Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Emerging Treatment Options For Cancer-Associated Cachexia: A Literature Review
Authors Naito T
Received 29 August 2019
Accepted for publication 8 October 2019
Published 29 October 2019 Volume 2019:15 Pages 1253—1266
DOI https://doi.org/10.2147/TCRM.S196802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Video abstract presented by Tateaki Naito.
Views: 4058
Tateaki Naito
Division of Thoracic Oncology, Shizuoka Cancer Center, Shizuoka, Japan
Correspondence: Tateaki Naito
Division of Thoracic Oncology, Shizuoka Cancer Center, 1007 Shimonagakubo, Nagaizumi, Sunto District, Shizuoka 411-0934, Japan
Tel +81-55-989-5222
Fax +81-55-989-5783
Email [email protected]
Abstract: Cachexia is a disease that has been recognized since antiquity; however, research in this area has recently increased. Promising new agents, including anamorelin hydrochloride, have been tested in large randomized controlled studies, and multidrug as well as multimodal approaches have been proposed as having the potential to improve outcomes in patients with cancer cachexia. However, standard treatment remains elusive. This review summarizes the current literature on treatment of cancer-associated cachexia, showing that there are challenges associated with conducting clinical trials in such patients. First, poor recruitment, retention, and compliance among cachectic patients cause research delays. Second, the lack of consensus regarding clinically meaningful endpoints impedes standardization of study designs and results. Further consideration is needed to identify the most suitable study design and endpoints, which can lead to the development of pharmacological and nonpharmacological interventions that improve patients’ prognosis and outcomes.
Keywords: cancer cachexia, physical function, anamorelin, multimodal intervention
Introduction
Cachexia is a wasting condition associated with chronic diseases that has been known since ancient time in Europe as well as East Asia.1 In Greece, Hippocrates precisely described the core pathogenesis of cachexia already in the fourth century BC saying, “the flesh is consumed and becomes water.” He considered cachexia as a sign of death.2 The detrimental impact of cachexia on prognosis in cancer patients has been recognized since the early 20th century.3 In 2011, the medical community achieved a landmark consensus on the diagnostic and staging criteria,4 which have allowed cancer cachexia to be recognized based on few anthropometric measurements and a quick interview. However, in spite of considerable research efforts, there is still no standard treatment for cancer cachexia. This report aimed to review recent literatures on the development of therapeutic interventions for cancer cachexia to propose future research directions.
Skeletal Muscle Metabolism And Clinical Outcomes In Cancer Cachexia
To understand the trends in emerging therapeutic interventions, examining the pathogenesis of cancer cachexia is essential. Cachexia involves multiple organs, including skeletal muscles, adipose tissues, and the digestive, immune, or central nervous system.5,6 Among them, altered skeletal muscle metabolism might play the most important role in worsening clinical outcomes (Figure 1). The chronic systemic inflammation is provoked by the presence of tumor and its microenvironment.7 Physical inactivity in cancer patients further increases systemic inflammation due to reduced anti-inflammatory effect of chronic exercise.8,9 Infrequent contractions of skeletal muscles due to physical inactivity reduce anabolic stimuli for muscle protein synthesis in myocytes.10 Relative shortage of amino acids in skeletal muscle restricted protein synthesis because amino acids are mainly consumed for production of acute phase protein in liver.11 In addition, hypogonadism in male cancer patients,12 and tissue resistance to ghrelin13,14 and growth factors,15 further impede muscle protein synthesis. Cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, induce insulin resistance in liver, skeletal muscle, and adipose tissue, which, in turn, produce anabolic resistance.16 Increased levels of these cytokines17 as well as the presence of ghrelin resistance14 also affect hypothalamic appetite control and induce anorexia. At the same time, muscle degeneration is enhanced by the ubiquitin-proteasome or autophagy-lysosome pathways, which are induced by other pro-inflammatory mediators or tumor-derived factors. These factors might include IL-6, TNF-α, TNF-related weak inducer of apoptosis, parathyroid hormone-related peptide, or transforming growth factor-β superfamily (e.g., activins and myostatin).5 Overall, the physical dysfunction in cachectic patients might be caused by both quantitative18,19 and qualitative20,21 reduction in skeletal muscle, which, combined, further impede the patient’s physical activity,22,23 resulting in a vicious cycle (Figure 1). Over time, this means cachectic cancer patients often have a disability, require a longer hospital stay, and generate larger medical costs than patients without cachexia.24,25 In addition, patients with cachexia are more susceptible to toxicity of chemotherapy26,27 and often unable to complete planned chemotherapy cycles.28,29 Consequently, the presence of cancer cachexia is associated with poor prognosis and low quality of life (QOL) from at the time of diagnosis,30 through treatment31 to near the end of the cancer trajectory.32 As cachexia is a complex disease, each component of its pathogenesis is a potential target for interventions to improve outcomes. In addition, the multifactorial processes associated with cachexia suggest a need for multidrug or multimodal approaches to this condition.
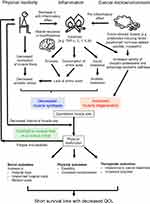 |
Figure 1 Skeletal muscle metabolism and clinical outcomes in cancer cachexia. Abbreviations: TNF, tumor necrosis factor; IL, interleukin; QOL, quality of life. |
Methods
Randomized controlled trials and systematic reviews for therapeutic interventions for cancer cachexia were identified by searching the PubMed using the following keywords in August 2019:
(cachexia[tiab] OR cachectic[tiab] OR Malnutrition[Mesh] OR malnutrition[tiab] OR ‘muscle wasting’[tiab] OR ‘muscular wasting’[tiab] OR ‘Muscle Weakness’[Mesh] OR ‘muscle weakness’[tiab] OR ‘muscular weakness’[tiab] OR sarcopenia[tiab] OR ‘Wasting syndrome’[MeSH:noexp] OR ‘wasting syndrome’[tiab] OR ‘weight loss’[tiab]) AND. (Neoplasms[MeSH] OR cancer[tiab] OR tumor[tiab] OR tumour[tiab] OR neoplas[tiab] OR malignan[tiab] OR carcinoma[tiab] OR adenocarcinoma[tiab] OR choricarcinoma[tiab] OR leukemia[tiab] OR leukaemia[tiab] OR metastat[tiab] OR sarcoma[tiab] OR teratoma[tiab])
The pre-specified inclusion criteria were articles in the English language; studies involving adults. Studies on hematologic malignancies, surgically operable cancers, cancer survivors, or noncancer populations were excluded. Regarding pharmacological interventions, agents which have been tested in phase 3 randomized controlled trials were mainly chosen. Information for ongoing trials were collected from trial registration site, reports of regulatory authority, or publications for study protocol. Entry criteria, cachectic status of participants, concurrent treatments, types of intervention, efficacy, and major toxicities in each study were summarized. Cachectic status was classified according to the consensus report.4
Results
Pharmacological Interventions
Randomized controlled trials with agents such as corticosteroids,33 progestins,34 nonsteroidal anti-inflammatory drugs (NSAIDs),35 thalidomide,36 and eicosapentaenoic acids (EPA)37 have been conducted to develop pharmacological interventions for cancer cachexia. Although each intervention improved some aspects of the condition, no reliable or clinically relevant effect on patient functioning or QOL was reported.38 In addition, some agents were associated with risks that outweighed their benefits.39 Previously reported treatment-associated complications included deep venous thrombosis or edema in progestins, glucose intolerance in corticosteroids, and gastrointestinal or renal toxicities in NSAIDs. As a result, no single agent was identified as a suitable standard treatment for cancer cachexia. However, recently, efforts have been made to develop novel agents or a way to combine available agents to improve treatment safety and effectiveness. Among various regimens, anamorelin hydrochloride, MABp1, enobosarm, and several multidrug combinations were tested in the phase 3 randomized controlled trials. Subsequent candidate agents were also tested in the recent phase 2 trials, which include espendrol40 (novel non-selective β blocker), testosterone,41 nabilone42 (cannabinoid), and LY249565543 (anti-myostatin antibody).
Anamorelin Hydrochloride
Anamorelin is a novel, orally active, selective ghrelin receptor agonist with appetite-enhancing and anabolic activity.44 It positively affects lean body mass (LBM) through increased secretion of the growth hormone, insulin-like growth factor 1, and insulin-like growth factor-binding protein 3 through activation of the ghrelin receptor. During phase 1 and 2 trials, anamorelin has been shown to enhance appetite and increase LBM, while maintaining a desirable tolerance profile (Table 1).45,46 Moreover, in two phase 2 studies based in Japan, anamorelin (100 mg daily for 12 weeks) was associated with an increase in LBM, body weight, and appetite in patients who had advanced non-small-cell lung cancer (NSCLC) with cachexia.47,48 Finally, two multinational phase 3 studies (ROMANA 1 and 2 trials) confirmed the effect of anamorelin (100 mg for 12 weeks) on increasing LBM and body weight, and improving anorexia/cachexia-specific QOL among patients with NSCLC and cachexia.49 Subsequently, an extension study (ROMANA 3), involving participants who had completed the ROMANA 1 and 2 trials, assessed the safety and feasibility of prolonged use of anamorelin over 24 weeks.50 Among the 345 patients who completed ROMANA 1 or 2 in the anamorelin group, 221 patients (64%) completed a 24-week of anamorelin 100 mg daily with a mean of 161.1 treatment days. Anamorelin was well tolerated and the incidence of treatment-related adverse events (AEs) was similar in the anamorelin (3.5%) and placebo (1.2%) groups without any grade ≥3 AEs. The most common treatment-related AEs in ROMANA 3 trial were hyperglycemia (1.2%), which is consistent with the results in ROMANA 1 (5.3%) and 2 (4.2%) trials. In addition, anamorelin, but not placebo, resulted in a progressive increase in body weight over the entire 24 weeks of the treatment period. Moreover, the alleviating effect of anamorelin on cachexia-related anorexia was maintained throughout the treatment period. Recently, anamorelin was further evaluated in patients with advanced gastrointestinal cancer and cachexia in a non-randomized single-arm study.51 This study also showed a positive effect of anamorelin on LBM, body weight, and anorexia that was comparable to the aforementioned data in patients with lung cancer. Finally, two meta-analyses52,54 also strongly supported the positive effect of anamorelin on LBM and body weight. However, in these studies, compared to placebo, anamorelin did not improve physical functions, measured with hand-grip strength44–50,52,53 or a 6-min walk.48
MABp1
MABp1 is a human IgG1 monoclonal antibody specific to human interleukin-1α. In an open-label, phase 1 dose-escalation study, 52 patients with 18 types of refractory malignancies were enrolled.54 MABp1 was well-tolerated and no dose-limiting toxicities were observed. For 30 patients whose data could be accessed, LBM increased by a mean of 1.02 kg during the 8-week treatment period. In a multinational double-blind, placebo-controlled phase 3 study, 333 patients with advanced colorectal cancer who have failed oxaliplatin- and irinotecan-based chemotherapy and had debilitating symptoms were randomly assigned to receive intravenous infusion of MABp1 (7.5 mg/kg) or placebo given every 2 weeks for 8 weeks until disease progression or unacceptable toxicity.55 Concomitant chemotherapy and radiotherapy were restricted. A responder was defined as a patient having a stable or increased LBM, and maintenance or improvement in two of three other symptoms (pain, fatigue, or anorexia). In this trial, MABp1 was significantly associated with a higher response rate than placebo (33% vs 19%). However, there were no significant differences between the MABp1 and the placebo arms in the LBM or QOL change from baseline to week 8. Physical function was not measured. Twenty-six patients experienced treatment-related AEs including edema, nausea, and anemia. The majority were grade 1or 2, and only few were grade 3, with no grade 4 or 5 cases included. However, there was an imbalance in infection-related AEs (11.6 vs 7.8%) and severe AEs (2.4 vs 0%) in the MABp1 and placebo arm, although these were reported as “not related” to treatment.56 Furthermore, another phase 3 study was planned to compare overall survival between MABp1 and megestrol acetate (MA) in 656 patients with metastatic colorectal cancer and cachexia. However, the study was terminated early because the study crossed the prospective futility boundary of the primary endpoint.57
Enobosarm
Enobosarm is an oral nonsteroidal selective androgen receptor modulator.58 In a phase 2, double-blind, placebo-controlled study in healthy postmenopausal women and elderly men, enobosarm increased LBM with a clinically meaningful improvement in physical performance measured by the Stair Climb Test.59 In a phase 2b, double-blind, placebo-controlled study, 159 patients with advanced cancer of different types and cachexia (≥2% weight loss in the preceding 6 months) were randomized to receive enobosarm 1 mg, enobosarm 3 mg, or placebo for up to 113 days.60 The primary endpoint was change in LBM from baseline. Both enobosarm arms significantly increased LBM compared to baseline; no improvement was observed in the placebo arm. In addition, performance on the Stair Climb Tests significantly improved in the enobosarm arms but not in the placebo arm. However, there was no significant difference in handgrip strength. No direct comparison between treatment arms was presented. No specific treatment-related or androgenic AEs were reported including negative effects on the prostate, virilization, or hirsutism. Finally, two large phase 3 studies (POWER 1 and 2 trials) enrolled 641 men or postmenopausal women with advanced NSCLC.61–63 Patients were randomized at initiation of first-line chemotherapy based upon the planned chemotherapy regimen: platinum + taxane (POWER 1, n=321) or platinum + non-taxane (POWER 2, n=320) and received either enobosarm 3 mg or placebo for 5 months. The primary outcomes were the percentage of responders with stair climb power change ≥10% or LBM change ≥10% from baseline at day 84. Patient accrual and data collection were reportedly completed on May 2013.62,63 However, the results have not been published to-date. Limited data from the POWER 1 trial are available from the trial registration site at the time of writing.62 According to this data, the response rate in stair climb power was 29.4% (95% confidence interval [CI]: 22.4–37.1) in enobosarm and 24.2% (95% CI: 17.8–31.6) in the placebo group. The response rate in LBM was 41.9% (95% CI: 34.1–49.9) in enobosarm and 30.4% (95% CI: 23.4–38.2) in the placebo group. The final study results are currently awaited.
Multidrug Combination
As shown in Figure 1, systemic inflammation is the central mechanism of cancer cachexia. Much effort has been made to develop multidrug combinations, including different types of anti-inflammatory agents, such as the MA, EPA, thalidomide, and NSAIDs (Table 2). In 2010, a large randomized phase 3 study showed that a combination of classic anticachectic medications is more effective than any one of these medications on its own.64 A total of 332 patients with advanced cancer and cachexia were randomly assigned to one of the five treatment arms: (1) medroxyprogesterone (500 mg/day) or MA (320 mg/day); (2) EPA; (3) L-carnitine; (4) thalidomide; and (5) a combination of all agents. The primary endpoints were: an increase in LBM, a decrease in resting energy expenditure (REE), and decrease in fatigue. After two interim analyses, arms 1 and 2 were withdrawn due to significant inferiority for primary endpoints. A post hoc analysis showed a superiority of arm 5 over the others for all primary and secondary endpoints, including appetite, IL-6, Glasgow Prognostic Score (GPS), physical activity, and performance status. Several studies since have also reported that combination treatments are more effective than monotherapy.65,66 For example, Wen et al reported results of a randomized controlled study comparing MA with MA + thalidomide for 8weeks in 102 cachectic patients with advanced cancer.65 The combination arm showed significantly greater improvements than the MA arm in body weight, hand grip strength, performance status, QOL, GPS, and fatigue. Toxicity was relatively negligible in both arms. Moreover, Macciò et al also reported the combination of MA + L-carnitine, celecoxib, and antioxidants was more effective than MA alone with respect to LBM, REE, fatigue, and global QOL in a randomized phase 3 study involving 104 patients with advanced gynecological tumor and cachexia.66
 |
Table 1 Trials Of Anamorelin Hydrochloride In Patients With Cancer Cachexia |
 |
Table 2 Trials Of Multidrug Combinations In Patients With Cancer Cachexia |
However, more is not always the better.67–69 In a large randomized controlled study comparing EPA, MA, and EPA + MA for cachectic patients with advanced cancer, there was no significant difference between groups in body weight and appetite.67 Madeddu et al reported that a two-drug combination of L-carnitine + celecoxib was non-inferior to a three-drug combination of L-carnitine + celecoxib + MA, with both arms showing a significant increase from baseline in LBM and physical performance.68 In addition, Kouchaki et al recently reported that two-drug combination of MA + celecoxib was not superior to MA + placebo in a randomized phase 3 study of 96 patients with advanced gastrointestinal cancer and cachexia.69 Differences in outcomes among these studies might depend on tumor types, concomitant treatments, or interactions between combined medications. Although the combination treatment, especially the MA-containing regimens, appears to have the most potential to alleviate signs and symptoms of cancer cachexia, no standard combination treatment has been established to-date.
Nonpharmacological Interventions
The consensus report on cancer cachexia proposed early introduction of combined nutritional, physical, and psychosocial interventions.4 Following the publication of this consensus report, nonpharmacological interventions have been widely tested.1 Moreover, many clinical trials selected study populations at an earlier stage of the cachexia and included patients with precachexia or those at high risk for cachexia. As a result, interventions tended to start earlier, alongside active cancer treatment.
Monomodal Intervention
Although cancer cachexia cannot be treated with nutritional therapy alone,70,71 optimum nutrition is an important part of any multimodal intervention aimed at increasing energy intake39 and alleviating psychosocial stress.72 In addition, nutritional supplements enriched with n-3 polysaturated fatty acids, such as EPA, might have benefits in cancer cachexia patients.73,74 Furthermore, exercise might also be an important part of an anticachectic intervention. Physical inactivity in cancer patients may largely contribute to systemic inflammation and altered muscle metabolism. Exercise may improve muscle mass and strength, physical function, fatigue, and QOL in patients with advanced cancer.75–77 Exercise may also directly prevent unfavorable consequences of cancer cachexia, including disability, which, overall, might prevent the effect of a vicious cycle described in the introduction to this review (Figure 1). However, evidence from well-designed clinical studies on exercise interventions for patients with cancer cachexia is limited.78 Low recruitment, high attrition rate, and poor compliance with exercise interventions79,80 are some of the challenges associated with clinical trials in cachectic patients with advanced cancer.
Multimodal Intervention
At the time of writing, there is no standard multimodal intervention combining nutrition and exercise for patients with cancer cachexia (Table 3). However, Solheim et al81 recently reported results of their multinational randomized phase 2 study (Pre-MENAC study) of multimodal intervention in patients who had advanced non-small-cell lung cancer and pancreatic cancer with or without cachexia. Their interventions consisted of nutritional counseling, exercise intervention, celecoxib, and supplements rich in EPA. The primary endpoint was feasibility. It took 30 months to recruit 46 patients; the recruitment rate was 11.5%. A total of 8% dropped out in the treatment arm. Overall compliance was 76% for celecoxib, 60% for exercise, and 48% for supplements. However, compliance for combination of two or three treatments was only 20–48% or 12%, respectively, suggesting there is a trade-off between number of interventions and a level of compliance that can be expected. Nevertheless, the efficacy of this intervention is currently being tested in a large randomized controlled study (MENAC study), where body weight is the primary endpoint.82 Meanwhile, Uster et al reported results of a single-center randomized controlled trial involving combined nutritional and exercise intervention for patients with advanced gastrointestinal or lung cancer with or without cachexia.83 The aim of the trial was to assess any clinically relevant improvement to global QOL. It took 32 months to recruit 58 patients; the recruitment rate was 13.0%. The overall attrition rate was 14.2%, while 67% of the patients allocated to the intervention arm were compliant with the intervention. Although there was an increase in the amount of protein intake, there was no improvement in the global QOL, weight, fatigue, or physical function, including HGS, sit-to-stand test, and leg strength measures. These reports showcase the challenges associated with trial recruitment and achieving compliance with nutritional or exercise interventions in patients with advanced cancer.84 The burden of multiple assessments, extra effort, and time spent on engaging with the intervention might have decreased compliance. Inclusion of patients at later disease stages may further limit the feasibility and efficacy of the potentially promising interventions.
 |
Table 3 Trials Of Multimodal Interventions For Patients With High-Risk For Cancer Cachexia |
To overcome these hurdles, another type of nonpharmacological multimodal intervention for cancer cachexia has been developed: The Nutrition and Exercise Treatment for Advanced Cancer (NEXTAC) program.85 This program combined nutritional counseling, low‑intensity home‑based resistance training, and counseling focused on encouraging physical activity. It was designed to prevent disability in elderly patients at risk for cachexia, newly diagnosed with advanced NSCLC or pancreatic cancer, due to start systemic chemotherapy. Results of the Phase I feasibility study of this new intervention (NEXTAC‑ONE) have already been reported.85 It took 9 months to recruit 30 patients, and the recruitment rate was 63%. The attrition rate was 3%. Participants showed excellent attendance (96.7%) and compliance with each intervention (≥90%) in the program. The majority of patients also applied the insights from health education and changed their health‑related behavior by, for example, increasing indoor or outdoor activity.86 No severe AEs were reported. Subsequently, a prospective, multicenter, randomized phase 2 clinical study (NEXTAC‑TWO) has been launched, aiming to increase disability-free survival in elderly patients with advanced cancer.87 A total of 130 patients are planned to be randomized to usual care or usual care plus NEXTAC in a 1:1 ratio. It has been hypothesized that the NEXTAC prolongs disability-free survival by 4 months compared to the usual care (80% power). Other multimodal interventions in different tumor types and clinical setting are currently being tested.84,88 The results of these ongoing studies are awaited because there was no report on nutrition/exercise interventions which definitively improved muscle mass or physical function in patients with cancer cachexia to-date. If one of these multimodal programs is shown as feasible and effective, it might be combined with newly emerging pharmacological interventions for cachexia to further improve functional prognosis and socioeconomic outcomes.
Discussion
Trial Challenges: Recruitment, Attrition, And Compliance
There are several challenges to conducting clinical studies of cancer cachexia. Trial recruitment is likely to be low,79–81 compliance is likely to be low, and dropout rates are likely to be high in clinical trials of pharmacological89 and nonpharmacological interventions.90 These challenges are partially accounted for by the vulnerability of cachectic cancer patients. For example, Temel et al reported results of a feasibility study of structured, moderate-intensity exercise program for patients with advanced NSCLC.91 Twenty-four percent of participants withdrew before attending any sessions; only 44% completed all planned study sessions. The reasons for withdrawal or noncompliance included deterioration of health status, feeling unwell, concerns about the amount of travel involved, and hospitalizations. As a result, it has been suggested that future trials in cancer cachexia patients should have less stringent entry criteria, and involve less exhaustive outcome measures.89
Lack Of Widely Accepted Endpoints
Despite increase in the number and scope of cancer cachexia studies, the ultimate goal of these trials, and cancer cachexia care, remains to be established.1 Selected endpoints, variables and measurements of interest, and statistical analyses used change, depending on the tested hypothesis, or preference of researchers or study sponsors. Such variation in endpoints decreases comparability of trial results and impede standardization of study results. In addition, there is no consensus among researchers, pharmaceutical companies, and regulatory authorities regarding constitutes a “clinically relevant” outcome.92 For example, although anamorelin has been associated with a significant increase in LBM, weight, and appetite among patients with advanced NSCLC,46–50 the drug was refused marketing authorization by the European Medicines Agency (EMA) due to undesirable risk-benefit profile.93 The EMA concluded that efficacy of anamorelin had not been established as there was only a marginal effect on LBM and no reliable and clinically relevant effect on patient functioning or QOL. A similar decision was made regarding MABp1. Although a well-designed phase 3 randomized controlled study met its primary endpoints,55 a recent EMA opinion refused to authorize its marketing due to the lack of clear improvements in LBM or QOL, and risk of infection considered “unacceptable”.56 Further regulatory review is pending in Europe. This regulatory reluctance to grant approval based on currently used endpoints suggests there is an urgent need to reconsider what is “clinically relevant” in cancer cachexia research and care, and meet the demands of patients, researchers, and regulatory authorities. Although concomitant improvement in skeletal muscle mass, physical function, QOL, and overall survival may be the “ideal” endpoint, these parameters do not always co-occur. For example, gain in LBM was not always associated with improvement in physical function46–49,60,66,94,95 or QOL.60,64,95 We have to determine the priority for outcomes among various endpoints used in the previous clinical studies including body weight, LBM, symptoms, physical functions, prognosis, or QOL.
Limitation
This review has several limitations. First, the literature search was carried out using only PubMed. Second, a single reviewer (T.N.) carried out the selection of articles for inclusion. These drawbacks may result in a potential selection bias in the establishment of a reference list. Finally, information for ongoing trials was obtained from trial registration site, reports of regulatory authority, or publications for study protocol at the time of writing (Aug 2019). Based on these limitations, we should pay careful attention while interpreting the contents.
Conclusion
Clinical trials evaluating treatments for cancer cachexia are increasing in number. Promising new agents, including anamorelin, MABp1, and enobosarm are being investigated. Multidrug and multimodal approaches are expected to improve poor outcomes in patients with cancer cachexia. However, there are several challenges to conducting clinical trials and developing treatment standards in this area. The most meaningful endpoint of cachexia care might be prolonging active life with satisfying QOL. Although the established endpoint, such as body mass increase, might be an important outcome, it may not always contribute as a true endpoint. Thus, a novel study design and a high-priority endpoint are required before a combination of pharmacological and nonpharmacological interventions that improve functional patient outcomes can be delivered.
Acknowledgments
The author would like to thank the investigators and staff as the Thoracic Oncology Division of the Shizuoka Cancer Center, Japan. The author would like to acknowledge Toshiaki Takahashi, MD, Koichi Takayama, MD, Kazuo Tamura, MD, Ms. Kanae Sasaga, and Ms. Hiromi Sakakibara for their generous instruction and support in conducting research in cancer cachexia.
Disclosure
Tateaki Naito received honoraria from ONO pharmaceutical company. The author reports no other conflicts of interest in this work.
References
1. Naito T. Evaluation of the true endpoint of clinical trials for cancer cachexia. Asia Pac J Oncol Nurs. 2019;6(3):227–233. doi:10.4103/apjon.apjon_68_18
2. KATZ AM, KATZ PB. Diseases of the heart in the works of hippocrates. Br Heart J. 1962;24:257–264. doi:10.1136/hrt.24.3.257
3. Warren S. The immediate cause of death in cancer. Am J Med Sci. 1932;184:610–619. doi:10.1097/00000441-193211000-00002
4. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi:10.1016/S1470-2045(10)70218-7
5. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi:10.1038/nrdp.2017.105
6. Argiles JM, Busquets S, Stemmler B, et al. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100–106.
7. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659.
8. Timmerman KL, Flynn MG, Coen PM, et al. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise?.J Leukoc Biol. 2008;84(5):1271–1278.
9. Koelwyn GJ, Quail DF, Zhang X, et al. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17(10):620–632.
10. Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol (1985). 2009;106(4):1374–1384. doi:10.1152/japplphysiol.91397.2008
11. Lundholm K, Edstrom S, Ekman L, Karlberg I, Bylund AC, Scherstén T. A comparative study of the influence of malignant tumor on host metabolism in mice and man: evaluation of an experimental model. Cancer. 1978;42(2):453–461. doi:10.1002/1097-0142(197808)42:2<453::aid-cncr2820420212>3.0.co;2-t
12. Garcia JM, Li H, Mann D, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106(12):2583–2591. doi:10.1002/cncr.21889
13. Shimizu Y, Nagaya N, Isobe T, et al. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9(2):774–778.
14. Garcia JM, Garcia-Touza M, Hijazi RA, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90(5):2920–2926. doi:10.1210/jc.2004-1788
15. Crown AL, Cottle K, Lightman SL, et al. What is the role of the insulin-like growth factor system in the pathophysiology of cancer cachexia, and how is it regulated? Clin Endocrinol (Oxf). 2002;56(6):723–733. doi:10.1046/j.1365-2265.2002.01540.x
16. Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol. 2018;29(suppl_2):ii18–ii26. doi:10.1093/annonc/mdx815
17. Burfeind KG, Michaelis KA, Marks DL. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol. 2016;54:42–52. doi:10.1016/j.semcdb.2015.10.038
18. Naito T, Okayama T, Aoyama T, et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer. 2017;17(1):571. doi:10.1186/s12885-017-3562-4
19. Kinsey E, Ajazi E, Wang X, Johnston MAM, Crawford J. Predictors of physical and functional loss in advanced-stage lung cancer patients receiving platinum chemotherapy. J Thorac Oncol. 2018;13(9):1294–1301. doi:10.1016/j.jtho.2018.05.029
20. Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front Physiol. 2017;8:87. doi:10.3389/fphys.2017.00087
21. Ruas JL, White JP, Rao RR, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151(6):1319–1331. doi:10.1016/j.cell.2012.10.050
22. Moses AW, Slater C, Preston T, Barber MD, Fearon KCH. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3fatty acids. Br J Cancer. 2004;90(5):996–1002. doi:10.1038/sj.bjc.6601620
23. Morikawa A, Naito T, Sugiyama M, et al. Impact of cancer cachexia on hospitalization-associated physical inactivity in elderly patients with advanced non-small-cell lung cancer. Asia Pac J Oncol Nurs. 2018;5(4):377–382. doi:10.4103/apjon.apjon_20_18
24. Arthur ST, Van Doren BA, Roy D, Noone JM, Zacherle E, Blanchette CM. Cachexia among US cancer patients. J Med Econ. 2016;19(9):874–880. doi:10.1080/13696998.2016.1181640
25. Naito T, Okayama T, Aoyama T, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer. 2017;17(1):800. doi:10.1186/s12885-017-3795-2
26. Chowdhry SM, Chowdhry VK. Cancer cachexia and treatment toxicity. Curr Opin Support Palliat Care. 2019. [Epub ahead of print]. doi:10.1097/SPC.0000000000000450
27. Fujio T, Nakashima K, Naito T, et al. Platinum combination chemotherapy is poorly tolerated in malnourished advanced lung cancer patients with poor performance status. Nutr Cancer. 2019;71(5):767–771. doi:10.1080/01635581.2018.1559941
28. Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90(10):1905–1911. doi:10.1038/sj.bjc.6601781
29. Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34(4):503–509. doi:10.1016/s0959-8049(97)10090-9
30. Takayama K, Atagi S, Imamura F, et al. Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients-Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support Care Cancer. 2016;24(8):3473–3480. doi:10.1007/s00520-016-3156-8
31. Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015;23(6):1699–1708. doi:10.1007/s00520-014-2534-3
32. Kubo Y, Naito T, Mori K, Osawa G, Aruga E. Skeletal muscle loss and prognosis of breast cancer patients. Support Care Cancer. 2017;25(7):2221–2227. doi:10.1007/s00520-017-3628-5
33. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31(25):3076–3082. doi:10.1200/JCO.2012.44.4661
34. Ruiz-Garcia V, Lopez-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia-anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444–452. doi:10.1002/jcsm.12292
35. Solheim TS, Fearon KC, Blum D, Kaasa S. Non-steroidal anti-inflammatory treatment in cancer cachexia: a systematic literature review. Acta Oncol. 2013;52(1):6–17. doi:10.3109/0284186X.2012.724536
36. Reid J, Mills M, Cantwell M, et al. Thalidomide for managing cancer cachexia. Cochrane Database Syst Rev. 2012;(4):CD008664.
37. Lavriv DS, Neves PM, Ravasco P. Should omega-3 fatty acids be used for adjuvant treatment of cancer cachexia? Clin Nutr ESPEN. 2018;25:18–25. doi:10.1016/j.clnesp.2018.02.006
38. Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol. 2014;25(8):1492–1499. doi:10.1093/annonc/mdu085
39. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi:10.1016/j.clnu.2016.07.015
40. Stewart Coats AJ, Ho GF, Prabhash K, et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: a randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial). J Cachexia Sarcopenia Muscle. 2016;7(3):355–365. doi:10.1002/jcsm.12126
41. Wright TJ, Dillon EL, Durham WJ, et al. A randomized trial of adjunct testosterone for cancer-related muscle loss in men and women. J Cachexia Sarcopenia Muscle. 2018;9(3):482–496. doi:10.1002/jcsm.12295
42. Turcott JG, Del Rocío Guillen Núñez M, Flores-Estrada D, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. Support Care Cancer. 2018;26(9):3029–3038. doi:10.1007/s00520-018-4154-9
43. Golan T, Geva R, Richards D, et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J Cachexia Sarcopenia Muscle. 2018;9(5):871–879. doi:10.1002/jcsm.12331
44. Garcia JM, Polvino WJ. Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncologist. 2007;12(5):594–600. doi:10.1634/theoncologist.12-5-594
45. Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21(1):129–137. doi:10.1007/s00520-012-1500-1
46. Garcia JM, Boccia RV, Graham CD, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16(1):108–116. doi:10.1016/S1470-2045(14)71154-4
47. Takayama K, Katakami N, Yokoyama T, et al. Anamorelin (ONO-7643) in Japanese patients with non-small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer. 2016;24(8):3495–3505. doi:10.1007/s00520-016-3144-z
48. Katakami N, Uchino J, Yokoyama T, et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer. 2018;124(3):606–616. doi:10.1002/cncr.31128
49. Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17(4):519–531. doi:10.1016/S1470-2045(15)00558-6
50. Currow D, Temel JS, Abernethy A, Milanowski J, Friend J, Fearon KC. ROMANA 3: a phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann Oncol. 2017;28(8):1949–1956. doi:10.1093/annonc/mdx192
51. Hamauchi S, Furuse J, Takano T, et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer. 2019. [Epub ahead of print]. doi:10.1002/cncr.32406
52. Nishie K, Yamamoto S, Nagata C, Koizumi T, Hanaoka M. Anamorelin for advanced non-small-cell lung cancer with cachexia: systematic review and meta-analysis. Lung Cancer. 2017;112:25–34. doi:10.1016/j.lungcan.2017.07.023
53. Bai Y, Hu Y, Zhao Y, et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Support Care Cancer. 2017;25(5):1651–1659. doi:10.1007/s00520-016-3560-0
54. Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15(6):656–666. doi:10.1016/S1470-2045(14)70155-X
55. Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18(2):192–201.
56. European Medicines Agency. Assessment report. Human IGG1 monoclonal antibody specific for human interleukin-1 alpha XBiotech. EMA/CHMP/552293/2017; September 14, 2017 Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/human-igg1-monoclonal-antibody-specific-human-interleukin-1-alpha-xbiotech.
57. U.S. National Library of Medicine. ClinicalTrials.gov: a Phase III study of Xilonix in PATIENTS With Advanced Colorectal Cancer (XCITE). Available from: https://clinicaltrials.gov/ct2/show/NCT01767857. Accessed August 25, 2019.
58. Chen J, Hwang DJ, Chung K, et al. In vitro and in vivo structure-activity relationships of novel androgen receptor ligands with multiple substituents in the B-ring. Endocrinology. 2005;146(12):5444–5454.
59. Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2(3):153–161.
60. Dobs AS, Boccia RV, Croot CC, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14(4):335–345.
61. Crawford J, Prado CM, Johnston MA, et al. Study design and rationale for the Phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER trials). Curr Oncol Rep. 2016;18(6):37.
62. U.S. National Library of Medicine. ClinicalTrials.gov: Phase III study of the effect of GTx-024 on muscle wasting in patients with Non-Small Cell Lung Cancer (NSCLC). Available from: https://clinicaltrials.gov/ct2/show/NCT01355484.
63. U.S. National Library of Medicine. ClinicalTrials.gov: effect of GTx-024 on muscle wasting in patients with Non-Small Cell Lung Cancer (NSCLC) on first line platinum. Available from: https://clinicaltrials.gov/ct2/show/NCT01355497. Accessed August 25, 2019.
64. Mantovani G, Maccio A, Madeddu C, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15(2):200–211.
65. Wen HS, Li X, Cao YZ, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy. 2012;58(6):461–467.
66. Maccio A, Madeddu C, Gramignano G, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol. 2012;124(3):417–425.
67. Jatoi A, Rowland K, Loprinzi CL, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol. 2004;22(12):2469–2476.
68. Madeddu C, Dessi M, Panzone F, et al. Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib ± megestrol acetate for patients with cancer-related anorexia/cachexia syndrome. Clin Nutr. 2012;31(2):176–182.
69. Kouchaki B, Janbabai G, Alipour A, et al. Randomized double-blind clinical trial of combined treatment with megestrol acetate plus celecoxib versus megestrol acetate alone in cachexia-anorexia syndrome induced by GI cancers. Support Care Cancer. 2018;26(7):2479–2489.
70. Baldwin C. The effectiveness of nutritional interventions in malnutrition and cachexia. Proc Nutr Soc. 2015;74(4):397–404.
71. Blackwood HA, Hall CC, Balstad TR, et al. A systematic review examining nutrition support interventions in patients with incurable cancer. Support Care Cancer. 2019. [Epub ahead of print]. doi:10.1007/s00520-019-04999-4
72. Oberholzer R, Hopkinson JB, Baumann K, et al. Psychosocial effects of cancer cachexia: a systematic literature search and qualitative analysis. J Pain Symptom Manage. 2013;46(1):77–95.
73. Murphy RA, Yeung E, Mazurak VC, et al. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br J Cancer. 2011;105(10):1469–1473.
74. de van der Schueren MAE, Laviano A, Blanchard H, et al. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. 2018;29(5):1141–1153.
75. Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297–2305.
76. Heywood R, McCarthy AL, Skinner TL. Efficacy of exercise interventions in patients with advanced cancer: a systematic review. Arch Phys Med Rehabil. 2018;99(12):2595–2620.
77. Stene GB, Helbostad JL, Balstad TR, et al. Effect of physical exercise on muscle mass and strength in cancer patients during treatment–a systematic review. Crit Rev Oncol Hematol. 2013;88(3):573–593.
78. Grande AJ, Silva V, Riera R, et al. Exercise for cancer cachexia in adults. Cochrane Database Syst Rev. 2014;(11):CD010804.
79. Maddocks M, Murton AJ, Wilcock A. Therapeutic exercise in cancer cachexia. Crit Rev Oncog. 2012;17(3):285–292.
80. Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–132.
81. Solheim TS, Laird BJA, Balstad TR, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(5):778–788.
82. Solheim TS, Laird BJA, Balstad TR, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care. 2018;8(3):258–265.
83. Uster A, Ruehlin M, Mey S, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. 2018;37(4):1202–1209.
84. Hall CC, Cook J, Maddocks M, et al. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: a systematic review. Support Care Cancer. 2019;27(7):2371–2384.
85. Naito T, Mitsunaga S, Miura S, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle. 2019;10(1):73–83.
86. Mouri T, Naito T, Morikawa A, et al. Promotion of behavioral change and the impact on quality of life in elderly patients with advanced cancer: a physical activity intervention of the multimodal nutrition and exercise treatment for advanced cancer program. Asia Pac J Oncol Nurs. 2018;5(4):383–390.
87. Miura S, Naito T, Mitsunaga S, et al. A randomized phase II study of nutritional and exercise treatment for elderly patients with advanced non-small cell lung or pancreatic cancer: the NEXTAC-TWO study protocol. BMC Cancer. 2019;19(1):528.
88. Hall CC, Norris L, Dixon L, et al. A randomised, phase II, unblinded trial of an Exercise and Nutrition-based Rehabilitation programme (ENeRgy) versus standard care in patients with cancer: feasibility trial protocol. Pilot Feasibility Stud. 2018;27(4):192.
89. Yennurajalingam S, Willey JS, Palmer JL, et al. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: results of a double-blind placebo-controlled randomized study. J Palliat Med. 2012;15(10):1059–1064.
90. Baldwin C, Weekes CE. Dietary counselling with or without oral nutritional supplements in the management of malnourished patients: a systematic review and meta-analysis of randomised controlled trials. J Hum Nutr Diet. 2012;25(5):411–426.
91. Temel JS, Greer JA, Goldberg S, et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(5):595–601.
92. Fearon K, Argiles JM, Baracos VE, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. 2015;6(4):272–274.
93. European Medicines Agency. Adlumiz: assessment Report. EMA/647868/2017; September 14, 2017. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/adlumiz.
94. Gordon JN, Trebble TM, Ellis RD, et al. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut. 2005;54(4):540–545.
95. Ramage MI, Skipworth RJE. The relationship between muscle mass and function in cancer cachexia: smoke and mirrors? Curr Opin Support Palliat Care. 2018;12(4):439–444.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
