Back to Journals » Infection and Drug Resistance » Volume 9
Emerging resistant serotypes of invasive Streptococcus pneumoniae
Authors Shamseldin Elshafie S, Taj-Aldeen SJ
Received 13 December 2015
Accepted for publication 7 March 2016
Published 29 June 2016 Volume 2016:9 Pages 153—160
DOI https://doi.org/10.2147/IDR.S102410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Sittana Elshafie,1,2 Saad J Taj-Aldeen2,3
1Qatar Orthopedic and Sports Medicine Hospital, Aspetar, Doha, Qatar; 2Weill Cornell Medicine-Qatar, 3Department of Laboratory Medicine and Pathology, Microbiology Division, Hamad Medical Corporation, Doha, Qatar
Background: Streptococcus pneumoniae is the leading cause of meningitis and sepsis. The aim of the study was to analyze the distribution, vaccine serotype coverage, and antibiotic resistance of S. pneumoniae serotypes isolated from patients with invasive diseases, after the introduction of pneumococcal 7-valent conjugated vaccine (PCV-7).
Methods: A total of 134 isolates were collected from blood and cerebrospinal fluid specimens at Hamad Hospital during the period from 2005 to 2009. Isolate serotyping was done using the Quellung reaction. The prevaccination period was considered before 2005.
Results: The most common serotypes for all age groups were 3 (12.70%), 14 (11.90%), 1 (11.90%), 19A (9.00%), 9V (5.20%), 23F (5.20%), and 19F (4.50%). Coverage rates for infant <2 years for PCV-7, the 10-valent conjugated vaccine (PCV-10), and the 13-valent conjugated vaccine (PCV-13) were 34.78%, 52.17%, and 78.26%, respectively. Coverage rates of these vaccines were 50%, 67.86%, and 75% for the 2–5 years age group; 27.12%, 40.68%, and 64.41% for the age group 6–64 years; and 25%, 33.33%, and 66.67% for the ≥65 years age group, respectively. The percentage of nonsusceptible isolates to penicillin, cefotaxime, and erythromycin were 43.86%, 16.66%, and 22.81%, respectively. Thirty-seven isolates (32.46%) were multidrug resistant (MDR) and belonged to serotypes 14, 19A, 19F, 23F, 1, 9V, 12F, 4, 6B, 3, and 15A. Compared to previous results before the introduction of PCV-7, there was a significant reduction in penicillin-nonsusceptable S. pneumoniae from 66.67% to 43.86%, and a slight insignificant reduction in erythromycin nonsusceptible strains from 27.60% to 22.8%, while there was a significant increase in cefotaxime nonsusceptible strains from 3.55% to 16.66%.
Conclusion: Invasive pneumococcal strains and the emergence of MDR serotypes is a global burden that must be addressed through multiple strategies, including vaccination, antibiotic stewardship, and continuous surveillance.
Keywords: Streptococcus pneumoniae, Qatar, vaccine, serotype distribution, antibiotic resistance
Introduction
Streptococcus pneumoniae is one of the most common human pathogens, and prior upper respiratory tract colonization of the nasopharynx by S. pneumoniae is a prerequisite for invasive pneumococcal diseases (IPD).1 It is the most common cause of community-acquired pneumonia, bacteremia, meningitis, otitis media, and other invasive diseases worldwide and is responsible for significant morbidity and mortality, particularly in young children.2
The World Health Organization (WHO) in 2005 estimated that pneumococcal disease is responsible for 1.6 million deaths annually, >800,000 of which are in children ≤5 years of age mostly from developing countries.3–6 The spread of human immunodeficiency virus, which increases the risk of pneumococcal diseases up to 40-fold, has exacerbated the situation in some of these countries.7
There are 94 pneumococcal serotypes on the bases of their reactions with serum antibodies,8,9 and these vary according to age, race, and geography. Globally, 20 serotypes were associated with over 80% of IPD in different age groups, with 13 most common serotypes causing 70%–75% of invasive diseases in children. As a result, the WHO recommended the inclusion of pneumococcal conjugate vaccines in national immunization programs.6 At present, there are three pneumococcal conjugated vaccines, pneumococcal 7-valent conjugated vaccine (PCV-7), which includes serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, responsible for much pediatric IPD in many Western countries,10 10-valent conjugated vaccine (PVC-10) (PCV-7 plus serotypes 1, 5, and 7F), and 13-valent conjugated vaccine (PCV-13) (PCV-10 plus serotypes 3, 6A, and 19A). PCV-7 was licensed in Qatar in August 2005, and PCV-13, in November 2010.
Initial treatment of pneumococcal disease was with optichin, a drug related to quinine that, although effective, had to be abandoned for clinical use because of ocular toxicity;11 currently, it is used as a diagnostic tool for the identification of S. pneumoniae in the laboratory. Thereafter and for many years, penicillin provided very effective treatment for S. pneumoniae infections. The emergence of S. pneumoniae strains with resistance not only to penicillin, but also to other antibiotics has been reported in the last two decades.12,13 This has contributed to treatment failure, at least in pneumococcal meningitis.14 In the most recent Spanish cohort study, nonsusceptibility to penicillin was detected in 4.2% of IPD patients.15
Penicillin-resistant S. pneumoniae (PRSP) was first reported in 1967;16 subsequently, S. pneumoniae isolates with high level resistance to penicillin and multidrug resistance were reported in South Africa in the late 1970s.17 Higher prevalence rates of PRSP were reported in Europe and USA in 1990.18,19
The resistance pattern of S. pneumoniae continues to evolve under the selective pressure of multiple factors, such as the indiscriminate use of antimicrobials,20 clonal spread,21 and vaccination.22–24 More recently, certain emerging serotypes exhibit decreased susceptibility to several agents including ceftriaxone,25 with the expansion of the multidrug resistant (MDR) population.26
The aim of this study was to analyze serotype distribution of 134 invasive S. pneumoniae isolates in Qatar over a 4-year period, in the 7-, 10-, and 13-valent vaccines in different age groups and assess their antimicrobial susceptibility pattern compared to that before the introduction of the PCV-7 vaccine.
Materials and methods
Patients and specimen collection
The isolates used in this study were collected from patients attending Hamad Hospital and emergency clinics at Hamad Medical Corporation, Qatar, during the period between January 2005 and March 2009. IPD was defined according to the Centers for Disease Control definition as isolation of S. pneumoniae from a normally sterile body site (eg, blood and cerebrospinal fluid [CSF]). Isolates of this study were isolated from blood and CSF by culturing on 5% sheep blood agar and incubating at 37°C in the presence of 5% CO2 for 24 hours. Alpha-hemolytic colonies were subcultured and further identified as S. pneumoniae by their colony morphology, sensitivity to optochin (ethylhydrocupreine hydrochloride), bile-solubility, and catalase negative test. The 134 invasive strains of S. pneumoniae were isolated from blood and CSF of patients aged 4 months to 90 years. All isolates were stored in cryovials at –80°C until further analysis. Hamad Medical Corporation Research and Ethics Committee (Ref #7072/07) granted ethical approval to conduct this study and they wavered the need for patient consent.
Antimicrobial susceptibility
For 118 isolates, susceptibility to chloramphenicol, co-trimoxazole erythromycin, and vancomycin was determined by the Kirby–Bauer disc diffusion method, and the results were interpreted according to CLSI 2012 guidelines.27 The minimum inhibitory concentration (MIC) of penicillin and cefotaxime were determined using E-test method (AB Biodisk, Solna, Sweden) as recommended by CLSI. Reference strain with known susceptibility (S. pneumoniae ATCC 49619) was included as control. The MDR organism phenotype was defined as having diminished susceptibility to three or more antibiotics. Interpretation of the results was done according to CLSI 2012 recommendation for meningitis and nonmeningitis cases.
Serotyping
Serotyping of the isolates was done at the Naval American Medical Research Unit Number 3 (NAMRU3) in Cairo, Egypt, using the Quellung reaction.
Results
The serotype was determined for 122/134 (91%) invasive pneumococcal isolates (Table 1 and Figure 1); the most common seven serotypes were 3 (12.7%), 14 (11.9%), 1 (11.9%), 19A (9%), 9V (5.2%), 23F (5.2%), and 19F (4.5%). The most common serotypes in children ≤5 years were 14, 19F, 23F, 19A, 1, and 3, and for the 6–64 years age group were 14, 1, 19A,12F, 3, 9V, 8, and 23F, while in elderly patients ≥65 years, the most common serotypes were 19A, 3, 1, 14, and 9V.
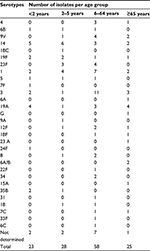  | Table 1 Serotype distribution of 134 invasive pneumococcal isolates by age group |
  | Figure 1 Serotype distribution of 134 invasive pneumococcal isolates. Note: Others: 18C, 7F, 6A, G, 9A, 23A, 24F, 22F, 15A, 31, 33F, and 6C. Abbreviation: NT, not determined. |
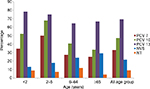
Vaccine coverage is shown in Figure 2. For patients <2 years, vaccine coverage rates for PCV-7, PCV-10, and PCV-13 were 34.78%, 52.17%, and 78.26%, respectively; coverage rates of these vaccines were 50%, 67.86%, and 75% for those aged 2–5 years age; 27.12%, 40.68%, 64.41% for those aged 6–64 years; and 25%, 33.33%, and 66.67% for the ≥65 years age group, respectively.
Susceptibility pattern is available for 118 strains against penicillin, cefotaxime, chloramphenicol, erythromycin, co-trimoxazole, tetracycline, and vancomycin and is listed in Table 2. Penicillin MIC for all isolates ranged from 0.008 to 4.0 μg, and, for cefotaxime, MIC ranged from 0.008 to 3.0 μg. (Figure 3). On the basis of the oral penicillin breakpoints, 66 (55.93%) were susceptible to penicillin, 40 (33.90%) were intermediate resistant, and 12 (10.17%) resistant to penicillin. A total of 52 isolates (44.06%) were nonsusceptible to penicillin (resistant and intermediate resistant; penicillin-nonsusceptable S. pneumonia [PNSP]). On the basis of the meningitis breakpoints, 97 (83.05%) were susceptible to cefotaxime, 19 (15.25%) were intermediate resistant, and 2 (1.69%) were resistant, ie, 17.80% cefotaxime nonsusceptible S. pneumoniae (CNSP). While applying the nonmeningitis breakpoints 107 (90.68%) were susceptible, 5 (4.24%) were intermediate resistant, and 2 (1.69%) were resistant to cefotaxime (5.93% CNSP). Resistance to chloramphenicol, co-trimoxazole, erythromycin, and tetracycline was 11.86%, 56.78%, 22.85%, and 38.14%, respectively. None of the isolates was resistant to vancomycin.
  | Table 2 Antimicrobial susceptibility of 118 invasive pneumococcal isolates |
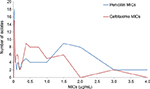  | Figure 3 MICs (μg/mL) of 118 invasive pneumoccocal isolates for penicillin and cefotaxime. Abbreviation: MICs, minimum inhibitory concentrations. |
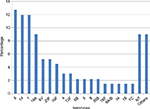
Table 2 shows that penicillin-nonsusceptible SP isolates were more likely to be resistant to other antibiotics than susceptible strains, with the exception of chloramphenicol, which showed good susceptibility to both penicillin-susceptible and penicillin-nonsusceptible isolates. Thirty-nine (33.05%) isolates were MDR. Serotyping was available only for 31 isolates. Serotypes distribution of these MDR isolates is shown in Figure 4. Predominant MDR serotypes were 14, 19A, 19F, and 23F (58.97% of all MDR isolates).
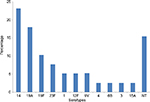  | Figure 4 Percentage distribution of 31 MDR serotypes of invasive pneumococcal isolates. Abbreviations: MDR, multidrug resistant; NT, not determined. |
The susceptibility rate to penicillin, cefotaxime, and erythromycin of the isolates in this study was compared with the result of the previous study (1999–2000).28 There was a significant reduction in PNSP from 66.67% to 43.86%, and a slight insignificant reduction in erythromycin nonsusceptible strains from 27.60% to 22.8%, while there was a significant increase in cefotaxime nonsusceptible strains from 3.55% to 17.80%.
Discussion
Diseases caused by S. pneumoniae are a major public health problem worldwide. The increasing difficulty in the management of pneumococcal disease has led to adoption of preventative measures against infection by S. pneumoniae. In the developing countries, young children and the elderly are most affected, and resistance to antimicrobials is a serious and rapidly increasing problem worldwide. In a previous study28 conducted in Qatar in 1999–2000, the PCV-7 vaccine provided only 52% coverage for infants <2 years. The most prevalent serotypes in this age group during that study were 4,6A, 6B, 11A, 14, 19A, 19F, and 23F. In this study, there is increased isolation of serotypes 1 and 3 that are covered by PCV-13, and PCV-10 covers only serotype 1, whereas PCV-7 does not cover serotypes 1 and 3. The most common serotypes reported in the region from infants <2 years were 6B, 6A, 14, 5, and 23F in Egypt, and 3, 4, 6, 14, and 23 in Saudi Arabia,29,30 while in this study, the most common serotypes seen in infants <2 years were 14, 19A, 19F, 1, and 3. The heptavalent vaccine only covers the second most common serotype of S. pneumonia.
Interestingly, this study also showed marked reduction of PCV-7 coverage in the <2 years age group from 52% to 34.78%. After mass vaccination has been introduced, IPD due to the vaccine serotypes, such as 6A, have tended to decrease in both young children and other age groups due to herd immunity. This marked decrease in PCV-7 serotypes was reported recently in France.31 This suggests that the use of the PCV-7 may have prevented infection caused by the vaccine serotypes. However, reduction of PCV-7 types was accompanied by the emergence of non-PCV-7 serotypes, a phenomenon that was observed by others.30,32–35 This information supports the switch from PCV-7 vaccination to PCV-13 immunization program. The rise in non-PCV-7 types may have been a consequence of the disappearance of the PCV-7 types. Nonetheless, this approach has the advantage of acknowledging that the serotype epidemiology has changed, as well as indicating that continued prevention of PCV-7 types is important.36 Overall coverage of PCV-13 to all age groups is up to 69.40%, and for the <2 years age group it was 78.26%.
Prevalence of PNSP varied widely among countries. In Asia, PNSP rates in 2000–2001 ranged from 78% to 92%,37 and markedly decreased to 25% during 2008–2009.38 Other regions also showed moderately high rates of PNSP based on the pre-2008 breakpoints. In Southern and Eastern Mediterranean regions, the overall PNSP rate was 25% in 2003–2005,39 and in Latin America, PNSP rates was 30.5%–38.8%.40
In Qatar, the rate of PNSP was reported previously in 1999–2000 as 60%28 (applying the 2012 CLSI interpretative criteria, it will be 66.67%), while in this study the rate was 44.07%. During the study period, injectable antibiotics were used only in the inpatients, but for the outpatients oral antibiotics were over the counter, which may have some impact on resistance. This decline in penicillin nonsusceptibility may be due to the introduction of the PCV-7 vaccine in 2005. This phenomenon was reported by others who demonstrated a substantial decline in PNSP prevalence after the introduction of PCV-7 vaccine, followed by an increase in prevalence as vaccine serotypes were replaced by nonvaccine serotypes.41–45 The use of cefotaxime/ceftriaxone instead of penicillin for empirical treatment of S. pneumoniae infections and the replacement of penicillin prophylaxis with vaccination may have also contributed to this significant reduction in PNSP.
Erythromycin nonsusceptible S. pneumoniae (ENSP) levels decreased slightly from 30% in 1999–2000 to 23.73% in this study. Stable or increasing ENSP were observed in countries without introduction of PCV-7 vaccine in the national immunization program,28,46,47 but declined in countries like Germany following the introduction of PCV-7 in 2006.48 CNSP increased from 2.6% (or 3.55% using the 2012 meningitis breakpoints) to 17.80% in this study.
Previous studies have shown that levels of antimicrobial resistance are directly proportional to antibiotic consumption in the community.38,49 Some of the first isolates of PRSP were found in a village in New Guinea after prophylactic penicillin had been used in an attempt to reduce the incidence of pneumococcal disease.50 Additional associations were found between uses of other antibiotics, ie, cross-resistance between antibiotic classes, eg, macrolide, other β-lactams.40
MDR S. pneumoniae infection developed in two vaccinated patients. One was a 12 years old female who received the 23-valent vaccine; she developed sever sinusitis with septicemia due to serotype 12F. The second patient was a 4 months old female vaccinated with the PCV-7 vaccine; she developed severe meningitis due to serotype 19A, which is not included in the PVC-7 but included in the PVC-13.
An alarming situation is the increase in resistance to third-generation cephalosporins – the mainstay agent for the treatment of meningitis, severe cases of pneumonia, and other serious diseases. The important strategy to minimize the development of resistance is to further decrease the indiscriminate use of antibiotics,51 and introduce a vaccine that covers more serotypes, eg, PVC-13.
The introduction of PCV-7 conjugate vaccine not only resulted in reduction of the prevalence of covered serotypes but also resulted in the emergence of serotypes not included in the PCV-7. The usage of antibiotics and the uptake of PCV-7 vaccine were associated with changes in the prevalence of antibiotic-resistant S. pneumoniae as well. Most countries reported a decrease in antibiotic-resistant S. pneumoniae after the introduction of the PCV-7, and at the same time, the overall usage of antibiotics decreased. Although penicillin and erythromycin resistance is decreasing, cefotaxime resistance is on the rise due to the increased use of this antibiotic. The introduction of the PCV-13, which offers 78.26% protection for infants younger than 2 years, and 66.67% for elderly patients ≥65 years of age, is recommended. Routine immunization in children may also give protection to others by herd immunity.52
Regular surveillance is necessary since changes in serotypes may occur naturally with time as well as with replacement by nonvaccine serotypes in response to vaccine pressure. After the introduction of PCV-7, a serotype replacement phenomena and persistence of PCV-7 serology were documented.53
Conclusion
In conclusion, IPD and the development of emerging antibiotic-resistant serotypes of S. pneumoniae is a global burden that must be addressed through multiple strategies, including national immunization program, antibiotic stewardship, and ongoing surveillance.
Disclosure
The authors report no conflicts of interest in this work.
References
Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–855. | ||
Rosen JB, Thomas AR, Lexau CA, et al. CDC Emerging Infections Program network. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clin Infect Dis. 2011;53(2):137–143. | ||
Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–154. | ||
Cornick JE, Everett DB, Broughton C, et al. Invasive Streptococcus pneumoniae in children, Malawi 2004–2006. Emerg Infect Dis. 2011;17(6):1107–1109. | ||
Zhou L, Yu SJ, Gao W, Yao KH, Shen AD, Yang YH. Serotype distribution and antibiotic resistance of 140 pneumococcal isolates from pediatric patients with upper respiratory infections in Beijing, 2010. Vaccine. 2011;29(44):7704–7710. | ||
Pneumococcal conjugate vaccine for childhood immunization-WHO position paper. Wkly Epidemiol Rec. 2007;82(12):93–104. | ||
Austrian R. Confronting drug-resistant pneumococci. Ann Intern Med. 1994;121(10):807–808. | ||
Calix JJ, Dagan R, Pelton SI, Porat N, Nahm MH. Differential occurrence of Streptococcus pneumonia serotype 11E between asymptomatic carriage and invasive pneumococcal disease isolates reflects a unique model of pathogen microevolution. Clin Infect Dis. 2012;54(6):794–799. | ||
Calix JJ, Nahm MH. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J Infect Dis. 2010;202(1):29–38. | ||
Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal diseases and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14(3):e197–e209. | ||
Levine O S, O’Brien KL, Knoll M, et al. Pneumococcal vaccination in developing countries. Lancet. 2006;367(9526):1880–1882. | ||
Ubukata K, Chiba N, Hanada S, et al. Invasive Pneumococcal Diseases Surveillance Study Group. Serotype changes and drug resistance in invasive pneumococcal diseases in adults after vaccinations in children, Japan, 2010–2013. Emerg Infect Dis. 2015;21(11):1956–1965. | ||
Jaiswal N, Singh M, Das RR, et al. Distribution of serotypes, vaccine coverage, and antimicrobial susceptibility pattern of Streptococcus pneumoniae in children living in SAARC countries: a systematic review. PLoS One. 2014;9(9):e108617. | ||
Weingarten RD, Marckiewicz Z, Gilbert DN. Meningitis due to penicillin-resistant Streptococcus pneumoniae in adults. Rev Infect Dis. 1990;12(1):118–124. | ||
de Egea V, Muñoz P, Valerio M, et al. GAMES Study Group. Characteristics and outcome of Streptococcus pneumoniae Endocarditis in the XXI Century: a systematic review of 111 cases (2000–2013). Medicine (Baltimore). 2015;94(39):e1562. | ||
Hansman D, Bullen MM. A resistant pneumococcus. Lancet. 1967;2:264–265. | ||
Jacobs MR, Koomhof HJ, Robins-Browne, et al. Emergence of multiple resistant pneumococci. N Engl J Med. 1978;299(14):735–740. | ||
Goldstein FW, Acar JF. The Alexander Project Collaborative Group. Antimicrobial resistance among lower respiratory tract isolates of Streptococcus pneumoniae: results of a 1992–93 Western Europe and USA collaborative study. J Antimicrob Chemother. 1996;38(Suppl A):71–84. | ||
Johnson DM, Stilwell MG, Fritsche TR, Jones RN. Emergence of multi-drug resistant Streptococcus pneumoniae: report from the SENTRY antimicrobial surveillance program (1999–2003). Diagn Microbiol Infect Dis. 2006;56(1):69–74. | ||
Riedel S, Beckmann SE, Heilmann KP, et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. Eur J Clin Microbiol Infect Dis. 2007;26(7):485–490. | ||
Sá-Leáo R, Tormasz A, delencastre H. Multilocus sequence typing of Streptococcus pneumoniae clones with unusual drug resistance patterns: generic backgrounds and relatedness to other epidemic clones. J Infect Dis. 2001;184(9):1206–1210. | ||
Gertz RE Jr, Li Z, Pimenta FC, et al. Increased penicillin nonsusceptibility of nonvaccine serotypes other than 19A and 6a invasive pneumococci in post 7-valent conjugate vaccine era. J Infect Dis. 2010;201(5):770–775. | ||
Jacob MR, Good CE, Bajaksouzian S, Windau AR. Emergence of Streptococcus pneumoniae serotypes 19a, 6C, and 22f and serogroup 15 in Cleveland, Ohio, in relation to introduction of the protein-conjugated pneumococcal vaccine. Clin Infect Dis. 2008;47(11):1388–1395. | ||
Li CF, Liu MF, Shi ZY, et al. Changing trends in antimicrobial susceptibility of Streptococcus pneumoniae isolates in Taiwan, 2006–2007. J Microbiol Immunol Infect. 2012;45(4):305–310. | ||
Mendes RE, Biek D, Critchley IA, Farrell DJ, Sader HS, Jones RN. Decreased ceftriaxone susceptibility in Emerging (35B and 6C) and persisting (19A) Streptococcus pneumoniae serotypes in the United States, 2011–2012: ceftriaxone remains active in vitro among β-lactam agents. Antimicrob Agent Chemother. 2014;58(8):4923–4927. | ||
Hulten KG, Kaplan SL, Lamberth LB, et al. Changes in Streptococcus pneumoniae serotypes 19A invasive infections in children from 1993 to 2011. J Clin Microbiol. 2013;51(4):1294–1297. | ||
Clinical Laboratories Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow aerobically: Approved Standard. 12th Ed CLSI document M7-A7. Wayne, PA: CLSI; 2012. | ||
Al-Khal A, Elshafie S, Al-Kuwari J, Bener A. Streptococcus pneumoniae serotypes in newly developed State of Qatar: consideration for conjugate vaccine. Qatar Med J. 2007;16:25–28. | ||
Shibl A, Memish Z, Pelton S. Epidemiology of pneumococcal disease in the Arabian Peninsula and Egypt. Int J Antimicrob Agents. 2009;33(5):410. e1–e9. | ||
Zangeneh TT, Baracco G, Al-Tawfiq JA. Impact of conjugate pneumococcal vaccines on the changing epidemiology of pneumococcal infections. Expert Rev Vaccines. 2011;10(3):345–353. | ||
Janoir C, Cohen R, Levy C, et al. Observatoires Régionaux du Pneumocoque (ORP) network. Clonal expansion of themacrolide resistant ST386 within pneumococcal serotype 6C in France. PLoS One. 2014;9(3):e90935. | ||
Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455–1463. | ||
Wagenvoort GH, Sanders EA, Vlaminckx BJ, et al. Invasive pneumococcal disease: clinical outcomes and patient characteristics 2–6 years after introduction of 7-valent pneumococcal conjugate vaccine compared to the pre-vaccine period, the Netherlands. Vaccine. 2016;34(8):1077–1085. | ||
Gounder PP, Brewster M, Bruce MG, et al. Impact of the pneumococcal conjugate vaccine and antibiotic use on nasopharyngeal colonization by antibiotic nonsusceptible Streptococcus pneumoniae, Alaska, 2000–2010. Pediatr Infect Dis J. 2015;34(11):1223–1229. | ||
Azzari C, Moriondo M, Di Pietro P, et al. The burden of bacteremia and invasive diseases in children aged less than five years with fever in Italy. Ital J Pediatr. 2015;41:92. | ||
Hausdorff WP, Hoet B, Adegbola RA. Predicting the impact of new pneumococcal conjugate vaccines: serotype composition is not enough. Expert Rev Vaccines. 2015;14(3):413–428. | ||
Song JH, Jung SI, KO KS, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ASNORP study). Antimicrob Agent Chemother. 2004;48(6):2101–2107. | ||
Song JH, Dagan R, Klugman KP, Fritzell B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine. 2012;30(17):2728–2737. | ||
Borg MA, Tiemersma E, Scicluna E, et al. Prevalence of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae isolates reported by laboratories in the Southern and Eastern Mediterranean Region. Clin Microbiol Infect. 2009;15(3):232–237. | ||
Castañeda E, Agudelo CI, Regueira M, et al. Laboratory- based surveillance of Streptococcus pneumoniae invasive disease in children in 10 latin American countries: a SIREVA 11 project, 2000–2005. Pediatr Infect Dis J. 2009;28(9):e265–e270. | ||
Mudhune S, Wamae M. Network for surveillance of pneumococcal disease in the East African Region. Report on invasive disease and meningitis due to Haemophilus influenzae and Streptococcus pneumoniae from the network for surveillance of pneumococcal disease in the East African Region. Clin Infect |Dis. 2009;48(Suppl 2):s147–s152. | ||
Van de Sande-Bruinsma N, Grundmann H, Verloo D, et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14(11):1722–1730. | ||
Cohen R, Levy C, Bonnet E, et al. Dynamic of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 introduction in France. Vaccine. 2010;28(37):6114–6121. | ||
Aguiar SI, Serrano I, Pinto FR, Melo-Cristino J, Ramirez M. Portuguese surveillance group for the study of respiratory pathogens. Changes in Streptococcus pneumoniae serotypes causing invasive disease with non-universal vaccination coverage of the seven-valent conjugate vaccine. Clin Microbiol Infect. 2008;14(9):835–843. | ||
Vestrheim DF, Høiby EA, Bergsaker MR, Rønning K, Aaberge IS, Caugant DA. Indirect effect of conjugate pneumococcal vaccination in a 2+1 dose schedule. Vaccine. 2010;28(10):2214–2221. | ||
Farrell DJ, Felmingham D, Shackcloth J, et al. Non-susceptibility trends and serotype distributions among Streptococcus pneumoniae from community-acquired respiratory tract infections and from bacteremias in the UK and Ireland, 1999–2007. J Antimicrob Chemother. 2008;62(Suppl 2):ii87–ii95. | ||
Wolter N, Von Gottberg A, Du Plessis M, De Gouveia L, Klugman KP. Group for Enteric, Respiratory and Meningeal disease Surveillance in South Africa. Molecular basis and clonal nature of increasing pneumococcal macrolide resistance in South Africa, 2000–2005. Int J Antimicrob Agents. 2008;32(1):62–67. | ||
Imöhl M, Reinert RR, Mutscher C, Van der Linden M. Macrolide susceptibility and serotype specific macrolide resistance of invasive isolates of Streptococcus pneumoniae in Germany from 1992 to 2008. BMC Microbiol. 2010;10:299. | ||
Dias R, Canica M. Trends in resistance to penicillin and erythromycin of invasive pneumococci in Portugal. Epidemiol Infect. 2008;136(7):928–939. | ||
Hansman D, Glasgow H, Sturt J, Devitt L, Douglas R. Increased resistance to penicillin of pneumococci isolated from man. N Engl J Med. 1971;284(4):175–177. | ||
Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10(3):195–203. | ||
Desa MN, Lin TK, Yasin RM, Parasakthi N. Penicillin susceptibility and molecular characteristics of clinical isolates of Streptococcus pneumoniae at the University of Malaya Medical center Kuala Lumpur Malaysia. Int J Infect Dis. 2003;7(3):190–197. | ||
Gaviria-Agudelo CL, Jordan-Villegas A, Garcia C, McCracken GH Jr. The effect of 13-valent pneumococcal conjugate vaccine on the serotype distribution andantibiotic resistance profiles in children with invasive pneumococcal disease. J Pediatric Infect Dis Soc. Epub February 22, 2016. DOI: 10.1093/jpids/piw005. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.