Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Emerging Data on the Safety and Efficacy of Ripretinib for the Treatment of Gastrointestinal Stromal Tumors
Authors Thirasastr P , Somaiah N
Received 11 August 2022
Accepted for publication 18 January 2023
Published 10 February 2023 Volume 2023:16 Pages 11—19
DOI https://doi.org/10.2147/CEG.S351839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Santosh Shenoy
Prapassorn Thirasastr, Neeta Somaiah
Department of Sarcoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Correspondence: Neeta Somaiah, Department of Sarcoma Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0450, Houston, TX, 77030, USA, Tel +1 713 792-3626, Email [email protected]
Abstract: In patients with gastrointestinal stromal tumors (GIST), systemic treatment after disease progression on imatinib is challenging. Sunitinib and regorafenib are approved in the second- and third-line setting, respectively, with activity against certain secondary mutations with comparatively much lower response rates and survival increment compared to imatinib. All three of these drugs were serendipitously found to have activity in GIST, starting with imatinib, which was formulated for its ability to inhibit BCR-ABL in chronic myelogenous leukemia. Ripretinib is a drug that was specifically developed as a more potent KIT tyrosine kinase inhibitor (TKI), with broad-spectrum activity against the mutations encountered in GIST. Encouraging responses in early and later lines of treatment in the Phase 1 trial of ripretinib in GIST led to the rapid development of this novel drug. In a Phase 3 randomized clinical trial with cross-over, ripretinib demonstrated superior PFS and overall survival (OS) in 4th-line treatment and beyond compared to placebo. This established 150 mg once daily ripretinib as the standard of care in this setting. Ripretinib is generally well tolerated, with common adverse effects of hair loss, diarrhea, cramps, fatigue and nausea. The favorable safety profile and efficacy of ripretinib prompted its evaluation in a randomized phase 3 trial in the 2nd-line treatment setting. However, it did not result in a longer PFS duration than sunitinib. Although the efficacy of ripretinib in this unselected patient population was not significantly different from that of sunitinib, the tolerability profile was better. This review article aims to review the efficacy and tolerability profile of ripretinib, together with its role in the setting of unresectable or metastatic GIST.
Keywords: ripretinib, GIST, systemic treatment in GIST, imatinib-resistant mutation
Introduction
Gastrointestinal stromal tumor (GIST), the most common type of soft tissue sarcoma, originates from the interstitial cells of Cajal, which are mesenchymal cells located in the muscle layers of the intestinal tract. The underlying molecular alterations of GIST are well characterized. C-KIT proto-oncogene and platelet-derived growth factor receptor alpha (PDGFRA)-activating mutations account for 75–80% and 10% of GIST cases, respectively.1 Other minor alterations include succinate dehydrogenase (SDH) deficiency (10%), rat sarcoma virus (RAS)/v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutation (2%), receptor tyrosine kinase (RTK) translocation (0.1%), and loss-of-function neurofibromatosis type 1 (NF1) gene mutation (0.1%).
Since the development of imatinib, the first tyrosine kinase inhibitor (TKI) used to treat GIST, there have been vast advances in systemic treatment. The US Food and Drug Administration (FDA) approved imatinib, sunitinib, and regorafenib for the 1st-, 2nd-, and 3rd-line treatment of unselected patients with unresectable or metastatic GIST on the basis of the results of previous randomized studies.2–4
In May 2020, ripretinib, a new TKI against KIT and PDGFRA with dual blockade mechanism, was approved for 4th-line treatment of unresectable or metastatic GIST on the basis of an improvement in PFS duration compared to placebo in the 4th- or later line treatment setting in the phase 3 INVICTUS study, which compared ripretinib to placebo in advanced GIST patients who received at least imatinib, sunitinib, and regorafenib.5 This review discusses the efficacy and safety of this recently approved drug in GIST treatment.
Systemic Treatment in Advanced GISTs: Pre-Ripretinib
KIT and PDGFRA are type III transmembrane tyrosine kinase receptors that are found in the interstitial cells of Cajal. Activating mutations of these receptors lead to the activation of the receptors without the binding of stem cell factor and initiation of downstream pathways, causing cell proliferation and growth progression that subsequently result in GIST development. The major domains of KIT/PDGFRA are the extracellular Ig-like domain (Figure 1A), which is responsible for stem cell factor binding and homo-dimerization; the transmembrane helix domain; and the intracellular domains. The intracellular parts include the juxtamembrane domain (550–586) or juxtamembrane region (JMR), which has an autoinhibitory function, kinase insert domain, and kinase domain. The kinase domain has a bilobed conformation, beginning with the small N-lobe in the antiparallel beta-sheet configuration and ending with the large C-terminal lobe. The N-lobe has an ATP-binding region, while the C-lobe has an activation loop and binding site for the protein substrate.6 The activation loop begins with Asp810 and has a conserved region, the Asp810-Phe811-Gly812 (DFG) motif, that is important to defining active and inactive conformation.7 The catalytic site of kinase is situated in the cleft between the N and C lobes.
The catalytic mechanism involves changes in the A-loop, JMR, and αC-helix. In the absence of activating ligand, KIT is in dormant form with JMR, which has auto-inhibitory function inserts between the N and C lobes that cause the αC-helix, a part of the small N-lobe, to move into dormant conformation.6 The activation loop (A-loop) is in inactive form (non-extended form) or the “DFG out” state. In this form, the catalytically important sidechain is protected away from the ATP-binding pocket. When the type III transmembrane tyrosine kinase receptors are activated with their ligands, stem cell factor in KIT and PDGFs in PDGFRA, receptor homodimerization occurs, resulting in phosphorylation of JMR and the 1–3 residues of the A-loop, including Tyr823. JMR then moves out from the small (αC-helix) and large lobes of the kinase domain, allowing the A-loop to extend into active conformation with “DFG in”. This conformation allows Asp810 of the A-loop to interact with the ATP substrate through Mg2+ coordination. The phosphorylation of Tyr823 further stabilizes KIT in the active conformation.
Imatinib
Imatinib was the first TKI used in GIST and was a breakthrough treatment, with a response rate (RR) of 51% and median PFS duration as high as 20.4 months.2 In the Phase 2 B2222 imatinib study, the median overall survival (OS) duration of the total population was 57 months; 7–9% of patients experienced a >10-year PFS duration. Patients with KIT exon 11 mutation had a longer and deeper response, while those with exon 9 mutation experienced more benefit from a higher dose (800 mg vs 400 mg).2
Resistance to imatinib can be grouped as primary or secondary. The major cause of primary resistance is D842V PDGFRA mutation, which constitutes about 5% of overall GIST cases. This mutation is located in exon 18 of PDGFRA, which is situated in the activation loop inside the C-terminal lobe of the tyrosine kinase domain (Figure 1A). The modification at D842 residue interferes with the swinging movement of the activation loop leads to a conformational shift of the ATP binding pocket, and affects imatinib binding.8
Secondary resistance usually occurs after 20–24 months of imatinib treatment. In the clinical setting, it is defined by disease progression after 6 months of initial clinical benefit.9 These patients eventually develop resistance to imatinib because of secondary mutations in subpopulations of the tumor, 85–90% of which occur in the ATP-binding pocket (exon 13, 14 of KIT) and activation loop (exon 17, 18 of KIT).1,10
Sunitinib
Similar to imatinib, sunitinib belongs to the tyrosine kinase inhibitor type II group, which inhibits tumor cell growth through binding to an induced-fit hydrophobic pocket formed by conformation changing of the FDG motif, which is located adjacent to the ATP-binding site.11 Imatinib targets the DFG-out conformation (inactive form), and while sunitinib has been reported to be ATP-competitive, it also demonstrates more activity in the inactive form of KIT.12 Although mutation in KIT exons 13 and 14 creates an alteration of the hydrophobic pocket that hinders the binding ability of imatinib to the pocket, sunitinib still exerts activity in these mutations since it does not access the deep hydrophobic pocket (Figure 1A).
Sunitinib is the approved 2nd-line treatment for metastatic GIST. Data from a phase 3 study demonstrated significant time to tumor progression (median, 27.3 weeks), PFS (median, 24.1 weeks), and OS (median, 73.9 weeks) benefits compared to placebo and an overall RR of 6.8% in patients whose disease was resistant to or intolerant of previous treatment with imatinib.3,13 Sunitinib can be given 50 mg a day (QD) 4/2 (4 weeks on, 2 weeks off), as in the phase 3 study, or 37.5 mg QD continuously, as evaluated in a multicenter phase 2 trial, with a median PFS duration of 34 weeks and median OS duration of 107 weeks; the results were comparable to the PFS and OS durations in the pivotal phase 3 study.14
Regorafenib
While sunitinib has activity against secondary mutations in the ATP-binding pocket (exon 13, 14 of KIT), regorafenib, which was approved for 3rd-line treatment, has activity against activation-loop (exon 17 of KIT) mutation, including residues 820 and 822, but not the D816V substitution.15,16 Furthermore, regorafenib also has poor activity against KIT exon 13 V654A mutation. Regorafenib resulted in a longer PFS duration than did placebo in metastatic or unresectable GIST that progressed after imatinib and sunitinib treatment, with a median PFS duration of 4.8 months and 0.9 months in the regorafenib and placebo arms, respectively (HR = 0.27, 95% CI = 0.19–0.39, p < 0.0001).4 There was no difference in OS duration because of a crossed-over rate of 84.8%. The efficacies of sunitinib and regorafenib in 2nd- and 3rd-line treatment were substantially lower than that of imatinib in 1st-line treatment.
Avapritinib
Avapritinib, or BLU-285, is the first type I TKI against KIT/PDGFRA. It was designed to target the resistant PDGFRA exon 18 D842V and KIT D816V activation loop mutations but has weak inhibition against V654A and T670I KIT mutations.17 Avapritinib showed high selectivity and had a good tolerability profile in preclinical studies.18,19 In the phase 1 NAVIGATOR trial, avapritinib had impressive activity against PDGFRA D842V, with an RR as high as 88%, and encouraging activity against non-PDGFRA D842V after 3 or more lines of treatment, with an RR of 17%.20,21 However, it failed to improve PFS duration compared to regorafenib in the 3rd- or later line treatment setting in unselected patients with advanced GIST in the phase 3 VOYAGER trial.20,22 The results of the NAVIGATOR study led to the FDA approval of avapritinib in PDGFRA D842V mutation, regardless of the line of treatment.
The emergence of cross-resistant tumor subpopulations is considered a major cause of drug resistance in GISTs. All of the TKIs described above have selective activity across the common KIT and PDGFRA mutations, allowing for emergence of resistant tumor clones and often a shorter PFS with subsequent lines of therapy (Figure 2). Novel therapeutic agents that have broad activity against multiple KIT mutations are needed and actively being investigated. Ripretinib is one such drug with broad activity against GIST mutations.
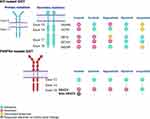 |
Figure 2 Distribution of mutated exons in GIST and drug sensitivity data reported in in-vitro studies. *Data from Serrano et al, 2019.23 (half maximal inhibitory concentration (IC50) > 500 nM suggests resistant), Smith et al, 2019.17 (IC50 >1000 nM suggests resistant), Gebreyohannes et al, 2019,18 Apsel Winger et al, 2019,24 and Heinrich et al, 2018.25 Image created with BioRender.com. **D816 in this figure includes D816E and D816H. |
Ripretinib
Mechanism of Action
Ripretinib, as an TKI type 2, has higher potency against the inactive form of RTKs, similar to imatinib, sunitinib, and regorafenib. In the context of secondary mutations that are resistant to imatinib, mutations are mainly located in the ATP-binding domain, consisting of the ATP-binding pocket (KIT exon 13, V654) and gatekeeper residues (KIT exon 14, T670) and the activation loop (KIT exon 17, 18). All of these mutations interfere with drug binding and induce a conformational shift to the active form, which then hinders protein interaction with the type 2 TKI. In one study, the most frequent secondary mutation hotspots were found in V654 (39.6%), T670 (11.1%), and exon 17 (41%).23
Although ripretinib is a type 2 inhibitor, it demonstrated a broad inhibitory profile against a large panel of KIT and PDGFRA mutations in a preclinical study explained through its action as a “switch-control” inhibitor.17 Ripretinib antagonizes the active state by binding to the switch pocket and activation loop, stabilizing the switch in an inactive conformation (Figure 1B). The “switch pocket” is an area that is adjacent to the ATP pocket and is the binding site of the activation loop.
Ripretinib showed broad activity toward secondary mutations including KIT exon 13 V654A, exon 14 T670I, exon 17 D816, and exon 18 A829P in preclinical models (Figure 2). While demonstrating lower potency against KIT exon 13 V654A (compared to sunitinib), exon 14 T670I mutation (compared to sunitinib),23 and KIT D816V mutation (compared to avapritinib), ripretinib showed highest potency among FDA-approved drugs for GIST against KIT exon 18 A829P.17 In addition, ripretinib showed higher potency in PDGFRA D842V transfection models than imatinib, sunitinib, and regorafenib but much lower than avapritinib.
Clinical Efficacy
In the setting of post-imatinib and later lines of treatment, sunitinib and regorafenib have limited efficacy as explained by the activity against secondary mutations, with an RR of 6.8% and PFS duration of 5.6 months for sunitinib in 2nd-line treatment and 4.5% and 4.8 months for regorafenib in 3rd-line treatment.3,4 Ripretinib was developed as a broad-spectrum TKI to solve the problem of the inter- and intra-tumor heterogeneity of secondary mutation subclones.
Ripretinib demonstrated activities in all lines of treatment, with RRs of 30% in 2nd-, 23% in 3rd-, and 11% in ≥4th-line treatment, as reported in a phase 1 study (Table 1).26,27 In the updated phase 1 study, the median PFS durations were 46.4, 36.3, and 23.9 weeks in 2nd-, 3rd-, and ≥4th-line treatment, respectively. After a long stretch, a TKI was showing activity in the ≥4th-line setting.
 |
Table 1 Efficacy of Ripretinib in Different Lines of Treatment from Phase 1 and 3 Trials |
The phase 3 INVICTUS study evaluated ripretinib in 4th-line or later treatment of advanced GIST compared to placebo.5 Ripretinib resulted in longer PFS and OS durations than did placebo, with a median PFS duration of 6.3 months (placebo = 1 month; HR = 0.15; 95% CI = 0.09–0.25; P < 0.0001) and median OS duration of 15.1 months (placebo = 6.6 months; HR = 0.36; 95% CI = 0.20–0.63; P = 0.0004). The overall RR was 9.4%. The significant superiority of ripretinib led the FDA to approve its use in 4th- or later line treatment of unselected patients with locally advanced or metastatic GIST. An updated analysis 19 months after the primary analysis showed an even longer OS duration for ripretinib, with a median OS duration of 18.2 months compared to 6.3 months in the placebo arm.30 In addition, the RR was 11.8% for ripretinib compared to 0% for placebo.
Ripretinib was not statistically significantly superior to sunitinib in the 2nd-line treatment setting, as demonstrated in the phase 3 INTRIGUE study.28 The preliminary results showed RRs of 21.7% and 17.6% in the ripretinib and sunitinib arms, respectively. The median PFS durations were 8.0 and 8.3 months for ripretinib and sunitinib (HR = 1.05), respectively. In the KIT-mutation subgroup, the median PFS durations were 8.3 and 7.0 months for ripretinib and sunitinib (HR = 0.88), respectively. Although 2nd-line treatment with ripretinib did not result in a longer PFS duration, it was associated with fewer grade 3–4 toxicities (41.3% vs 65.6%) and lower rates of dose interruption (29.1 vs 41.6), dose reduction (20.2 vs 48.0), and treatment discontinuation (3.6 vs 7.7) as a result of treatment-emergent adverse events (TEAEs); a better quality of life (QOL) was also observed.29 Moreover, the PFS duration in the KIT exon 9 subgroups favored treatment with sunitinib compared to ripretinib (HR = 2.85, 95% CI = 1.48–5.48) but did not significantly differ in the KIT exon 11 (HR = 0.88, 95% CI = 0.82–1.33) and other mutation subgroups for ripretinib and sunitinib. However, the RR in the KIT exon 11 population favored ripretinib over sunitinib (23.9% vs 14.6%, difference = 9.3%, 95% CI = 0.7–17.8, p = 0.03).
Dosing and Side Effects
According to the results of a phase 1 dose-escalation study, the maximum tolerated dose was not reached at a maximum of 200 mg twice a day (BID). The recommended phase 2 dose of 150 mg once daily (QD) was established on the basis of safety, pharmacokinetic, and pharmacodynamic findings.31
The phase 1 dose escalation and expansion study revealed that ripretinib had a good tolerability profile, with 14% of patients experiencing dose reduction and 11% discontinuing treatment because of TEAEs.26 The most common adverse effects of any grade reported in phase 1 and phase 3 studies of ripretinib are hair loss, diarrhea, cramps, fatigue and nausea (Table 2). Grade 3–4 adverse effects with 150 mg of ripretinib QD included asymptomatic lipase increase (11%), anemia (7%), hypertension (6%), abdominal pain (5%), increased blood bilirubin (3%), and diarrhea (2%). Other side effects found in at least 1% of cases included fatigue, vomiting, dyspnea, decreased appetite, back pain, and hand-foot skin reaction. Ripretinib increases skin lesions and routine dermatology follow-up is required. Majority of new skin findings are benign, but the occasional skin cancer should be picked up and treated early. Rare but serious side effects included skin cancer (cutaneous squamous cell carcinoma, 4.7%, and melanoma, 2.4%) and congestive heart failure (1.2%).
 |
Table 2 Representative Treatment-Emergent Adverse Events (TEAEs) Observed in the Phase 3 INVICTUS Study and Phase 1 Intra-Patient Dose Escalation Study |
Dose escalation from 150 mg QD to 150 mg BID was an option in patients who participated in phase 1 dose escalation and phase 3 studies and experienced disease progression on 150 mg QD, as determined by RECIST 1.1.32 The median PFS1 duration, calculated from the date of the first dose of ripretinib (150 mg QD) to disease progression, was 11.0 months (95% CI = 3.5–22.1 months) for patients on 2nd-line treatment, 8.3 months (95% CI = 1.8–11.1 months) for 3rd-line treatment, and 5.5 months (95% CI = 2.1–8.1 months) for 4th-line or later treatment. The median PFS2 duration, calculated from the date of dose escalation to disease progression or death, was 5.6 months (95% CI=1.4-not estimable) for patients on 2nd-line treatment, 3.3 months (95% CI = 2.3–7.4 months) for 3rd-line treatment, and 4.6 months (95% CI = 2.8–5.6 months) for 4th-line or later treatment. Tumor metabolic response on PET scan was evaluated in 37 of 67 patients, per European Organization for the Research and Treatment of Cancer (EORTC) criteria.33 Thirteen patients (35.1%) experienced a partial metabolic response, while no new TEAEs were observed. Table 2 lists all TEAEs that were reported in >5% of patients in the phase 3 study or were worsening in the dose escalation study. Of note, dose escalation to 150 mg BID was associated with a higher incidence of a few grade 3–4 side effects.
In conclusion, adverse effects of ripretinib were mostly grade 1 and 2 and higher-grade adverse events reported in clinical studies were manageable. In addition, patients receiving ripretinib experience fewer grade 3–4 TEAEs as compared to sunitinib arm in the preliminary results of the INTRIGUE study reported at ASCO Plenary Series 2022.29
Quality of Life
In the phase 3 INVICTUS study, patient-reported outcomes were measured as a pre-specified secondary outcome5 using the EuroQol-5D (EQ-5D-5L) and the EORTC QOL Questionnaire (QLQ)-C30, and the change from the cycle 1, day 1 (baseline) to cycle 2, day 1 between ripretinib and placebo was compared.34 Patients receiving ripretinib reported consistently more stable and significantly better health status outcomes on the EQ-5D-5L and physical and role functioning tests than did those receiving placebo.
QOL was measured in patients who received ripretinib or sunitinib in the INTRIGUE study.29 These measures included the impact of skin toxicity on QOL as measured by Dermatology Life Quality Index (DLQI) and the percentage of patients with skin toxicity that had a moderate to extremely large impact on their lives and those with deterioration in role functioning (ability to engage in work or leisure activities during treatment) on the EORTC QLQ-C30. Fewer patients in the ripretinib arm experienced skin toxicity that affected QOL. Also, patients receiving ripretinib reported experiencing less decline in EORTC QLQ-C30 role functioning.
Conclusions
Ripretinib is a novel TKI against primary KIT/PDGFRA mutations and resistant secondary KIT mutations. It has potency against a wide range of KIT/PDGFRA mutations in GIST and can be of substantial benefit in case of subclone heterogeneity in later line treatment settings. According to the results of the phase 3 clinical trial (INVICTUS), 4th-line or later treatment with ripretinib conferred PFS and OS benefits compared to placebo.5 However, in the 2nd-line setting, ripretinib treatment was not superior to the current standard, sunitinib.29 A subgroup analysis from the INTRIGUE preliminary report showed that ripretinib had less PFS benefit in exon 9 KIT mutation but resulted in a superior RR in exon 11 KIT mutation. Although the efficacy of ripretinib in an unselected population was not different from that of sunitinib, the tolerability profile seems to be better.
In conclusion, ripretinib is an established standard of care in the 4th-line or higher setting in GIST, supported by the efficacy and tolerability data. As the field moves forward, with emerging data on the efficacy of TKIs according to mutation subgroups and our ability to identify the spectrum of mutations in each patient (through tumor and liquid biopsies), a more personalized treatment approach in selecting the sequence of TKIs might be feasible in the future (Figure 3).
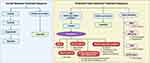 |
Figure 3 Current systemic treatment sequence and potential future treatment sequence with personalized approach. Image created with BioRender.com. |
Acknowledgments
We thank Editing Services, Research Medical Library, the University of Texas MD Anderson Cancer Center, for providing excellent language editing.
Disclosure
NS received an honorarium for advisory board participation from Boehringer Ingelheim, Deciphera, Epizyme, Aadi Biosciences, and Bayer. The authors report no other conflicts of interest in this work.
References
1. Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. 2021;14(1):2. doi:10.1186/s13045-020-01026-6
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG intergroup trial S0033. JAMA Oncol. 2017;3(7):944–952. doi:10.1001/jamaoncol.2016.6728
3. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. doi:10.1016/S0140-6736(06)69446-4
4. Demetri GD, Reichardt P, Kang Y-K, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. doi:10.1016/S0140-6736(12)61857-1
5. Blay J-Y, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923–934. doi:10.1016/S1470-2045(20)30168-6
6. Roskoski R
7. Liang L, Yan XE, Yin Y, Yun CH. Structural and biochemical studies of the PDGFRA kinase domain. Biochem Biophys Res Commun. 2016;477(4):667–672. doi:10.1016/j.bbrc.2016.06.117
8. Nannini M, Tarantino G, Indio V, et al. Molecular modelling evaluation of exon 18 His845_Asn848delinsPro PDGFRalpha mutation in a metastatic GIST patient responding to imatinib. Sci Rep. 2019;9(1):2172. doi:10.1038/s41598-018-38028-x
9. Mazzocca A, Napolitano A, Silletta M, et al. New frontiers in the medical management of gastrointestinal stromal tumours. Ther Adv Med Oncol. 2019;11:1758835919841946. doi:10.1177/1758835919841946
10. Napolitano A, Vincenzi B. Secondary KIT mutations: the GIST of drug resistance and sensitivity. Br J Cancer. 2019;120(6):577–578. doi:10.1038/s41416-019-0388-7
11. Norman RA, Ferguson AD, Ferguson AD. Structural approaches to obtain kinase selectivity. Trends Pharmacol Sci. 2012;33:1873–3735. doi:10.1016/j.tips.2012.03.005
12. Gajiwala KS. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci USA. 2009;2009:1091–6490.
13. Blay JY. Pharmacological management of gastrointestinal stromal tumours: an update on the role of sunitinib. Ann Oncol. 2010;21(2):208–215. doi:10.1093/annonc/mdp291
14. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–1968. doi:10.1016/j.ejca.2009.02.011
15. Yeh C-N, Chen M-H, Chen -Y-Y, et al. A Phase II trial of regorafenib in patients with metastatic and/or a unresectable gastrointestinal stromal tumor harboring secondary mutations of exon 17. Oncotarget. 2017;8(27):44121–44130. doi:10.18632/oncotarget.17310
16. Bauer S, George S, von Mehren M, Heinrich MC. Early and next-generation KIT/PDGFRA kinase inhibitors and the future of treatment for advanced gastrointestinal stromal tumor. Front Oncol. 2021;11:672500. doi:10.3389/fonc.2021.672500
17. Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738–51 e9. doi:10.1016/j.ccell.2019.04.006
18. Gebreyohannes YK, Wozniak A, Zhai ME, et al. Robust activity of avapritinib, potent and highly selective inhibitor of mutated KIT, in patient-derived xenograft models of gastrointestinal stromal tumors. Clin Cancer Res. 2019;25(2):609–618. doi:10.1158/1078-0432.CCR-18-1858
19. Evans EA-O, Gardino AK, Kim JA-O, et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med. 2017;2017:1946–6242.
20. Heinrich MC, Jones RL, von Mehren M, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21(7):935–946. doi:10.1016/S1470-2045(20)30269-2
21. George S, Jones RL, Bauer S, et al. Avapritinib in patients with advanced gastrointestinal stromal tumors following at least three prior lines of therapy. Oncologist. 2021;26(4):E639–E49. doi:10.1002/onco.13674
22. Kang Y-K, George S, Jones RL, et al. Avapritinib versus regorafenib in locally advanced unresectable or metastatic gi stromal tumor: a randomized, open-label phase III study. J Clin Oncol. 2021;39(28):3128–3139. doi:10.1200/JCO.21.00217
23. Serrano C, Marino-Enriquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2019;120(6):612–620. doi:10.1038/s41416-019-0389-6
24. Apsel Winger B, Cortopassi WA, Garrido Ruiz D, et al. ATP-competitive inhibitors midostaurin and avapritinib have distinct resistance profiles in exon 17-mutant KIT. Cancer Res. 2019;79(16):4283–4292. doi:10.1158/0008-5472.CAN-18-3139
25. Heinrich M, von Mehren M, Jones RL, Bauer S, Kang Y. Avapritinib is highly active and well-tolerated in patients with advanced GIST driven by diverse variety of oncogenic mutations in KIT and PDGFRA.
26. George S, Heinrich M, Chi P, et al. Initial results of Phase I study of DCC-2618, a broad-spectrum KIT and PDGFRa inhibitor, in patients (pts) with gastrointestinal stromal tumor (GIST) by number of prior regimens. Ann Oncol. 2018;29:viii576–viii7. doi:10.1093/annonc/mdy299.002
27. Chi P, Janku F, Heinrich M, et al. Abstract C077: updated results of phase 1 study of ripretinib (DCC-2618), a broad-spectrum KIT and PDGFRA inhibitor, in patients with gastrointestinal stromal tumor (GIST) by line of therapy (NCT02571036)2019. C077-C p. Mol Cancer Ther. 2019;18(12 Suppl):C077–C077. doi:10.1158/1535-7163.TARG-19-C077
28. Rose S. News in brief: testing ripretinib against sunitinib in GIST. Cancer Discov. 2022;2022:OF1–OF.
29. Heinrich MC, Jones RL, Gelderblom H, et al. INTRIGUE_A phase III_ randomized, open-label study to evaluate the efficacy and safety of ripretinib vs sunitinib in patients with advanced gastrointestinal stromal tumor.
30. Mehren M, Heinrich M, George S, et al. Clinical benefit with ripretinib as ≥4th-line treatment in patients with advanced gastrointestinal stromal tumor: long-term update from the phase 3 INVICTUS study.
31. Janku F, Abdul Razak AR, Chi P, et al. Switch control inhibition of KIT and PDGFRA in patients with advanced gastrointestinal stromal tumor: a phase I study of ripretinib. J Clin Oncol. 2020;38(28):3294–3303. doi:10.1200/JCO.20.00522
32. George S, Chi P, Heinrich MC, et al. Ripretinib intrapatient dose escalation after disease progression provides clinically meaningful outcomes in advanced gastrointestinal stromal tumour. Eur J Cancer. 2021;155:236–244. doi:10.1016/j.ejca.2021.07.010
33. Young H, Baum R, Cremerius U. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET study group. Eur, J Cancer. 1999;35(13):1773–1782. doi:10.1016/S0959-8049(99)00229-4
34. Heinrich MC, George S, Zalcberg J, et al. Quality of Life (QoL) and self-reported function with ripretinib in ≥4th-line therapy for patients with Gastrointestinal Stromal Tumors (GIST): analyses from INVICTUS. J Clin Oncol. 2020;38(Suppl):11535. doi:10.1200/JCO.2020.38.15_suppl.11535
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

