Back to Journals » Clinical Ophthalmology » Volume 17
Emerging Treatment Options for Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration
Authors Khan H, Aziz AA , Sulahria H , Khan H, Ahmed A , Choudhry N, Narayanan R, Danzig C, Khanani AM
Received 31 October 2022
Accepted for publication 9 January 2023
Published 23 January 2023 Volume 2023:17 Pages 321—327
DOI https://doi.org/10.2147/OPTH.S367089
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hannah Khan,1 Aamir A Aziz,1 Humza Sulahria,2 Huma Khan,2 Abrahim Ahmed,3 Netan Choudhry,4– 7 Raja Narayanan,8,9 Carl Danzig,10 Arshad M Khanani1,2
1University of Nevada, Reno School of Medicine, Reno, NV, USA; 2Sierra Eye Associates, Reno, NV, USA; 3Morsani College of Medicine, University of South Florida, Tampa, FL, USA; 4Department of Ophthalmology and Vision Sciences, University of Toronto, Toronto, Ontario, Canada; 5Vitreous Retina Macula Specialists of Toronto, Etobicoke, Ontario, Canada; 6Cleveland Clinic Canada, Toronto, Ontario, Canada; 7Octane Imaging Lab, Toronto, Ontario, Canada; 8Anant Bajaj Retina Institute, LV Prasad Eye Institute, Hyderabad, Telangana, India; 9Indian Health Outcomes, Public Health and Economics Research Centre (IHOPE), Hyderabad, Telangana, India; 10Rand Eye Institute, Deerfield Beach, FL, USA
Correspondence: Arshad M Khanani, Sierra Eye Associates, 950 Ryland Street, Reno, NV, USA, Tel +1 775 329-0286, Fax +1 775 329-0849, Email [email protected]
Abstract: Age-related macular degeneration (AMD) is characterized as a chronic, multifactorial disease and is the leading cause of irreversible blindness. Advanced AMD is classified as neovascular (wet) AMD and non-neovascular (dry) AMD. Dry AMD can progress to a more advanced form that manifests as geographic atrophy (GA), which significantly threatens vision, leading to progressive and irreversible loss of visual function. There are currently no approved therapeutics commercially available for GA patients. However, data from various clinical trials have demonstrated favorable results with significant reduction in GA lesion growth. Approaches to GA treatment vary from complement inhibitors to ocular gene therapy, some of which may delay disease progression, while others may reverse the disease. This review furthers the understanding of the pathophysiology of GA, as well as current clinical trial data on investigational therapeutics.
Keywords: complement system, gene therapy, neuroprotective agents, personalized treatment, anti-inflammatory agents, intravitreal injection
Introduction
Age-related macular degeneration (AMD) is a multifactorial, degenerative disease associated with progressive vision loss, and is more common in developed nations but is prevalent in all countries.1 AMD is categorized into two major subtypes: non-neovascular (dry or non-exudative) and neovascular (wet or exudative; nAMD) form.2 Macular neovascularization (MNV) in the retina or choroid is a characteristic of nAMD with associated acute vision loss. Anti-vascular endothelial growth factor (anti-VEGF) treatment has proven efficacious in nAMD patients as it targets neovascularization and treats associated intra- (IRF) and subretinal fluid (SRF). If untreated, MNV can induce leakage of fluid, lipids, and blood into the outer retina, resulting in irreversible vision loss.
Dry AMD can progress to an advanced degenerative stage manifesting as geographic atrophy (GA), characterized by irreversible vision loss due to loss of retinal pigment epithelium (RPE), photoreceptors and choriocapillaris in the macula.3 Visual acuity can remain intact with spared foveal involvement in some GA patients. Enlargement of GA is associated with furthering disease progression, ultimately leading to irreversible vision morbidity.4 Nonmodifiable risk factors, including genetics and age, and modifiable risk factors such as smoking status, and comorbidities such as hypertension, are inherently associated with GA progression.5 Studies have indicated ocular benefits and delayed disease progression in conjunction with a healthy lifestyle.6
Currently, there are no therapeutics approved for GA; however, numerous clinical trials are underway investigating potential therapeutics for reduction in GA lesion growth. In this review, we focus on the pathophysiology of GA, as well as current investigational therapeutics for the disease.
Methods
Publications regarding GA were identified via a systematic literature search on the PubMed/MEDLINE database and ClinicalTrials.gov website. The term “geographic atrophy” was used in conjunction with “treatments”, “diagnosis”, and “management.” In addition, literature cited in the reference lists of identified GA publications were also utilized. Current and recent therapeutics were compiled and summarized for this review.
Investigational Therapeutics
Complement Inhibitors
The complement system interacts with various proteins to result in a humoral immune response and has three pathways: classical, alternative, or mannose-binding lectin.7 The classical pathway activates via antibody-antigen recognition, and the mannose-binding lectin pathway activates upon mannose complexes binding to pathogens.8 The alternative pathway is constitutively active due to spontaneous C3 hydrolysis, but also has potential to amplify via a positive feedback loop.9 The alternative pathway is tightly regulated by proteins such as factor H and properdin, under normal conditions.10 Approximately 50% of dry AMD patients have mutations in genes encoding for regulatory complement proteins.11 Conserved among all three pathways is C3 convertase, which cleaves C3 into C3a for an inflammatory response and C3b for opsonization.7 C3b also plays a role in C5 convertase formation, which also splits into C5a for an inflammatory response and C5b, which is a component in the terminal membrane attack complex (MAC), thus responsible for cell death.4 Inhibiting an overactive complement system is a potential therapeutic approach for GA treatment (Figure 1).
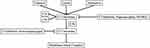 |
Figure 1 Development of GA therapeutics in targeting the complement cascade. |
Avacincaptad pegol (ACP), a pegylated RNA aptamer, binds specifically to complement C5 and prevents cleavage, slowing progression of complement-mediated inflammation and cell death as seen in GA.12 Administered intravitreally (IVT), ACP locally targets the complement system in the eye, decreasing MAC formation. RPE and photoreceptor atrophy is therefore reduced, ultimately slowing GA disease progression. Numerous clinical trials have demonstrated the therapeutic potential of ACP as a monotherapeutic and in combination with others (Table 1).
 |
Table 1 Clinical Trials Investigating ACP as a Therapeutic for Various Disease Processes |
Efficacy and tolerability of ACP has been investigated in patients with GA in a Phase 1/2a clinical trial, OPH2001 (ClinicalTrials.gov Identifier: NCT00950638), where 47 patients were enrolled and randomized 1:1:1 into two arms: 0.3mg ACP and 1mg ACP. ACP IVT injections were administered in both arms at day 0, week 4, week 8, and weeks 24 and 36, subsequently.13,14 Visual acuity, intraocular pressure changes and imaging via optical coherence tomography (OCT) and fundus autofluorescence (FAF), along with other safety assessments, were conducted. Results suggest the 1mg ACP arm demonstrated greater mean growth GA reduction than the 0.3mg arm. This trial met its primary endpoint, as ACP treatment was well-tolerated among GA patients with no adverse events (AEs) reported.14
GATHER1 Phase 2/3 trial (ClinicalTrials.gov Identifier: NCT02686658) investigated the safety and efficacy of ACP in 286 patients diagnosed with GA secondary to dry AMD. Patients were randomized into three arms: sham, 2mg monthly ACP or 4mg monthly ACP over 18 months. Patients demonstrated significant GA reduction in the 2mg (28.11%; p = 0.0072) and 4mg (29.97%; p = 0.0051) arms as early as 6 months into the trial.5,15 ACP has been well-tolerated across all patients with no drug-specific AEs reported.15
Efficacy of ACP in GA was further investigated in a confirmatory Phase 3 clinical trial, GATHER2 (ClinicalTrials.gov Identifier: NCT04435366) where 447 patients with only extrafoveal GA were enrolled in the study and randomized 1:1 into 2 arms: 2 mg ACP Q4W (n = 225) and sham (n = 222).16 Both study arms saw comparable patient retention rates and treatment fidelity rates above 90% through year one.17 At month 12, patients in the 2 mg ACP treatment arm demonstrated a 14.3% (p = 0.0064) reduction in mean rate of GA growth using square root transformation. Using the observed non-square root transformed data, patients treated with ACP saw a 17.3% (p = 0.0027) reduction in mean change in GA lesion area compared to the sham arm at month 12.17 ACP was well tolerated in GATHER2 without any cases of intraocular inflammation, endophthalmitis or ischemic optic neuropathy. There was a higher rate of CNV in patients treated with ACP compared to sham, 6.7% vs 4.1%. ACP is the first investigational therapeutic to treat GA that has achieved its prespecified 12-month primary endpoint of reduction in mean growth rate of GA in 2 separate pivotal Phase 3 clinical trials.17
Pegcetacoplan (APL-2) is a modified pegylated peptide that binds to C3 and C3b, ultimately blocking all three complement system pathways. The safety and tolerability of APL-2 was investigated in the Phase 2 FILLY trial (ClinicalTrials.gov Identifier: NCT02503332), in which 246 patients were randomized into four study arms: APL-2 15mg every month (PM) for 12 months; APL-2 15mg every other month (EOM) for 12 months; sham monthly Q4W; sham EOM for 12 months. Patients in the APL-2 15mg PM at 12 months demonstrated 29% reduction in GA growth, while patients APL-2 15mg EOM at 12 months demonstrated a 20% reduction on fundus autofluorescence (FAF) imaging.18 The Phase 3 trials, OAKS and DERBY, investigated the efficacy of IVT APL-2 in GA. A total of 1258 patients with GA secondary to AMD were randomized into 4 study arms: APL-2 15mg monthly (PM), APL-2 15mg every other month (PEOM), sham monthly and sham EOM.19 The OAKS study met the primary endpoint, demonstrating significant lesion growth reduction, but DERBY did not meet the same primary endpoint. Patients treated with APL-2 in the PM arm of the OAKS study saw 22% reduction (p = 0.0003) in mean change in growth of GA from baseline, while patients treated with APL-2 in the PEOM arm saw a 16% reduction (p = 0.0052) from baseline vs sham.18 In the DERBY study, patients in the PM arm saw a 12% reduction (p = 0.0528) while patients in the PEOM arm demonstrated a 11% reduction (p = 0.0750) in mean change of GA lesion growth.19 In terms of safety, the combined intraocular inflammation (IOI) rate in patients with ≥1 event of IOI was 9 (2.1%) in PM and 4 (1.0%) in PEOM groups. There were 3 cases of endophthalmitis in APL-2 treated groups. In addition, rate of CNV using reading center detected rates was 6.0% PM, 4.1% PEOM, and 2.4% sham.19 The GALE extension study will further investigate long-term safety and efficacy data for PM and PEOM dosing. APL-2 was granted Fast Track designation by the FDA. It is currently under review by the FDA with a decision regarding approval of this agent due in early 2023.
CATALINA (ClinicalTrials.gov Identifier: NCT04465955), a Phase 2 clinical trial investigated NGM621, a humanized immunoglobulin G1 monoclonal antibody that inhibits complement C3, reducing GA disease progression. Three hundred and twenty patients were randomized into 4 study arms: IVT administration of NGM621 every 4 weeks (Q4W), every 8 weeks (Q8W), sham Q4W, or sham Q8W for 52 weeks. Patients in the Q4W arm and Q8W arm demonstrated a 6.3% and 6.5% reduction in rate of change in GA lesion area, respectively.20 A favorable safety and tolerability profile was demonstrated, with low ocular inflammation rates and no drug-related severe AEs (SAEs) or endophthalmitis cases. CATALINA did not meet its primary endpoint due to low GA growth rate reduction.
Complement inhibitors have shown mixed results as therapeutic agents for the treatment of GA. Both avacincaptad pegol and pegcetacoplan have demonstrated meaningful reductions in GA lesion growth, while NGM621 unfortunately did not. Many therapeutics remain under investigation to delay disease progression and ultimately preserve macular function in patients with GA.
Neuroprotective Agents
Brimonidine, a selective α2 adrenergic (α2A) receptor agonist has demonstrated neuroprotection via various mechanisms, including upregulation of survival cell signaling and retinal ganglion cells releasing brain-derived neurotrophic factor (BDNF). Safety and efficacy of brimonidine was investigated (ClinicalTrials.gov Identifier: NCT00658619), in which 119 patients were randomized into three arms: IVT of 200µg brimonidine, IVT of 400µg brimonidine and sham.21 The 200µg and 400µg arms demonstrated an 18% reduction and 27% reduction in GA size, respectively. Brimonidine was well-tolerated with no AEs reported.21
BEACON, a Phase 2, double-blind clinical trial, is investigating the safety and efficacy of the brimonidine intravitreal implant in GA patients secondary to AMD (ClinicalTrials.gov Identifier: NCT02087085), where 310 patients are randomized into two treatment arms: 400µg brimonidine implant intravitreally administered on day 1, then Q3M through month 21 and sham treatment with needleless applicator.23,24 Results demonstrated a favorable safety profile with associated GA lesion reduction at primary endpoints; 10% (p = 0.047) at month 24 and 12% (p = 0.017) at month 30.22,23 Brimonidine was well-tolerated among patients with no unexpected AEs.25
Neuroprotective agents present the potential to delay photoreceptor death, which can delay the progression of GA. Safety and efficacy of neurotrophic factors with emphasis on their signaling cascades must be further investigated to prolong visual function in patients with GA.
Ocular Gene Therapy
One-time gene therapies may be a potential major advancement in retinal disease treatment. GT005, an AAV2-based, one-time investigational gene therapy that aims to restore complement system homeostasis by increasing complement factor I (CFI) protein production. Increased CFI expression reduces inflammation as it is a natural complement system down-regulator.26 The safety, dose response, and efficacy of GT005 administered via surgical, transvitreal subretinal injection (TVSI) in GA patients is currently being investigated in the Phase 1/2 FOCUS trial (ClinicalTrials.gov Identifier: NCT03846193).27 Patients were randomized into 3 GT005 dose levels: 2E10 vector genomes (vg), 5E10vg, and 2E11vg. GT005 demonstrated a positive safety profile at 2E10vg to 2E11vg. Mild AEs in the study eye were noted; however, there were no signs of SAEs or GT005-related inflammation.28 Ongoing Phase 2 clinical trials, EXPLORE (ClinicalTrials.gov Identifier: NCT04437368)29 and HORIZON (ClinicalTrials.gov Identifier: NCT04566445),30 are further investigating transvitreal subretinal administration of GT005 in patients with GA based on genotyping to identify patients with low CFI levels or a broader population, respectively.
Evidently, an overactive complement system plays a pivotal role in dry AMD development, and subsequently, GA disease progression. The accumulation of MAC on cell surfaces can lead to damage and atrophy, causing disease. CD59, a cell surface protein, blocks the formation of MAC, which is a pivotal step in complement-mediated cell lysis. Gene therapy requires one IVT injection with monthly monitoring visits for safety evaluation. A novel AAV2 gene therapy product, AAVCAGsCD59, was investigated as a gene therapy therapeutic to treat GA secondary to AMD in the Phase 1, dose-escalating HMR-1001 trial (ClinicalTrials.gov Identifier: NCT03144999).31 AAVCAGsCD59 increases the soluble form of CD59 (sCD59), which functionally mimics naturally-occurring CD59 (Figure 1). In the trial, 17 patients were randomized into 3 different arms with low, medium, and high IVT dosage. Patients in the high IVT dose arm demonstrated a decreased rate of GA progression compared to controls.32 Treatment among all study arms was well tolerated, with no dose-limiting toxicity or nAMD conversion.32
Ocular gene therapy has demonstrated a favorable safety profile and offers a life-changing treatment option among patients with GA. Further investigations are necessary to optimize delivery methods, as well as further evaluating risks of chronic gene expression.
Stem Cell Therapy
Stem cell therapy has demonstrated favorable potential in the treatment of GA, as transplantation of stem cells could regenerate RPE and compromised photoreceptors.33 Induced pluripotent stem (iPS) cells produce patient- and disease-specific cells that can derive RPE and photoreceptor cells and promote a lower risk for immune rejection.34 The safety and tolerability of OpRegen, human embryonic stem cell-derived RPE cells is being investigated in a current Phase 1/2a study (ClinicalTrials.gov Identifier: NCT02286089).35 Twenty-four subjects were enrolled and divided into four cohorts. Favorable safety signals were demonstrated with no reports of an inflammatory or immune response and increased visual functions among patients.
Careful consideration must be taken when determining an appropriate cell type for each patient, as cell derivation and transplantation techniques must be optimized. Safety and efficacy data, along with long-term clinical trial data are required to better equip ophthalmologists to incorporate this treatment option for GA patients.
A summary of the treatment modalities and associated clinical trials is depicted in Table 2.
 |
Table 2 Clinical Trials Investigating Therapeutics for GA via Various Treatment Modalities |
Conclusion
Ongoing clinical trials are investigating potential therapeutics for the treatment of GA secondary to AMD. Currently, there are no FDA approved treatments for GA, but we are inching closer to identifying therapeutic agents to help address this huge unmet need in the retina community. Several therapeutics have demonstrated promising results and preliminary data from various clinical trials have furthered investigation into therapeutics available to treat GA. Dry AMD and subsequent GA disease progression will require personalized treatment to determine the correct option for each patient. As investigators continue to study various treatment options for GA, ophthalmologists must carefully consider all treatment options and safety outcomes to determine the most efficacious modality. Improving the understanding of the pathophysiology of GA secondary to dry AMD, for patients, optometrists, and ophthalmologists alike, may further aid in correctly identifying and diagnosing patients, reduce disease burden among patients, and ultimately, prolong their visual function.
Disclosure
Dr. Netan Choudhry reports personal fees from Apellis for consulting, during the conduct of the study. Dr. Arshad M. Khanani and Dr. Carl Danzig are consultants for Iveric Bio and receive research grants. Dr. Carl Danzig also reports grants from Alexion, Adverum, Bayer, Gyroscope, Kodiak, Genentech, Regeneron, IvericBio, RegenxBio, Roche, and Novartis, outside the submitted work. Dr. Khanani is also a consultant for Apellis, Janssen, NGM BIO and Gyroscope and receives research grant funding. The authors report no other conflicts of interest in this work.
References
1. Harvey PT. Common eye diseases of elderly people: identifying and treating causes of vision loss. Gerontology. 2003;49:1–11. doi:10.1159/000066507
2. Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32(6):375–413. doi:10.1016/0039-6257(88)90052-5
3. Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren CM. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. doi:10.1016/j.ophtha.2013.11.023
4. Kawa MP, Machalinska A, Roginska D, Machalinski B. Complement system in pathogenesis of AMD: dual player in degeneration and protection of retinal tissue. J Immunol Res. 2014;2014:483960. doi:10.1155/2014/483960
5. Nebbioso M, Lambiase A, Cerini A, et al. Therapeutic approaches with intravitreal injections in geographic atrophy secondary to age-related macular degeneration: current drugs and potential molecules. Int J Mol Sci. 2019;20:1693. doi:10.3390/ijms20071693
6. Chiu C-J, Chang M-L, Zhang FF, et al. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol. 2014;158(1):118–127. doi:10.1016/j.ajo.2014.04.016
7. Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol. 2016;787:94–104. doi:10.1016/j.ejphar.2016.03.001
8. Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37:819–835. doi:10.1097/IAE.0000000000001392
9. Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi:10.1038/cr.2009.139
10. Cho H. Complement regulation: physiology and disease relevance. Korean J Pediatr. 2015;58:239–244. doi:10.3345/kjp.2015.58.7.239
11. Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576–586. doi:10.1016/j.ophtha.2020.08.027
12. Iveric Bio. Iveric Bio announces positive Zimura 18 month data supporting the 12 month efficacy findings: continuous positive treatment effect with favorable safety profile in geographic atrophy secondary to age-related macular degeneration in a phase 3 trial; 2020. Available from: https://investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-positive-zimura-18-month-data-supporting-12.
13. ClinicalTrials.gov. A phase I study of ARC1905 (anti-C5 aptamer) in subjects with dry age-related macular degeneration. ClinicalTrials.gov Identifier: NCT00950638. Available from: https://clinicaltrials.gov/ct2/show/NCT00950638.
14. Rezaei K A method for treating or preventing neovascular age-related macular degeneration. W O 2019/040397 A l ed: World Intellectual Property Organization – International Bureau; 2019.
15. Iveric Bio I. Iveric bio announces positive zimura 18 month data supporting the 12 month efficacy findings: continuous positive treatment effect with favorable safety profile in geographic atrophy secondary to age-related macular degeneration in a phase 3 trial; 2020. Available from: https://investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-positive-zimura-18-month-data-supporting-12.
16. ClinicalTrials.gov. A phase 3 safety and efficacy study of intravitreal administration of Zimura (complement C5 inhibitor) ClinicalTrials.gov identifier: NCT04435366. Available from: https://clinicaltrials.gov/ct2/show/NCT04435366.
17. Khanani AM, Patel SS, Staurenghi G, et al. GATHER2 pivotal phase 3 study results: efficacy of intravitreal avacincaptad pegol in geographic atrophy. Am Acad Ophthalmol. 2022;2022:1.
18. ClinicalTrials.gov. A phase II, multicenter, randomized, single-masked, sham-controlled study of safety, tolerability and evidence of activity of intravitreal APL-2 therapy in patients with geographic atrophy (GA). ClinicalTrials.gov Identifier: NCT02503332. Available from: https://clinicaltrials.gov/ct2/show/NCT02503332.
19. Heier J, Wykoff C, Singh R, et al. Efficacy of intravitreal pegcetacoplan in geographic atrophy: results from the DERBY and OAKS trials. Retina Society; 2021. Available from: https://investors.apellis.com/static-files/fe6d8c27-e1b2-4c87-b77f-aa5cb249bacf.
20. NGM bio announces topline results from the CATALINE phase 2 trial of NGM621 in patients with geographic atrophy (GA) secondary to age-related macular degeneration; 2022. Available from: https://ir.ngmbio.com/node/9586/pdf.
21. ClinicalTrials.gov. Safety and efficacy of brimonidine intravitreal implant in patients with geographic atrophy due to age-related macular degeneration (AMD) ClinicalTrials.gov identifier: NCT00658619. Available from: https://clinicaltrials.gov/ct2/show/NCT00658619.
22. Doozandeh A, Yazdani S. Neuroprotection in Glaucoma. J Ophthalmic Vis Res. 2016;11:209–220. doi:10.4103/2008-322X.183923
23. ClinicalTrials.gov. Safety and efficacy of brimonidine posterior segment drug delivery system in patients with geographic atrophy secondary to age-related macular degeneration clinicaltrials.gov identifier: NCT02087085. Available from: https://clinicaltrials.gov/ct2/show/NCT02087085.
24. Kuppermann BD, Patel SS, Boyer DS, et al.; Brimo DDS Gen 1 Study Group. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (brimo DDS) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina. 2021;41(1):144–155. doi:10.1097/IAE.0000000000002789
25. Freeman WR, Bandello F, Souied EH, et al. Lopez; phase 2b study of brimonidine DDS: potential novel treatment for geographic atrophy. Invest Ophthalmol Vis Sci. 2019;60(9):971.
26. Waheed NK FOCUS interim results: GT005 gene therapy phase I/II study for the treatment of geographic atrophy. angiogenesis, exudation, and degeneration; 2021. Available from: https://www.gyroscopetx.com/wp-content/uploads/2021/02/Waheed-Focus_FINAL.pdf.
27. ClinicalTrials.gov. Focus: first in human study to evaluate the safety and efficacy of GT005 administered in subjects with dry AMD” clinicaltrials.gov identifier: NCT03846193. Available from: https://clinicaltrials.gov/ct2/show/NCT03846193.
28. Nielsen J, MacLaren RE, Heier JS, et al. Preliminary results from a first-in-human phase I/II gene therapy study (FOCUS) of subretinally delivered GT005, an investigational AAV2 vector, in patients with geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2022;6`7):1504.
29. ClinicalTrials.gov. HORIZON: a Phase II study to evaluate the safety and efficacy of two doses of GT005. ClinicalTrials.gov Identifier: NCT04566445. Available from: https://clinicaltrials.gov/ct2/show/NCT04566445.
30. ClinicalTrials.gov. EXPLORE: a phase II study to evaluate the safety and efficacy of two doses of GT005. ClinicalTrials.gov Identifier: NCT04437368. Available from: https://clinicaltrials.gov/ct2/show/NCT04437368.
31. ClinicalTrials.gov. A phase 1, open-label, multi-center, dose-escalating, safety and tolerability study of a single intravitreal injection of AAVCAGsCD59 in patients with advanced non-exudative (dry) age-related macular degeneration with geographic atrophy. ClinicalTrials.gov Identifier: NCT03144999. Available from: https://clinicaltrials.gov/ct2/show/NCT03144999.
32. Dugel PU Data on a gene therapy for dry and wet AMD A phase 1 clinical trial program is targeting both disease states. Available from: https://www.retinalphysician.com/issues/2020/april-2020/clinical-trial-download-data-on-a-gene-therapy-for.
33. Cho MS, Kim SJ, Ku SY, et al. Generation of retinal pigment epithelial cells from human embryonic stem cell-derived spherical neural masses. Stem Cell Res. 2012;9:101–109. doi:10.1016/j.scr.2012.05.002
34. Du H, Lim SL, Grob S, Zhang K. Induced pluripotent stem cell therapies for geographic atrophy of age-related macular degeneration. Semin Ophthalmol. 2011;263:216–224. doi:10.3109/08820538.2011.577498
35. ClinicalTrials.gov. Safety and efficacy study of opregen for treatment of advanced dry-form age-related macular degeneration clinicalTrials.gov identifier: NCT02286089. Available from: https://clinicaltrials.gov/ct2/show/NCT02286089.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
