-based systems.
Conclusion and Future Perspectives
Colloidal delivery systems have played a crucial role in the development of radiopharmaceuticals with better efficacy and efficiency. Emulsion technology is one of the most potent means of creating colloidal delivery systems with well-defined compositions and structures. In particular, the composition, size, shape, and surface characteristics of emulsion droplets can be tuned for specific applications. Clinical successes have already been demonstrated for several emulsion-based formulations for hydrophobic drug delivery and for the development of adjuvants for vaccines. The success of these systems has encouraged further development of emulsion-based delivery systems in other areas of medicine. The ability to formulate emulsions from GRAS excipients, including oils, emulsifying agents, and stabilizers, can help to ensure they are safe for clinical applications. There are also a wide range of methods available to create emulsions, including high- and low-energy methods, which allows systems with different properties to be produced.
The thermodynamic instability of nanoemulsions raises some issues for drug delivery applications. The nanoemulsion is indeed not thermodynamic stable but rather a kinetic trap state in which the movement of the particles through Brownian’s motion and the repulsion from surfactants prevent or delay destabilization events. The kinetic stability of nanoemulsions can be improved by using appropriate stabilizers, such as emulsifiers, weighting agents, ripening inhibitors, and thickening agents. These stabilizers increase the resistance of nanoemulsions to gravitational separation and aggregation.124 As a result, nanoemulsions may be stable for months or years, thereby improving their applications in pharmaceuticals.
In nuclear medicine, the emulsion technology has been introduced for targeted radionuclide therapy (TRT). Emulsion-based delivery systems developed for this purpose have mainly focused on treatment of hepatocellular carcinoma (HCC), but there is growing evidence that they can also be used for other forms of cancer. Plus, emulsion-based systems can be used to deliver radiosensitizer molecules, thereby enhancing radiotherapy efficacy for cancer cells with hypoxia conditions. This technology can be combined with TRT to provide synergetic effects against hypoxia tumors. Emulsion-based systems also have potential applications in imaging especially for multimodal imaging techniques. The application of emulsion technology in nuclear medicine is still fairly new with limited examples and applications. However, this technology has already demonstrated that it has great potential in this area. In future, it will be important to develop and test a wider range of emulsion-based delivery systems for specific diagnostic and/or therapeutic applications in nuclear medicine for more understanding of their behaviors such as non-specific accumulation, effect of protein corona on its surface modifications, bio-distribution, and metabolism. Emulsions containing droplets with different compositions, sizes, shapes, and surface characteristics can be prepared, which means their properties can be tuned for specific purposes, giving them a broad range of potential applications in this field.
Acknowledgment
This publication is funded by Chulalongkorn University under contact number of ReinUni_65_03_30_15. We would like to thank Professor Supatporn Tepmongkol, MD, for her valuable suggestions on this work.
Disclosure
The authors declare that they have no known competing interests that could have appeared to influence the work reported in this paper.
References
1. Kramer-Marek G, Capala J. The role of nuclear medicine in modern therapy of cancer. Tumor Biol. 2012;33(3):629–640. doi:10.1007/s13277-012-0373-8
2. Aboagye EO, Barwick TD, Haberkorn U. Radiotheranostics in oncology: making precision medicine possible. CA Cancer J Clin. 2023;73(3):255–274. doi:10.3322/caac.21768
3. Schofield R, Menezes L, Underwood SR. Nuclear cardiology: state of the art. Heart. 2021. doi:10.1136/heartjnl-2019-315628
4. Anagnostopoulos C, Underwood R. Nuclear cardiology. Clin Med. 2012;12(4):373–377. doi:10.7861/clinmedicine.12-4-373
5. Wang J, Jin C, Zhou J, et al. PET molecular imaging for pathophysiological visualization in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2023;50(3):765–783. doi:10.1007/s00259-022-05999-z
6. Khalatbari H, Shulkin BL, Parisi MT. Emerging trends in radionuclide imaging of infection and inflammation in pediatrics: focus on FDG PET/CT and immune reactivity. Semin Nucl Med. 2023;53(1):18–36. doi:10.1053/j.semnuclmed.2022.10.002
7. Oh JS. Nuclear medicine physics: review of advanced technology. Prog Med Phys. 2020;31(3):81–98. doi:10.14316/pmp.2020.31.3.81
8. Kaushik D, Jangra P, Verma R, et al. Radiopharmaceuticals: an insight into the latest advances in medical uses and regulatory perspectives. J Biosci. 2021;2021:46.
9. Ramnaraign B, Sartor O. PSMA-targeted radiopharmaceuticals in prostate cancer: current data and new trials. Oncologist. 2023;28(5):392–401. doi:10.1093/oncolo/oyac279
10. Pallares RM, Abergel RJ. Development of radiopharmaceuticals for targeted alpha therapy: where do we stand? Front Med. 2022;9:1020188. doi:10.3389/fmed.2022.1020188
11. Handkiewicz-Junak D, Poeppel TD, Bodei L, et al. EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides. Eur J Nucl Med Mol Imaging. 2018;45(5):846–859. doi:10.1007/s00259-018-3947-x
12. Goldsmith SJ. Targeted radionuclide therapy: a historical and personal review. Semin Nucl Med. 2020;50(1):87–97. doi:10.1053/j.semnuclmed.2019.07.006
13. Pellico J, Gawne PJ, de Rosales R. Radiolabelling of nanomaterials for medical imaging and therapy. Chem Soc Rev. 2021;50(5):3355–3423. doi:10.1039/D0CS00384K
14. Wu S, Helal-Neto E, Matos APDS, et al. Radioactive polymeric nanoparticles for biomedical application. Drug Deliv. 2020;27(1):1544–1561. doi:10.1080/10717544.2020.1837296
15. Nehoff H, Parayath NN, Domanovitch L, Taurin S, Greish K. Nanomedicine for drug targeting: strategies beyond the enhanced permeability and retention effect. Int J Nanomedicine. 2014;9(1):2539–2555. doi:10.2147/IJN.S47129
16. Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24(6):1159–1166. doi:10.1016/j.copbio.2013.02.020
17. Lepareur N, Lacœuille F, Bouvry C, et al. Rhenium-188 labeled radiopharmaceuticals: current clinical applications in oncology and promising perspectives. Front Med. 2019;6:132. doi:10.3389/fmed.2019.00132
18. Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi:10.1016/j.addr.2010.04.009
19. Lee W, Il An G, Park H, et al. Imaging Strategy that achieves ultrahigh contrast by utilizing differential esterase activity in organs: application in early detection of pancreatic cancer. ACS Nano. 2021;15(11):17348–17360. doi:10.1021/acsnano.1c05165
20. Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. Biotech. 2015;5(2):123–127. doi:10.1007/s13205-014-0214-0
21. Díez-Villares S, Pellico J, Gómez-Lado N, et al. Biodistribution of (68/67)Ga-radiolabeled sphingolipid nanoemulsions by PET and SPECT Imaging. Int J Nanomedicine. 2021;16:5923–5935. doi:10.2147/IJN.S316767
22. Kim H, Lee SK, Kim YM, et al. Fluorescent iodized emulsion for pre- and intraoperative sentinel lymph node imaging: validation in a preclinical model. Radiology. 2014;275(1):196–204. doi:10.1148/radiol.14141159
23. Holman R, Lorton O, Guillemin PC, Desgranges S, Contino-Pépin C, Salomir R. Perfluorocarbon emulsion contrast agents: a mini review. Front Chem. 2021;9:810029. doi:10.3389/fchem.2021.810029
24. Sánchez-López E, Guerra M, Dias-Ferreira J, et al. Current applications of nanoemulsions in cancer therapeutics. Nanomater. 2019;9(6). doi:10.3390/nano9060821
25. Zhang T, Li M, Yang R, et al. Therapeutic efficacy of lipid emulsions of docetaxel-linoleic acid conjugate in breast cancer. Int J Pharm. 2018;546(1–2):61–69. doi:10.1016/j.ijpharm.2018.05.032
26. Qiu Z-H, Zhang -W-W, Zhang -H-H, Jiao G-H. Brucea javanica oil emulsion improves the effect of radiotherapy on esophageal cancer cells by inhibiting cyclin D1-CDK4/6 axis. World J Gastroenterol. 2019;25(20):2463–2472. doi:10.3748/wjg.v25.i20.2463
27. Jain S, Winuprasith T, Suphantharika M. Design and synthesis of modified and resistant starch-based oil-in-water emulsions. Food Hydrocoll. 2019;89:153–162. doi:10.1016/j.foodhyd.2018.10.036
28. Karthik P, Ezhilarasi PN, Anandharamakrishnan C. Challenges associated in stability of food grade nanoemulsions. Crit Rev Food Sci Nutr. 2017;57(7):1435–1450. doi:10.1080/10408398.2015.1006767
29. Zhu Y, Ye J, Zhang Q. Self-emulsifying drug delivery system improve oral bioavailability: role of excipients and physico-chemical characterization. Pharm Nanotechnol. 2020;8(4):290–301. doi:10.2174/2211738508666200811104240
30. Wilson RJ, Li Y, Yang G, Zhao C-X. Nanoemulsions for drug delivery. Particuology. 2022;64:85–97. doi:10.1016/j.partic.2021.05.009
31. Salawi A. Self-emulsifying drug delivery systems: a novel approach to deliver drugs. Drug Deliv. 2022;29(1):1811–1823. doi:10.1080/10717544.2022.2083724
32. Dragulska SA, Chen Y, Wlodarczyk MT, et al. Tripeptide-stabilized oil-in-water nanoemulsion of an oleic acids–platinum (II) conjugate as an anticancer nanomedicine. Bioconjug Chem. 2018;29(8):2514–2519.
33. Patel NR, Piroyan A, Ganta S, et al. In vitro and in vivo evaluation of a novel folate-targeted theranostic nanoemulsion of docetaxel for imaging and improved anticancer activity against ovarian cancers. Cancer Biol Ther. 2018;19(7):554–564. doi:10.1080/15384047.2017.1395118
34. Chen L, Ao F, Ge X, Shen W. Food-grade pickering emulsions: preparation, stabilization and applications. Molecules. 2020;25(14). doi:10.3390/molecules25143202
35. Zhang W, Fu L, Yang H. Micrometer-scale mixing with pickering emulsions: biphasic reactions without stirring. Chem Sus Chem. 2014;7(2):391–396. doi:10.1002/cssc.201301001
36. Zhang J, Zhu J, Cheng Y, Huang Q. Recent advances in pickering double emulsions and potential applications in functional foods: a perspective paper. Foods. 2023;12(5). doi:10.3390/foods12050992
37. Ganta S, Singh A, Patel NR, et al. Development of EGFR-targeted nanoemulsion for imaging and novel platinum therapy of ovarian cancer. Pharm Res. 2014;31(9):2490–2502. doi:10.1007/s11095-014-1345-z
38. Natesan S, Sugumaran A, Ponnusamy C, Thiagarajan V, Palanichamy R, Kandasamy R. Chitosan stabilized camptothecin nanoemulsions: development, evaluation and biodistribution in preclinical breast cancer animal mode. Int J Biol Macromol. 2017;104:1846–1852.
39. Qadir A, Faiyazuddin MD, Talib Hussain MD, Alshammari TM, Shakeel F. Critical steps and energetics involved in a successful development of a stable nanoemulsion. J Mol Liq. 2016;214:7–18. doi:10.1016/j.molliq.2015.11.050
40. Singh Y, Meher JG, Raval K, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi:10.1016/j.jconrel.2017.03.008
41. Song B, Wu S, Li W, Chen D, Hu H. Folate modified long circulating nano-emulsion as a promising approach for improving the efficiency of chemotherapy drugs in cancer treatment. Pharm Res. 2020;37(12):242. doi:10.1007/s11095-020-02811-1
42. Saani SM, Abdolalizadeh J, Heris SZ. Ultrasonic/sonochemical synthesis and evaluation of nanostructured oil in water emulsions for topical delivery of protein drugs. Ultrason Sonochem. 2019;55:86–95.
43. Masalova I, Tshilumbu NN, Mamedov E, Sanatkaran N. Effect of oil type on stability of high internal phase water-in-oil emulsions with super-cooled internal phase. Chem Eng Commun. 2018;205(1):1–11. doi:10.1080/00986445.2017.1367669
44. Gomes A, Costa ALR, Cunha RL. Impact of oil type and WPI/Tween 80 ratio at the oil-water interface: adsorption, interfacial rheology and emulsion features. Colloids Surf B Biointerfaces. 2018;164:272–280. doi:10.1016/j.colsurfb.2018.01.032
45. Izgelov D, Shmoeli E, Domb AJ, Hoffman A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int J Pharm. 2020;580:119201. doi:10.1016/j.ijpharm.2020.119201
46. Reddy MR, Gubbiyappa KS. Formulation development, optimization and characterization of Pemigatinib-loaded supersaturable self-nanoemulsifying drug delivery systems. Futur J Pharm Sci. 2022;8(1):45. doi:10.1186/s43094-022-00434-4
47. Lee Y, Lee D, Park E, et al. Rhamnolipid-coated W/O/W double emulsion nanoparticles for efficient delivery of doxorubicin/erlotinib and combination chemotherapy. J Nanobiotechnology. 2021;19(1):411. doi:10.1186/s12951-021-01160-4
48. Handa M, Ujjwal RR, Vasdev N, Flora SJS, Shukla R. Optimization of surfactant-and cosurfactant-aided pine oil nanoemulsions by isothermal low-energy methods for anticholinesterase activity. ACS omega. 2020;6(1):559–568.
49. Galvão KCS, Vicente AA, Sobral PJA. Development, characterization, and stability of O/W pepper nanoemulsions produced by high-pressure homogenization. Food Bioprocess Technol. 2018;11(2):355–367. doi:10.1007/s11947-017-2016-y
50. Kumar M, Bishnoi RS, Shukla AK, Jain CP. Techniques for formulation of nanoemulsion drug delivery system: a review. Prev Nutr Food Sci. 2019;24(3):225.
51. Heidari F, Jafari SM, Ziaiifar AM, Malekjani N. Stability and release mechanisms of double emulsions loaded with bioactive compounds; a critical review. Adv Colloid Interface Sci. 2022;299:102567. doi:10.1016/j.cis.2021.102567
52. Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3(3):145–150. doi:10.1038/nnano.2008.30
53. Manzanares D, Ceña V. Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics. 2020;12(4). doi:10.3390/pharmaceutics12040371
54. Foroozandeh P, Aziz AA. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. 2018;13. doi:10.1186/s11671-018-2728-6
55. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2). doi:10.3390/pharmaceutics10020057
56. Nandhakumar S, Dhanaraju MD, Sundar VD, Heera B. Influence of surface charge on the in vitro protein adsorption and cell cytotoxicity of paclitaxel loaded poly(ε-caprolactone) nanoparticles. Bull Fac Pharm Cairo Univ. 2017;55(2):249–258. doi:10.1016/j.bfopcu.2017.06.003
57. Ghobrial J, Gibson CM, Pinto DS. Delayed clopidogrel transit during myocardial infarction evident on angiography. J Invasive Cardiol. 2015;27(5):E68–9.
58. Chen X, Zhu W, Liu H, Deng F, Wang W, Qin L. Preparation of injectable clopidogrel loaded submicron emulsion for enhancing physicochemical stability and anti-thrombotic efficacy. Int J Pharm. 2022;611:121323. doi:10.1016/j.ijpharm.2021.121323
59. Abd-Elhakeem E, Teaima MHM, Abdelbary GA, El Mahrouk GM. Bioavailability enhanced clopidogrel-loaded solid SNEDDS: development and in-vitro/in-vivo characterization. J Drug Deliv Sci Technol. 2019;49:603–614.
60. Jadvar H. Targeted radionuclide therapy: an evolution toward precision cancer treatment. Am J Roentgenol. 2017;209(2):277–288. doi:10.2214/AJR.17.18264
61. Sousa D, Ferreira D, Rodrigues JL, Rodrigues LR. Nanotechnology in targeted drug delivery and therapeutics. In: In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, editors. Micro and Nano Technologies. Elsevier; 2019:357–409.
62. Umeda I, Hamamichi S, Fujii H. High tumor accumulation and rapid background clearance by using radionuclide-carrying liposomes for targeted radionuclide therapy and theranostics. J Nucl Med. 2017;58(supplement 1):458458.
63. Zhao L, Zhu M, Li Y, Xing Y, Zhao J. Radiolabeled dendrimers for nuclear medicine applications. Molecules. 2017;22(9):1350. doi:10.3390/molecules22091350
64. Ognjanović M, Radović M, Mirković M, et al. 99mTc-, 90Y-, and 177Lu-labeled iron oxide nanoflowers designed for potential use in dual magnetic hyperthermia/radionuclide cancer therapy and diagnosis. ACS Appl Mater Interfaces. 2019;11(44):41109–41117. doi:10.1021/acsami.9b16428
65. Ngwa W, Kumar R, Sridhar S, et al. Targeted radiotherapy with gold nanoparticles: current status and future perspectives. Nanomedicine. 2014;9(7):1063–1082. doi:10.2217/nnm.14.55
66. Suhail N, Alzahrani AK, Basha WJ, et al. Microemulsions: unique properties, pharmacological applications, and targeted drug delivery. Front Nanotechnol. 2021;2021:3.
67. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
68. J-g L, Wang A-Y, Wei -Y-Y, Lui W-Y, Chi C-W, Chan W-K. Preparation of [131I]lipiodol as a hepatoma therapeutic agent. Int J Radiat Appl Instrumentation Part a Appl Radiat Isot. 1992;43(12):1431–1435. doi:10.1016/0883-2889(92)90168-E
69. Park C, Choi SI, Kim H, Yoo HS, Lee YB. Distribution of Lipiodol in hepatocellular carcinoma. Liver. 1990;10(2):72–78. doi:10.1111/j.1600-0676.1990.tb00439.x
70. Raoul J, Guyader D, Bretagne J, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled–iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26(5):1156–1161. doi:10.1002/hep.510260511
71. Raoul JL, Bourguet P, Bretagne JF, et al. Hepatic artery injection of I-131-labeled lipiodol. Part I. Biodistribution study results in patients with hepatocellular carcinoma and liver metastases. Radiology. 1988;168(2):541–545. doi:10.1148/radiology.168.2.2839866
72. Jouneau S, Vauléon E, Caulet-Maugendre S, et al. 131I-labeled lipiodol-induced interstitial pneumonia: a series of 15 cases. Chest. 2011;139(6):1463–1469. doi:10.1378/chest.10-1591
73. de Baere T, Zhang X, Aubert B, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology. 1996;201(3):731–735. doi:10.1148/radiology.201.3.8939223
74. Garin E, Denizot B, Roux J, et al. Effect of stabilized iodized oil emulsion on experimentally induced hepatocellular carcinoma in rats. J Vasc Interv Radiol. 2005;16(6):841–848. doi:10.1097/01.RVI.0000156192.89569.0C
75. Shih YH, Lin XZ, Yeh CH, et al. Preparation and therapeutic evaluation of (188)Re-thermogelling emulsion in rat model of hepatocellular carcinoma. Int J Nanomedicine. 2014;9:4191–4201. doi:10.2147/IJN.S66346
76. Luo T-Y, Shih Y-H, Chen C-Y, et al. Evaluating the potential of 188Re-ECD/lipiodol as a therapeutic radiopharmaceutical by intratumoral injection for hepatoma treatment. Cancer Biother Radiopharm. 2009;24(5):535–541. doi:10.1089/cbr.2008.0603
77. Kitson LS, Cuccurullo V, Moody ST, Mansi L. Radionuclide antibody-conjugates, a targeted therapy towards cancer. Curr Radiopharm. 2013;6(2):57–71. doi:10.2174/1874471011306020001
78. Grieve ML, Paterson BM. The evolving coordination chemistry of radiometals for targeted alpha therapy. Aust J Chem. 2022;75(2):65–88. doi:10.1071/CH21184
79. Silindir-Gunay M, Karpuz M, Ozer AY. Targeted alpha therapy and nanocarrier approach. Cancer Biother Radiopharm. 2020;35(6):446–458. doi:10.1089/cbr.2019.3213
80. Du Y, Cortez A, Josefsson A, et al. Preliminary evaluation of alpha-emitting radioembolization in animal models of hepatocellular carcinoma. PLoS One. 2022;17(1):e0261982. doi:10.1371/journal.pone.0261982
81. Miyazaki T, Kai T, Ishida E, Kawashita M, Hiraoka M. Fabrication of yttria microcapsules for radiotherapy from water/oil emulsion. J Ceram Soc Japan. 2010;118(1378):479–482. doi:10.2109/jcersj2.118.479
82. Kawashita M, Takayama Y, Kokubo T, Takaoka GH, Araki N, Hiraoka M. Enzymatic preparation of hollow yttrium oxide microspheres for in situ radiotherapy of deep-seated cancer. J Am Ceram Soc. 2006;89(4):1347–1351. doi:10.1111/j.1551-2916.2005.00867.x
83. Memon K, Lewandowski RJ, Riaz A, Salem R. Yttrium 90 microspheres for the treatment of hepatocellular carcinoma. In: Vauthey JN, Brouquet A, editors. Multidisciplinary Treatment of Hepatocellular Carcinoma. Berlin Heidelberg: Springer; 2013:207–224.
84. Park YN, Yang C-P, Fernandez GJ, Cubukcu O, Thung SN, Theise ND. Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol. 1998;22(6):1.
85. Padia SA, Shivaram G, Bastawrous S, et al. Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. J Vasc Interv Radiol. 2013;24(3):301–306. doi:10.1016/j.jvir.2012.11.023
86. Sridharan DM, Asaithamby A, Bailey SM, et al. Understanding cancer development processes after HZE-Particle exposure: roles of ROS, DNA damage repair and inflammation. Radiat Res. 2015;183(1):1–26. doi:10.1667/RR13804.1
87. Brown JM. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain memorial award lecture. Cancer Res. 1999;59(23):5863–5870.
88. Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett. 2013;341(1):63–72. doi:10.1016/j.canlet.2012.11.019
89. Peitzsch C, Perrin R, Hill RP, Dubrovska A, Kurth I. Hypoxia as a biomarker for radioresistant cancer stem cells. Int J Radiat Biol. 2014;90(8):636–652. doi:10.3109/09553002.2014.916841
90. Wang H, Mu X, He H, Zhang X-D. Cancer radiosensitizers. Trends Pharmacol Sci. 2018;39(1):24–48. doi:10.1016/j.tips.2017.11.003
91. Krafft MP. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv Drug Deliv Rev. 2001;47(2):209–228. doi:10.1016/S0169-409X(01)00107-7
92. Johnson JLH, Dolezal MC, Kerschen A, Matsunaga TO, Unger EC. In vitro comparison of Dodecafluoropentane (DDFP), Perfluorodecalin (PFD), and Perfluoroctylbromide (PFOB) in the facilitation of oxygen exchange. Artif Cells Blood Sub Biotechnol. 2009;37(4):156–162. doi:10.1080/10731190903043192
93. Suyama T, Yokoyama K, Naito R. Development of a perfluorochemical whole blood substitute (Fluosol-DA, 20%)--an overview of clinical studies with 185 patients. Prog Clin Biol Res. 1981;55:609–628.
94. Rockwell S, Irvin CG, Kelley M, et al. Effects of hyperbaric oxygen and a perfluorooctylbromide emulsion on the radiation responses of tumors and normal tissues in rodents. Int J Radiat Oncol. 1992;22(1):87–93. doi:10.1016/0360-3016(92)90986-R
95. Evans RG, Kimler BF, Morantz RA, et al. A phase I/II study of the use of Fluosol® as an adjuvant to radiation therapy in the treatment of primary high-grade brain tumors. Int J Radiat Oncol. 1990;19(2):415–420. doi:10.1016/0360-3016(90)90551-T
96. Police AM, Waxman K, Tominaga G. Pulmonary complications after Fluosol administration to patients with life-threatening blood loss. Crit Care Med. 1985;13(2):1.
97. Johnson JLH, Leos RA, Baker AF, Unger EC. Radiosensitization of Hs-766T pancreatic tumor xenografts in mice dosed with dodecafluoropentane nano-emulsion-preliminary findings. J Biomed Nanotechnol. 2015;11(2):274–281. doi:10.1166/jbn.2015.1903
98. Jayaraman MS, Graham K, Unger EC. In vitro model to compare the oxygen offloading behaviour of dodecafluoropentane emulsion (DDFPe). Artif Cells Nanomed Biotechnol. 2019;47(1):783–789. doi:10.1080/21691401.2019.1577882
99. Johnson JLH. Oxygen carriers: are they enough for cellular support? In: Lapchak PA, Zhang JH, editors. Neuroprotective Therapy for Stroke and Ischemic Disease. Springer International Publishing; 2017:621–640.
100. Correas J-M, Meuter AR, Singlas E, Kessler DR, Worah D, Quay SC. Human pharmacokinetics of a perfluorocarbon ultrasound contrast agent evaluated with gas chromatography. Ultrasound Med Biol. 2001;27(4):565–570. doi:10.1016/s0301-5629(00)00363-x
101. Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomedicine. 2018;13:6049–6058. doi:10.2147/IJN.S140462
102. Komatsu H, Kandeel F, Mullen Y. Impact of oxygen on pancreatic islet survival. Pancreas. 2018;47(5):533–543. doi:10.1097/MPA.0000000000001050
103. Jalani G, Jeyachandran D, Bertram Church R, Cerruti M. Graphene oxide-stabilized perfluorocarbon emulsions for controlled oxygen delivery. Nanoscale. 2017;9(29):10161–10166. doi:10.1039/C7NR00378A
104. Fu X, Ohta S, Kamihira M, Sakai Y, Ito T. Size-controlled preparation of microsized perfluorocarbon emulsions as oxygen carriers via the shirasu porous glass membrane emulsification technique. Langmuir. 2019;35(11):4094–4100. doi:10.1021/acs.langmuir.9b00194
105. Fu X, Ohta S, Kawakatsu T, Kamihira M, Sakai Y, Ito T. Bioinspired perfluorocarbon-based oxygen carriers with concave shape and deformable shell. Adv Mater Technol. 2022;7(3):2100573. doi:10.1002/admt.202100573
106. Kaoui B, Biros G, Misbah C. Why do red blood cells have asymmetric shapes even in a symmetric flow? Phys Rev Lett. 2009;103(18):188101. doi:10.1103/PhysRevLett.103.188101
107. Hein AL, Ouellette MM, Yan Y. Radiation-induced signaling pathways that promote cancer cell survival (review). Int J Oncol. 2014;45(5):1813–1819. doi:10.3892/ijo.2014.2614
108. Mansouri K, Rasoulpoor S, Daneshkhah A, et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20(1):791. doi:10.1186/s12885-020-07256-8
109. Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23(8):1599–1607. doi:10.1038/sj.onc.1207284
110. Amekyeh H, Alkhader E, Sabra R, Billa N. Prospects of curcumin nanoformulations in cancer management. Molecules. 2022;27(2). doi:10.3390/molecules27020361
111. Jiang T, Liao W, Charcosset C. Recent advances in encapsulation of curcumin in nanoemulsions: a review of encapsulation technologies, bioaccessibility and applications. Food Res Int. 2020;132:109035. doi:10.1016/j.foodres.2020.109035
112. Minafra L, Porcino N, Bravatà V, et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci Rep. 2019;9(1):11134. doi:10.1038/s41598-019-47553-2
113. Lundsten S, Spiegelberg D, Raval NR, Nestor M. The radiosensitizer Onalespib increases complete remission in (177)Lu-DOTATATE-treated mice bearing neuroendocrine tumor xenografts. Eur J Nucl Med Mol Imaging. 2020;47(4):980–990. doi:10.1007/s00259-019-04673-1
114. Tian M, He X, Jin C, et al. Transpathology: molecular imaging-based pathology. Eur J Nucl Med Mol Imaging. 2021;48(8):2338–2350. doi:10.1007/s00259-021-05234-1
115. Carrete LR, Young JS, Cha S. Advanced imaging techniques for newly diagnosed and recurrent gliomas. Front Neurosci. 2022;16:1.
116. Israel O, Pellet O, Biassoni L, et al. Two decades of SPECT/CT – the coming of age of a technology: an updated review of literature evidence. Eur J Nucl Med Mol Imaging. 2019;46(10):1990–2012. doi:10.1007/s00259-019-04404-6
117. Vaquero JJ, Kinahan P. Positron emission tomography: current challenges and opportunities for technological advances in clinical and preclinical imaging systems. Annu Rev Biomed Eng. 2015;17:385–414. doi:10.1146/annurev-bioeng-071114-040723
118. Boschi A, Uccelli L, Marvelli L, Cittanti C, Giganti M, Martini P. Technetium-99m radiopharmaceuticals for ideal myocardial perfusion imaging: lost and found opportunities. Molecules. 2022;27(4). doi:10.3390/molecules27041188
119. Liu M, Anderson R-C, Lan X, Conti PS, Chen K. Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer. Med Res Rev. 2020;40(3):909–930. doi:10.1002/med.21642
120. Nagachinta S, Bouzo BL, Vazquez-Rios AJ, Lopez R. Sphingomyelin-Based Nanosystems (SNs) for the development of anticancer miRNA therapeutics. Pharmaceutics. 2020;12(2):189. doi:10.3390/pharmaceutics12020189
121. Takino T, Konishi K, Takakura Y, Hashida M. Long circulating emulsion carrier systems for highly lipophilic drugs. Biol Pharm Bull. 1994;17(1):121–125. doi:10.1248/bpb.17.121
122. Huang G, Huang H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J Control Release. 2018;278:122–126. doi:10.1016/j.jconrel.2018.04.015
123. Wang C, Leach BI, Lister D, et al. Metallofluorocarbon nanoemulsion for inflammatory macrophage detection via PET and MRI. J Nucl Med. 2021;62(8):1146–1153. doi:10.2967/jnumed.120.255273
124. Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2–3):85–103. doi:10.1016/j.micron.2011.07.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

