Back to Journals » International Journal of Nanomedicine » Volume 18
Emerging Anesthetic Nanomedicines: Current State and Challenges
Authors Zhang S, Wang Y, Zhang S, Huang C, Ding Q, Xia J, Wu D, Gao W
Received 19 April 2023
Accepted for publication 10 July 2023
Published 19 July 2023 Volume 2023:18 Pages 3913—3935
DOI https://doi.org/10.2147/IJN.S417855
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Shuo Zhang,1,* Yishu Wang,1,* Shuai Zhang,1 Chengqi Huang,1 Qiyang Ding,2 Ji Xia,2 Daocheng Wu,1 Wei Gao2
1The Key Laboratory of Biomedical Information Engineering of Ministry of Education, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an, 710049, People’s Republic of China; 2Department of Anesthesiology & Center for Brain Science & Center for Translational Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710049, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Daocheng Wu, The Key Laboratory of Biomedical Information Engineering of Ministry of Education, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an, 710049, People’s Republic of China, Tel/Fax +86-29-82663941, Email [email protected] Wei Gao, Department of Anesthesiology & Center for Brain Science & Center for Translational Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710049, People’s Republic of China, Tel +86 15209225834, Email [email protected]
Abstract: Anesthetics, which include both local and general varieties, are a unique class of drugs widely utilized in clinical surgery to alleviate pain and promote relaxation in patients. Although numerous anesthetics and their traditional formulations are available in the market, only a select few exhibit excellent anesthetic properties that meet clinical requirements. The main challenges are the potential toxic and adverse effects of anesthetics, as well as the presence of the blood-brain barrier (BBB), which makes it difficult for most general anesthetics to effectively penetrate to the brain. Loading anesthetics onto nanocarriers as anesthetic nanomedicines might address these challenges and improve anesthesia effectiveness, reduce toxic and adverse effects, while significantly enhance the efficiency of general anesthetics passing through the BBB. Consequently, anesthetic nanomedicines play a crucial role in the field of anesthesia. Despite their significance, research on anesthetic nanomedicines is still in its infancy, especially when compared to other types of nanomedicines in terms of depth and breadth. Although local anesthetic nanomedicines have received considerable attention and essentially meet clinical needs, there are few reported instances of nanomedicines for general anesthetics. Given the extensive usage of anesthetics and the many of them need for improved performance, emerging anesthetic nanomedicines face both unparalleled opportunities and considerable challenges in terms of theory and technology. Thus, a comprehensive summary with systematic analyses of anesthetic nanomedicines is urgently required. This review provides a comprehensive summary of the classification, properties, and research status of anesthetic nanomedicines, along with an exploration of their opportunities and challenges. In addition, future research directions and development prospects are discussed. It is hoped that researchers from diverse disciplines will collaborate to study anesthetic nanomedicines and develop them as a valuable anesthetic dosage form for clinical surgery.
Keywords: anesthesia, nanocarriers, blood-brain barrier, drugs
Introduction
The modern era of anesthetic medicines was pioneered by William T.G. Morton, an American dentist who, in 1846, successfully demonstrated the use of medical anesthesia with diethyl ether through inhalation, marking a significant turning point in surgical procedures. Since then, anesthetics have transformed surgery from a terrifying and agonizing experience to a painless and peaceful process, saving countless lives worldwide each year. As a result, anesthetics have become a crucial class of drugs used in clinical surgery.1 Anesthesia typically involves the temporary inhibition of central and/or peripheral nervous system function through oral, inhalational, topical, intravenous, or intramuscular anesthetics. Electromyography studies have shown that anesthetics temporarily and reversibly disrupt the connections between cortical and thalamic neurons. This results in the absence of pain sensation, even during invasive procedures involving certain body tissue owing to the effects of the anesthetics.2 As new medicines, biotechnologies, innovations, and drug discoveries emerge, the development of anesthetics has continued to make significant progress.
In clinical practice, anesthetics are currently divided into two main categories of local anesthesia and general anesthesia, with related anesthetics also falling under these categories as local and general anesthetics.3 Local anesthesia involves the temporary numbing of a specific area of the body, typically used for minor surgical, topical or dental procedures. Subcategories of local anesthesia include surface anesthesia, local infiltration anesthesia, intravenous local anesthesia, peripheral nerve blocks, and spinal canal anesthesia.4 Traditional local anesthetics, also referred to as surface anesthetics, are commonly available in the form of ointments, gels, lotions, or sprays.5 In comparison with general anesthetics, local anesthetics have a lesser impact on the physiological state of the body. Therefore, the widespread use of local anesthetics can generally meet the needs of local surgeries.6 Although local anesthetics have less impact on the physiological state of the body compared to general anesthetics, their toxicity must be taken into account.7 Improper use of local anesthetics can not only damage local tissues but also harm nerve cells.8 Currently, the local anesthetics and their traditional formulations available on the market can essentially meet clinical demand, but there is still a need to improve their efficacy and reduce their toxicity.9 General anesthetics are typically administered through inhalation of gases or vapors, known as total inhalational anesthesia, or via a combination of inhalants and injectables, known as balanced anesthesia. Alternatively, they can be administered through intravenous or intramuscular injections only, known as total intravenous anesthesia. These methods work by inhibiting the central nervous system, resulting in loss of consciousness without pain.10 It is important to note that general anesthetics must pass through the BBB to exert their effects on the central nervous system.11 As a result, the challenge of efficiently and rapidly passing through the BBB remains a significant hurdle in the development of new and effective general anesthetics. Intravenous injection is currently the most commonly used administration method for general anesthesia.12 Even though many general anesthetics are effective, they may also have significant side effects and toxicity, posing risks to patients undergoing surgery. Due to the large number of general anesthetics used in clinics, inappropriate usage can lead to severe negative effects and even death. The risk factor increases with the dosage of anesthetics.13 As a result, a significant number of anesthesia accidents and fatalities occur worldwide each year due to improper use of general anesthetics. Finding the balance between the toxicity, dosage, and efficacy of anesthetics to achieve optimal anesthesia is challenging.14 Therefore, there is an urgent need to develop new types of general anesthetics that have high efficacy and low toxicity. Additionally, improving existing general anesthetics to reduce their toxicity and increase their efficacy presents a significant opportunity for drug research and development.
In recent years, precision medicine has emerged as a trend in the development of clinical anesthesia. Precision medicine aims to provide personalized healthcare by tailoring medical treatment to the individual needs and characteristics of each patient, thereby maximizing the quality of health care.15 As a result, precision anesthesia has increasingly replaced traditional anesthesia research and development goals. Precision anesthesia aims to tailor the anesthetic treatment to the unique needs of each patient, which requires the anesthetics to be delivered accurately to the target site. In some cases, multiple anesthetics may need to be delivered simultaneously. However, traditional drug formulations may not be able to meet these requirements. An alternative approach is to load the anesthetics onto nanocarriers, which can then be referred to as anesthetic nanomedicines, to achieve the desired precision.16 Nanocarrier technology allows for the modification of the size, charge, composition, and surface features of anesthetics, resulting in nanomedicines with specialized functionalities.17 Nanomedicine has gained popularity due to its excellent biocompatibility and stability, high curative effect, minimal side effects, prolonged residence period in the body, reduced injury to normal human tissue, and targeted therapy techniques that enable accurate treatment.18 Anesthetic nanomedicines can facilitate targeted delivery of anesthetics, especially to the brain, during general anesthesia procedures.19 They can also prolong the duration of anesthetic action, reduce toxic and side effects resulting from high concentrations of anesthetics, and release anesthetics as needed to achieve safe and effective analgesic and anesthetic effects.20 Anesthetic nanomedicines, which include both general and local anesthetic nanomedicines, have become a critical area of development for precision anesthesia. However, while there has been some research on local anesthetic nanomedicines, there are very few reports currently available on general anesthetic nanomedicines. There are several reasons why there are few reports on general anesthetic nanomedicines. Firstly, many general anesthetics are fat-soluble small molecules that can pass through the BBB, but their ability may be reduced after being made into nanomedicines.21 Secondly, the choice of nanomaterials used as carriers can impact the ability of anesthetic nanomedicines to enter the brain through the BBB, placing higher demands on carrier materials. Lastly, the addictive effect of general anesthetics may be stronger when made into nanomedicines, which presents a challenge for research in this field. Additionally, anesthetics have considerable toxic and radiative effects and may become addictive with long-term use, leading to strict regulation of their use and procurement in many countries. Consequently, the development, production, and operation of general anesthetics nanomedicines require specific approval and strict procedures, which makes this research relatively challenging. Therefore, the development of emerging anesthetic nanomedicines is riddled with numerous difficulties and challenges. Furthermore, due to the high demand for anesthetics worldwide, there is a vast market for anesthetic nanomedicines, which holds great potential for development and numerous opportunities. Anesthetic nanomedicines with modified features, such as reduced toxicity and addiction, and additional functionalities like imaging and multi-drug cooperation, are particularly promising. It is puzzling that anesthetic nanomedicines are one of the emerging field, but their research methods, including theory and technology, are very traditional. Many new theories and technologies of drugs have not been applied in this field. To fully explore this potential, it is necessary to systematically analyze the current state of clinical anesthetics, summarize recent progress in anesthetic nanomedicine research, identify possible future development directions, and encourage interdisciplinary collaboration among researchers in this field. This review aims to provide a comprehensive overview of the current status of clinical anesthetics and the potential of anesthetic nanomedicines. It will organize and analyze the existing research on anesthetic nanomedicines, highlighting their advantages, research progress, and challenges that must be addressed. By doing so, we hope to encourage more scientists to focus on this emerging area and collaborate to advance the development of anesthetics (Scheme 1).
 |
Scheme 1 This study primarily focuses on the introduction, applications, challenges, and future prospects of anesthetic nanomedicines. |
Local Anesthesia Nanomedicines
Current Status of Local Anesthetics
Local anesthetics are medications that can temporarily block the generation and transmission of sensory nerve impulses. They can lead to the reversible disappearance of pain in a specific area of the body while consciousness is maintained. The effect of local anesthetics is usually limited to the site of administration, and it dissipates quickly as the drug diffuses away. Cocaine, which is an alkaloid extracted from coca leaves in South America, was the first local anesthetic used. However, its use was restricted due to its high toxicity after absorption.22 Common local anesthetics have a chemical structure composed of three parts: a lipophilic benzene ring, an intermediate carboxylic ester (-COO-) or amide (-NCO-) chain for linkage, and a hydrophilic tertiary or secondary amine group (Scheme 1). 23 The mechanism of action for traditional local anesthetics involves the inhibition of voltage-gated sodium channels, which are responsible for conducting neuronal impulses.24 Local anesthetics are characterized by their dual properties of lipophilicity and hydrophobicity, as well as hydrophilicity and lipophobic. The lipophilic benzene ring of the chemical structure is hydrophobic, while the intermediate carboxylic ester or amide chain and the hydrophilic tertiary or secondary amine group are hydrophilic. Most local anesthetics are weak alkaline compounds with low solubility in water, so they are combined with acidic compounds to form water-soluble salts for administration. The physicochemical properties of local anesthetics are determined by the structure of the aromatic ring, the type of linkage chain group, and the nature of the alkyl group attached to the amine nitrogen. Local anesthetics can be classified into two main groups based on their intermediate chain: esters and amides.25 Ester local anesthetics are metabolized in the body to produce para-aminobenzoic acid (PABA), which can be recognized as a foreign substance by the immune system and trigger an allergic reaction, resulting in a high incidence of anaphylaxis. In contrast, amide local anesthetics do not undergo this metabolism and are less likely to cause allergic reactions. Therefore, patients who are allergic to ester local anesthetics should be administered amide local anesthetics instead.26 When multiple types of local anesthetics are mixed in a clinical setting, the concentration is often diluted. This may result in a faster onset of the anesthetic effect, but the duration may be shorter compared to using a single drug, due to possible competitive relationships between the same type of amide compounds. Furthermore, the metabolism of amide local anesthetics is mainly by the liver, while ester local anesthetics are primarily metabolized by plasma cholinesterase.27 Procaine and tetracaine are examples of ester local anesthetics, while lidocaine, bupivacaine, and ropivacaine are examples of amide local anesthetics. Compared to esters, amide local anesthetics have better chemical stability and a lower risk of causing allergic reactions.28 Bupivacaine and lidocaine hydrochloride, which is also called lignocaine, are commonly used amide local anesthetics. Bupivacaine exhibits a slower onset of action, typically occurring about 5–10 minutes after injection, but offers a longer duration of action (lasting between 4–8 hours), and has a greater lipophilic effect, which enhances tissue permeability and lipid solubility. Its minimal dosage (around 0.40–0.50 mg/kg) and higher potency makes it an effective option for post-operative analgesia in prolonged surgical procedures.24 In contrast, Lignocaine has a rapid onset of action, taking effect within 2–5 minutes of administration, and offers an intermediate duration of action, lasting up to 3 hours. It has less of a lipophilic effect, but inherent potency and antiarrhythmic properties. Lignocaine has an average dosage of 1–1.5mg/kg, rapid absorption in tissues, and negligible cardiotoxicity. Bupivacaine is generally administered in prolonged operative procedures, providing better anesthetic and analgesic properties but with greater cardiotoxicity risks. Lidocaine, on the other hand, is provided for short-term procedures with minimal post-operative risks.25
Local anesthetics are often administered directly to the affected nerve cells, producing rapid but short-lived effects. To address this limitation, numerous preparations of local anesthetics have been developed and applied in diverse industries and fields. These drugs find frequent use in dentistry, ophthalmology, cosmetic surgery, and other fields that involve minor or localized procedures. The availability of different dosage forms, such as gels, sprays, creams, powders, and injections, enables local anesthetics to cater to a wide range of market needs. Various dosage forms of bupivacaine and lidocaine are available in clinical settings, including injectables, oral, and topical formulations such as gels, sprays, mucilages, cataplasms, patches, creams, powders, and eye drops.29 Each dosage form is suitable for specific symptoms and has unique properties. For example, lidocaine hydrochloride mucilage is often used as a local anesthetic during upper gastrointestinal endoscopy due to its ability to reduce drug and instrumental irritation, improve tissue absorption, increase analgesic potency, and prolong the duration of action.30 Previous studies have shown that the use of bupivacaine patches at the targeted site resulted in a 2.34-fold increase in anesthetic effect, indicating enhanced penetration and longer analgesic efficacy. In addition, the administration of lidocaine spray at the pulmonary target site has been found to reduce perioperative respiratory adverse events, with a rapid onset of action and localizing effect.31 While bupivacaine is commonly administered through injections, both forms of local anesthetics can lead to serious health hazards if overdosed.32 Therefore, the selection and use of local anesthetics should be based on the individual needs of each patient. Furthermore, the ongoing development of local anesthetics with improved effectiveness and minimal side effects is essential.
As described above, local anesthetics are classified into three main chemical groups based on their chemical structure and pharmacology: amino esters, amino amides, and a newer group with guanidine-like structure that includes biotoxins such as saxitoxin and tetrodotoxin. The use of biotoxins has led to the development of novel molecular structures in local anesthetics in recent years.33,34 Biotoxins, such as saxitoxin and tetrodotoxin, possess a high affinity for sodium channels and effectively block the influx of sodium ions in the outer surface receptor of neuronal membranes, leading to nerve conduction occlusion. However, the clinical use and promotion of biotoxins have been limited due to the systemic toxicity and paralysis risks that have been identified in clinical trials. An instance of the extreme toxicity of biotoxins can be seen in the lethal dose 50 (LD50) of tetrodotoxin, which was found to be 10.7 ng/g with intraperitoneal injection and 12.5 ng/g with subcutaneous inoculation in mice.35 Additionally, the narrow treatment window further highlights the fact that these drugs are not ideal in terms of safety, which can be daunting for new drug developers and medical workers.
In summary, although local anesthetics are considered traditional drugs with suitable delivery methods and low toxicity compared to general anesthetics, their potential toxic and addictive effects cannot be ignored, such as cocaine and tetrodotoxin. Moreover, despite the availability of many different types of local anesthetics in various dosage forms, only a few are widely used and personalized treatment options are still lacking. Hence, there is a need for continued research into the development of high-performance, effective, and comfortable local anesthetics in diverse dosage forms. Nanotechnology can play a crucial role in improving existing local anesthetics and advancing this field.
Local Anesthetic Nanomedicines
In contrast to traditional local anesthetics, the ideal local anesthetics would have longer duration of action, low systemic toxicity, high potency, rapid onset, non-irritating properties, reversible effects, prominent anesthetic and analgesic effects. To achieve this, scientists have been developing new local anesthetic nanomedicines, including lipid-based, polymer-based, and lipid-polymer hybrid local anesthetic nanomedicines. These nanomedicines utilize various carrier materials, such as liposomes, nanohybrid hydrogels, lipid particles, dendrimers, polymer micelles, mesoporous silica, metallic nanoparticles, quantum dots, and polymer-based nanomaterials, as seen in Table 1.
 |
Table 1 Properties of Local Anesthetic Nanomedicines |
Lipid-Based Local Anesthetic Nanomedicines
Lipid-based nanoparticles typically consist of a lipid core that contains the pharmaceutical drug (in this case, a local anesthetic) and surfactants as stabilizers. These nanoparticles can be divided into six categories based on their structure: liposomes, lipid nanoemulsions, solid lipid nanoparticles (SLNs), niosomes, lipid nanoparticles (LNPs), and nanostructured lipid carriers (NLCs).45 Liposomes are composed of a phospholipid bilayer surrounding an aqueous core, while lipid nanoemulsions consist of a lipid monolayer surrounding a liquid lipid core. Solid lipid nanoparticles are composed of a surfactant (emulsifier) layer that encloses lipids in a solid state.46 Meanwhile, LNPs have a phospholipid monolayer, and their core consists of phospholipid molecules connected to each other and to the surfactant with the aid of oligonucleotides.47 NLCs consist of a mixture of solid and liquid lipids, with a layer of surfactant/emulsifier covering the outside.48 Niosomes are single-layered nonionic surfactants that encapsulate a hydrophilic core. Hydrophilic local anesthetics bind to the water-soluble regions of lipid-based nanoparticles, while lipophilic drugs bind to the hydrophobic regions that are lipid-soluble.49 The distribution of local anesthetics within lipid-based nanoparticles is dependent on the intended site, mode and mechanism of action. Lipid-based nanomedicines offer several distinctive benefits, such as excellent biocompatibility, biodegradability, increased drug loading efficacy and stability, precise targeting of specific sites, lower toxicity, simple high-yield production, improved drug permeability at the intended site, and more. Consequently, they are among the most widely considered biomimetic drug delivery systems, and also as one of the most commonly utilized nanocarriers for the delivery and application of local anesthetics.23 One notable researcher in the field of controlled release of local anesthetics using liposomes is Kohane. His team has made significant contributions to modifying liposome nanocarriers to achieve effective and multiple controlled release of encapsulated anesthetics using light sensitivity. Specifically, they attached gold nanorods (GNRs) to liposomes (Lip-GNRs) which can convert near-infrared (NIR) light into heat. This phototriggered phase transition causes the lipid bilayer to undergo a phase transition, resulting in the release of local anesthetics.37,50 Using this method could result in the quick release of anesthetics not triggered by light, which could lead to prolonged nerve block during local anesthesia. Consequently, the research team conducted further investigations and experiments. They connected local anesthetics to a large molecule carrier called coumarin, producing P407-CM-T nanoparticles. Although this macromolecular prodrug is not inherently anesthetic, when exposed to a low-power blue light emitting diode (LED), it exhibited a local anesthetic effect. According to observations, when exposed to a light intensity of 300 mW cm−2 for 5 minutes, the duration of anesthesia can extend up to 78.1 ± 24 minutes. The duration of the anesthesia appeared to be directly proportional to the light intensity, as illustrated in Figure 1a–c). Moreover, this approach is capable of being activated multiple times, allowing the anesthetic to be released and triggered by light up to five times after its initial effect has worn off, as depicted in Figure 1d and e. Thus, adjusting the intensity and duration of irradiation can result in on-demand drug delivery to alleviate pain.51
 |
Figure 1 (a) The duration of nerve block after administering tetracaine, along with 2-minute exposure to 300 mW/cm² irradiation immediately following injection. (b) The nerve block resulting from the irradiation of P407-CM-T at different irradiances and exposure durations. (c) The impact of energy density on the duration of the induced nerve block (n = 4). (d) The temporal profile of nerve block elicited by several instances of light activation was assessed, where the blue arrows indicate the triggering of LED for a period of 2 minutes at 300 mW/cm². (e) The average duration of nerve block following each triggering event in d (n = 4). Reproduced from Zhang W, Ji T, Li Y, et al. Light-triggered release of conventional local anesthetics from a macromolecular prodrug for on-demand local anesthesia. Nat Commun. 2020;11(1):2323. Creative Commons.51 |
Additionally, lipid nanoparticles have evolved from the original SLNs to a new generation of NLCs. The latter are composed of a blend of solid and liquid lipids in the core region.39,52 Zhang et al employed the layer-by-layer (LBL) technology to produce lidocaine loaded nanostructured lipid carriers (LBL-LA/NLCs). These particles exhibited nearly twice the skin permeability of the free drug during transdermal administration. Moreover, they displayed sustained release capabilities, providing anesthetic effects lasting up to 60 minutes, as opposed to the rapid release of free drugs, which peaks at 10 minutes.53 As demonstrated by the examples above, the appropriate use of lipid-based anesthetic nanoparticles can extend the duration of local anesthetics, enabling the same anesthetic effect to be achieved with a lower dosage and reduced toxicity. Currently, lipid-based anesthetic nanoparticles have been well-researched and are being employed in clinical settings. For instance, liposomal tetracaine nanoparticles (topical) were first utilized in humans in 1988, and the first successful treatment of postoperative pain through liposomal bupivacaine injection was reported in 1994. In 2011, liposomal encapsulated bupivacaine was approved by the US Food and Drug Administration (FDA) for postsurgical analgesia. Furthermore, lots of studies have highlighted that lipid-based nanoparticles as carriers for local anesthetics offer superior analgesic duration and enhanced invasiveness in comparison to traditional anesthetics.54
Polymer-Based Local Anesthetic Nanomedicines
Polymeric nanoparticles offer several advantages, including the controlled release of therapeutic agents at constant doses over prolonged periods, cyclic dosage capabilities, tunable release of both hydrophilic and hydrophobic drugs, bioimitative properties, enhanced stability, targeting, increased bioavailability, and reduced side effects.55 Natural and synthetic polymers can be combined with local anesthetics to produce polymer-based anesthetic nanomedicines. These nanomedicines are typically categorized into two groups based on their structure: nanospheres (matrix system) and nanocapsules (reservoir system). The most frequently employed biodegradable synthetic polymers include polyethylene glycol (PEG), polylactic acid (PLA), polyamino acids, polyglutamic acid (PGA), poly lactic glycolic acid (PLGA), and poly Ɛ-caprolactone (PCL). Natural polymers such as alginate, chitosan, gelatin, cellulose, and albumin are commonly utilized in the fabrication of polymer-based local anesthetic nanomedicines.56 Upon reaching the nerve periphery, local anesthetics delivered by the polymer carrier are released slowly, resulting in a prolonged nerve block and reduced total amount of local anesthetics entering the human circulation per unit time. The release of local anesthetics from polymeric nanocarriers enables a prolonged nerve block while also minimizing the likelihood of the drug entering the bloodstream.
A study conducted by Lalatsa et al demonstrated that the onset time of lidocaine hydrogel was significantly shorter compared to EMLA (an anesthesia cream), and a 5.2-fold increase in the flux of lidocaine through the skin of the human eyelid area was observed.40 Similarly, Weldon et al developed a bupivacaine micelle formulation of only 15nm (M-Bup). M-Bup provided local anesthesia twice as long as free bupivacaine, and patients treated with M-Bup had lower levels of bupivacaine detected in their blood, indicating less systemic toxicity.41 In addition, it was observed that liposomal bupivacaine (L-Bup) with a size of approximately 100 nm, at the same dose as M-Bup, did not produce any anesthesia. This demonstrates the biocompatibility and specificity of certain types of nanoparticles. Local anesthetics can be bound to the surface of polymeric nanoparticles through chemical bonds or physically through processes such as adsorption, dissolution, entrapment, embedment or encapsulation. Polymer prodrugs linked by chemical bonds are relatively stable, and the release pattern and loading efficiency of drugs in polymer-based local anesthetic nanomedicines are influenced by factors such as anesthetics ionicity, solubility nature (hydrophilic or lipophilic) and molecular mass of the polymer. The release of anesthetics from polymeric carriers can occur through several processes, including desorption, diffusion, biodegradation of the polymers, or a combination of these mechanisms. The anesthetics can be released through the channels within the polymer carrier or through the decomposition of the carrier molecules. On the other hand, a polymer prodrug that is chemically bonded is typically more stable For instance, Zhao et al demonstrated the use of a degradable polymer, TDP [poly(triol dicarboxylic acid)-co-poly(ethylene glycol)], connected through ester bonds to tetrodotoxin (TTX). The slow release of TTX was observed, and its derivatives acted as a chemical permeation enhancer (CPE) (Figure 2). This allowed TTX to penetrate the neurovascular barrier into the axoplasm, resulting in the release of 80 µg of TTX over three days. This approach significantly increased the therapeutic window for TTX, achieving high efficiency and low toxicity.57
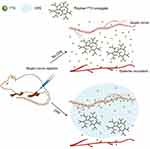 |
Figure 2 A polymer-TTX conjugate, which contains a high concentration of TTX and is designed for slow release, is positioned near a nerve. The delivery system includes a chemical permeation enhancer that facilitates the entry of TTX into the nerve. The hyperbranched structure of the polymer-TTX conjugate shown here has a TTX loading capacity that is significantly greater than the conjugates produced in the current experiment. Reproduced from Zhao C, Liu A, Santamaria CM, et al. Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity. Nat Commun. 2019;10(1):2566. Creative Commons.57 |
Lipid-Polymer Hybrid Local Anesthetic Nanomedicines
Lipid-polymer hybrid nanoparticles (LPHNPs) are a type of core-shell nanocarrier consisting of biodegradable polymer nanoparticles as the core and a lipid shell as the outer layer.58 Based on their structure, LPHNPs can be classified into five groups: monolithic LPHNPs, polymer core lipid shells, hollow core-shell nanoparticles, erythrocyte membrane camouflaged biomimetic lipid-polymer hybrids, and polymer-caged liposomes. Local anesthetics can either be covalently embedded on the lipid shell surface or encapsulated within the polymer core. Drug release is regulated by a variety of factors, such as the matrix composition, microenvironment pH, and surrounding temperature.58 As a delivery carrier for local anesthetics, LPHNPs offer the advantages such as small particle size, high encapsulation efficiency, and good biocompatibility. Furthermore, the polymer core of LPHNPs can enhance the sustained release effect of drugs, provide excellent storage stability, enable on-site drug delivery, and facilitate facile preparation. LPHNPs also offer significant prolonged duration of local anesthesia.
In a study by Li et al, lipid-polymer hybrid nanoparticles (LPHNPs) were synthesized using poly real (ethylene glycol)-distearoylphosphatidylethanolamine (PEG DSPE) as a hydrophilic lipid shell and PCL as the hydrophobic polymer core. Ropivacaine (RPV) was encapsulated in LPHNPs to create RPV-LPHNPs. Results from in vitro transdermal assays showed that RPV-LPHNPs had a cumulative RPV permeability of 907 ± 35µg/ cm2 after 48 hours, which was 2.6-fold higher than that of RVC injection (344 ± 23µg/cm2). In vivo experiments conducted by Li et al demonstrated that single administration of RPV-LPHNPs significantly prolonged the duration of analgesia in mice compared with RPV solution. The median duration of analgesia was 36 hours and 0.5 hours for RPV-LPHNPs and RPV solution, respectively.43 Additionally, Wang et al utilized the nanoprecipitation method to create lipid-polymer nanoparticles (LDC-LPHNPs) loaded with lidocaine (LDC). In vitro cytotoxicity experiments showed that LDC@LPHNPs reduced the cytotoxicity in mouse embryonic fibroblast NF-3T3 cells. Moreover, in vivo experiments demonstrated that LDC-@LPHNPs reduced injection pain and prolonged the mid-term duration of painless anesthesia to 20 hours, which was superior to LDC-@NPs or free LDC (Figure 3).42
 |
Figure 3 (a) The process of producing LDC@LPHNs for use in local anesthetics applications. (b) The effect of various concentrations of LDC (0, 1, 5, 10, 20 μM) on the proliferation of NIH-3T3 cells after 24 hours of treatment.; Evaluation of the anesthetic effects in a Swiss animal model in vivo; Observation of vocalization response phase progression triggered by electrical shocks in mice after subcutaneous administration of LDC alone, LDC@NPs, and LDC@LPHNs formulations as analgesics. Reprinted from Process Biochem, volume102, Wang Y, Qin M, Hou J, Chen Y. In vitro and in vivo evaluation of a lidocaine loaded polymer nanoparticle formulation co-loaded with lidocaine for local anesthetics effect. 333–340, copyright (2021), with permission from Elsevier.42 |
The above examples demonstrate that local anesthetic nanomedicines have shown promising results in achieving satisfactory anesthetic effects that meet the needs of local anesthesia. Nanotechnology offers several advantages for local anesthetics, including reducing drug toxicity by modifying the physicochemical properties to improve its anesthetic effect and lower dosage, which can result in less systemic toxicity. Prolonged anesthesia can be achieved by using anesthetic nanomedicines that effectively overcome biological barriers and allow the anesthetics to work more efficiently, leading to a longer duration of analgesia and numbness. Furthermore, nanomedicines can provide targeted and controlled release, allowing the anesthetic to be released on demand at a specific site after the corresponding stimulation, resulting in higher potency and minimal drug flow in the blood or surrounding areas, and reduced toxicity. The speed of drug release in anesthetic nanomedicines, whether they are fast or slow, have their corresponding clinical applications, and there is no inherent superiority or inferiority for clinic use. The advantages of anesthetic nanomedicines lie in their ability to decrease drug metabolism, allowing more drugs to reach the target site and reducing drug waste. In general, during clinical surgeries, it requires rapid onset of anesthesia, precise control of anesthesia maintenance duration, and swift recovery. Therefore, for surgeries require prolonged anesthesia, employing sustained-release anesthetic nanomedicines are excellent choice. This approach achieves both effective anesthesia and reduced toxicity. For surgeries with shorter duration, we utilize nanomedicines with effective control and release of drugs. Upon a specific external stimulus, these nanomedicines rapidly release the drug, achieving optimal anesthetic effects with minimal drug dosage, while enabling a quick return to the normal state. Besides traditional local anesthetics, modified biotoxins can also be utilized for local anesthesia. Nanotechnology-modified anesthetics are not only more efficient and safer but also easier to handle. With mature technology, their usage can significantly lower risks and labor costs associated with local anesthetics. This approach also holds promising clinical translation value and excellent research prospects.59 However, when applying local anesthetics, the metabolism of carrier materials should be carefully considered. If injected into muscle tissue or subcutaneous sites, drug metabolism may be slower and more challenging. Improper dosages may result in inflammation, proliferation, and other side effects.59 Another limitation in the field of local anesthetic nanomedicine is the limited variety of nanocarrier materials available. Currently, the materials used in this area are largely derived from those that have been established in other fields, and although they can enhance the anesthetic effect to some extent, significant breakthroughs have been difficult to achieve. Additionally, there has been a lack of research into combining local anesthetics with other innovative technologies. As a result, further exploration and research are needed in this area to fully tap into its potential. Overall, compared to other areas of drug research, the field of local anesthetic nanomedicine has not yet been extensively explored. Based on the aforementioned discussion, although local anesthetic nanomedicines have demonstrated some improvements in targeting, permeability, retention, and side effects, there have been no major breakthroughs. To meet the growing market and clinic demands for local anesthetics, further progress is required in drug design, novel drug formulations, combination therapies, and the application of advanced nanotechnologies in local anesthetic nanomedicines.
General Anesthesia Nanomedicines
Current Status of General Anesthetics
General anesthetics can be administered via three primary methods: intravenous, intramuscular, and inhalational. In some cases, a combination of intravenous and inhalational methods may be used as an adjunct.11 Intravenous general anesthetics are further sub-divided into various groups based on their chemical structure, pharmacokinetics and pharmacodynamics. These groups include barbiturates (such as sodium thiopental and methohexital), hydroxy acids, alkylphenols, phencyclidines, imidazoles, benzodiazepines (such as midazolam, lorazepam, and diazepam), alpha 2 adrenergic agonists, phenothiazines, butyrophenones, and aldehydes, as shown in Table 2.60 Inhalational anesthetics are typically divided into gaseous and volatile liquid anesthetics. Nitrous oxide and xenon are examples of gas anesthetics, while volatile liquid inhalational anesthetics include chloroform, sevoflurane, isoflurane, ethane, halothane, ether, desflurane, enflurane, and methoxyflurane, among others.61 Sevoflurane, halothane, desflurane, and isoflurane are currently the most commonly used volatile inhalational anesthetics due to their rapid onset of action and elimination from the body, reduced dosage requirement, and less negative impact on the respiratory system. However, some inhalational anesthetics have a pungent odor and can cause environmental pollution and global warming, especially desflurane, which requires high protocol equipment, making their use challenging at times.62 Because inhalational anesthetics do not require carriers and are easy to administer with little impact on the body, this review will mainly focus on intravenous anesthetics.
 |
Table 2 Properties of General Anesthetics |
Compared to inhalational anesthetics, intravenous anesthetics offer certain advantages such as rapid onset, ease of equipment use for induction and resuscitation, and strong analgesic effect. However, the processes of drug metabolism and post-operative recovery are slower.73,74 The mechanism of action for general anesthetics involves passing through the BBB or the placental barrier and inhibiting presynaptic voltage-gated sodium channels in glutamatergic synapses, primarily in the thalamus region of the brain, while analgesia is usually achieved at the spinal cord level. As shown in Table 2, the majority of anesthetics are highly lipophilic, which facilitates their easy delivery through the BBB to the target site.75 Table 2 highlights that most general anesthetics are lipophilic, but for intravenous administration, they are formulated as hydrochloride or sulfate salts to aid their stability in hydrophilic environments. Examples of such anesthetics include ketamine, midazolam, dexmedetomidine, promethazine, haloperidol, and droperidol.76,77 Additionally, some general anesthetics are administered in the form of fat emulsions, where the anesthetics are encapsulated by liposomes to reduce toxicity or irritation and improve their solubility. Examples of such highly lipophilic fat emulsion general anesthetics include propofol, etomidate, and flurbiprofen axetil.78 Table 2 indicates that regardless of the type of general anesthetics, clinical usage and solubility features, adverse effects such as cardiac arrest and the possibility of nerve injury still exist. According to Global Info Research, the enormous annual consumption and demand for general anesthetics is evident, with global revenue already reaching $774.28 million in 2021 and projected to reach $925.67 million in 2028.79 Anesthetic agents directly target neurons through intravenous injection or inhalation and can result in common side effects such as nausea, vomiting, hypotension, shivering, temporary confusion, interim memory loss, respiratory depression, and arrhythmia.80 Improper administration can cause severe damage to the central nervous system, vital organs, or even death, making accurate handling with professional measures essential. Therefore, further research and improvements to develop targeted, sustained, and controlled release of general anesthetics with minimal side effects, easy resuscitation, and prevention of adverse reactions are imperative.
As previously discussed, due to the intravenous route of administration for most general anesthetics, safety measures and dose precision are critical, resulting in limited drug variety and a relatively uniform formulation. Nevertheless, the widespread use and extensive market demand for general anesthetics present both an advantageous opportunity and a challenging dilemma for researchers. Various general anesthetics exhibit evident toxicity and radiation concerns that significantly restrict their clinical utility. Recently, some encouraging progress has been made in nano-modifications of general anesthetics, offering potential benefits on the future.
General Anesthetic Nanomedicines
Recent studies have shown significant progress in the practical application of general anesthetic nanomedicines, as highlighted in Table 3. These nanomedicines primarily include propofol and etomidate, and they utilize carrier materials made of biodegradable synthetic polymers or natural biomacromolecule materials such as PEG, PLA, polyamino acid, PGA, alginate, starch, amino acids, liquid crystal materials, micelle, among others.81 However, in comparison to local anesthetic nanomedicines, general anesthetic nanomedicines have not gained the traction in FDA approved applications, thus providing ample opportunities for further research, analysis, and future market applicability of these novel formulations.
 |
Table 3 Properties of General Anesthetic Nanomedicines |
To date, propofol and etomidate, two commonly used general anesthetics, have been the focus of research as general anesthetic nanomedicines.90 General anesthetic nanomedicines can enhance drug solubility, stability, and permeability, reduce injection pain, deliver anesthesia specifically to the target site, increase potency, and reduce the required dosage, thereby minimizing toxic effects.
Propofol Nanomedicine
Propofol lipid emulsion has been a reliable choice for more than 30 years in clinical settings, but it has some drawbacks. While it boasts several benefits, such as effective anesthesia, it can also cause a range of side effects, including respiratory distress, blurred vision, hypertriglyceridemia, and a decrease in arterial blood pressure. Injection pain during administration is also a common complaint among patients, affecting up to 28% to 90% of individuals, as per previous research.91 The lipid components present in the emulsion can also be a breeding ground for bacteria, leading to iatrogenic infections. Furthermore, prolonged and large infusions of propofol lipid emulsion can lead to hyperlipidemia, hypercholesterolemia, and glycerin toxicity, which in turn can cause acute pancreatitis, renal failure, and potentially fatal propofol infusion syndrome.84 These side effects have been linked to the specific lipid emulsion used, highlighting the need for modifications to the propofol agentia.
Liu et al developed a peptide-based carrier material called GQY, using atypical hydrophobic amino acids (HAAs), which contained many hydrophobic pockets for encapsulating highly concentrated hydrophobic anesthetics like propofol to form a stable suspension. The PRO-GQY formulation had greatly reduced the pain on injection effect compared to traditional propofol lipid emulsion. GQY was not only as effective as lipids in drug loading but also achieved anesthetic efficacy equivalent to lipid-based formulations while reducing the probability of side effects due to lipid-based formulations. Figure 4 illustrates the mechanism of the PRO-GQY formulation.85 The biocompatibility of GQY was tested using NRK-49F and L929 cells to assess its potential cytotoxicity. The results showed no significant cytotoxicity, indicating the safety of the peptide as a carrier material. Additionally, GQY did not exhibit in vitro hemolytic activity, further indicating its safety for intravenous injection. The anesthetic efficacy of GQY-based formulations was found to be superior to that of lipid or propylene glycol-based emulsions. These findings suggest that GQY, as a lipid-free carrier, holds promise as a hydrophobic anesthetic carrier. Assani et al developed a simple method to create a carrier system for water-insoluble anesthetics. They combined octanol with alginates to generate octanol-grafted alginate (Alg-C8), which was then utilized to produce nanoparticles for encapsulating propofol. The resulting propofol nanomedicines are transparent liquid with a particle size of less than 80 nm, smaller and more uniform than commercial lipid emulsions. The research showed that the nanomedicines exhibited superior chemical and physical stability at room temperature for at least 6 months, compared to the currently used lipid propofol emulsions. The study concluded that Alg-C8-encapsulated propofol nanomedicines could be the promising clinical intravenous general anesthetics for delivering poorly soluble propofol.82 In our previously research of general anesthetic nanomedicines, we has developed a method for rapid transport across the BBB. To accomplish this, propofol was loaded into propionylated amylose helix (HLPAH) nanoclusters to create propionylated amylose helix (HLPAH), which utilizes phosphatidylethanolamine-triggered release. The results indicated that the released propofol was able to cross the BBB of the central nervous system (CNS) through a concentration gradient and lipophilicity, as shown in Figure 5. The study comparing propofol loaded in PLPAH nanoclusters to propofol lipid emulsion group showed that the total amount of propofol in the former was only 0.13 times that of the latter. However, the induction time of the nanocluster group was 41 ± 10s, which was 0.22 times faster than that of the propofol emulsion group. Moreover, the recovery time of the nanocluster group was 50 ± 10s, which was 0.20 times faster than the propofol emulsion group. These results indicate that HLPAH nanoclusters possess high BBB permeability and specificity, rapid onset, short maintenance time, rapid recovery, and require a small drug dosage. Hence, they have the potential to be used in the treatment of central nervous system diseases.84 Additionally, researchers are exploring the use of ultrasound technology to achieve more efficient and rapid controlled drug release for general anesthesia. For example, Airan et al developed an ultrasound-gated nanodroplet of propofol, consisting of a polyethylene glycol-B-polycaprolactone encapsulating copolymer matrix, a liquid perfluorocarbon core, and propofol.83 The nanodroplets underwent a liquid-to-gas phase transition and released the drug when exposed to ultrasound. Experiments confirmed that ultrasound doubled the release rate of propofol without causing any harm to the brain tissue or disrupting the BBB. In addition, the nanoparticles induced potent neuromodulation using non-invasive focused ultrasound. This approach shows promise for achieving precise control of brain activity with spatial accuracy of millimeters and temporal accuracy of minutes, paving the way for clinical applications.
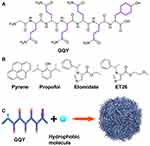 |
Figure 4 (A) GQY’s chemical structure. (B) Chemical structure of pyrene and general anesthetics, such as propofol, etomidate, and ET26. (C) Schematic depiction of GQY drug injection. The hydrophobic side chains of glutamine and tyrosine are depicted in purple. Reprinted with permission from Dove Medical Press. Liu J, Peng F, Kang Y, et al. High-loading self-assembling peptide nanoparticles as a lipid-free carrier for hydrophobic general anesthetics. Int J Nanomedicine. 2021;16:5317–5331.85 |
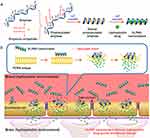 |
Figure 5 (a) The process of creating HLPAH nanoclusters. (b) The BBB-triggered HLPAH nanoclusters to be released in vivo. Reprinted from Biomaterials, volume 113, Gao W, Liu Y, Jing G, et al. Rapid and efficient crossing blood-brain barrier: hydrophobic drug delivery system based on propionylated amylose helix nanoclusters. 133–144, Copyright (2017), with permission from Elsevier.84 |
Etomidate Nanomedicine
Etomidate is an intravenous general anesthetic that has lipophilic properties and unique advantages, such as minimal impact on breathing and circulation, as well as notable hemodynamic stability, making it especially suitable for anesthesia induction in elderly patients.92 However, its side effects include suppression of corticosteroid synthesis in the adrenal cortex, and it can be highly lethal for critically ill patients. Moreover, the lipid emulsion of etomidate used in clinical practice has a high probability of catching bacterial contamination, and it is not easy to store for a longer time.93 Therefore, researchers have conducted relevant research to improve the efficacy and storage stability of these lipid-soluble general anesthetics.
Zhao et al developed a novel lipid emulsion, named ETM-ILE, for the intravenous administration of etomidate using high-pressure homogenization. The aim of the study was to compare the efficacy of ETM-ILE (a nanoformulation) with ETM-SOL (a commercially used etomidate injectable solution). Results indicated that ETM-ILE showed highly improved stability and drug loading efficiency, as well as reduced cytotoxic reactions compared to ETM-SOL. Furthermore, ETM-ILE exhibited long-term stability when stored at 4 ± 2 °C or 25 ± 2 °C for 12 months. The pharmacokinetic studies of both formulations were almost similar, but ETM-ILE was found to be safer, as it did not undergo hemolysis, showed no irritation to blood vessels, and had faster onset and clearance properties. Overall, the study concluded that ETM-ILE nanoemulsion has considerable potential for clinical applications.89 Wu et al investigated not only the stability but also the anesthetic properties of etomidate nanomedicines. They developed etomidate-loaded poloxamer micelles (Eto-SOPM) using a thin-film hydration method and enhanced its encapsulation with soybean oil (SO) to obtain etomidate nanomedicines. The release rate of this nanomedicine was found to be greater than that of the lipid emulsion, while maintaining the potency of etomidate. The speed of rats from loss to recovery of righting reflex was also 10% faster compared to the free group, resulting in a shortened action period of Eto-SOPM nanomedicines. These promising results suggest that further research into the potential of Eto-SOPM nanomedicines is warranted.88 In addition, A 2020 study demonstrated the development of a novel sequential nanoprecipitation technique to create ketamine-encapsulated single polymer PEG-PLGA nanoparticles and double polymer PEG-PLGA/shellac nanoparticles with a high drug loading of 41.8%. These drug-loaded nanoparticles showed sustained-release characteristics for over 5 days following intravenous injection in mice and for up to 21 days in vitro.86
The above reports indicate that research on general anesthetics is still in the early stages, and there are relatively fewer studies on non-propofol general anesthetics compared to propofol. Moreover, in propofol research, the focus has been on the overall properties of synthesized nanomedicine formulations rather than exploring the anesthetic effect. It is noteworthy that most general anesthetics are small molecules and lipid-soluble, with high BBB penetration rates when administered as nanomedicines. It should be noted that some hydrophilic anesthetics, like 4-Hydroxybutanoic acid sodium salts, face difficulties in crossing the BBB. How to better balance anesthesia effect and toxicity, the principle is to use fewer doses of anesthetics to achieve better anesthetic effects. At present, there are mainly two methods, one is the combined use of multiple anesthetics, through the combined use of two or more anesthetics, reduce the toxicity of a single anesthetic to specific organs of the body, and achieve better anesthetic effects. The second is to use nanomedicines that have the ability to decrease the metabolism rate of anesthetics, allowing a greater accumulation of these drugs at the target site. For example, targeted molecules like lactoferrin, special carriers such as starch carriers, surface modified ligands like apolipoprotein E derived peptide (ApoE), angiopep 2 peptide, transferrin.94–96 and technical means like ultrasound can be utilized to enhance drug delivery. Additionally, nanomedicines can extend the duration of drug action. As a result, we can significantly reduce the dosage and toxicity of anesthetics without compromising the anesthesia effectiveness. This approach aligns with the objective of minimizing toxicity while maximizing efficacy. By leveraging these new nanocarriers, technologies, and methods, it may be possible to achieve improved analgesic and anesthetic effects while reducing toxicity, which represents an important goal for future research. However, the effect of these methods is not very satisfactory now, so it needs to continue to explore.
Opportunities, Challenges and Prospects
In recent years, the focus on precision medicine has grown among doctors and patients alike. In surgery, the need for precise dosage and targeted delivery of anesthetics has become increasingly important, but current preparations and methods are falling short. Nanofabrication of anesthetics has emerged as a crucial method for improving their effectiveness. However, research on general anesthetics is still limited, with only a few studies focusing on nano-sized local anesthetics. This presents unprecedented opportunities for the development of emerging anesthetic nanomedicines for several reasons. Firstly, there are numerous theoretical and practical problems in the study of anesthetic mechanisms that require urgent solutions. Secondly, anesthetics have vast clinical applications and offer significant market potential for research and development. Finally, the use of nanomedicines can help overcome existing anesthesia techniques’ limitations, such as BBB penetration. The field of anesthetics presents both opportunities and challenges:
- The significant challenge is the lack of understanding of the mechanism of action of anesthetics and the scarcity of research on the mechanism of general anesthetic nanomedicines. Such research is crucial for the advancement of human medicine and warrants further exploration.
- The BBB is the main obstacle, which restricts the entry of lipophobic general anesthetics into the brain, resulting in wastage. While ultrasound has shown promise in opening the BBB, it has yet to be combined with anesthetics for targeted drug delivery to the brain. The limited research and development of anesthetics and the difficulty in developing new anesthetics due to their long development cycle also pose significant challenges.
- Most general anesthetics are small-molecular drugs, making them difficult to load during the preparation of nanomedicines. Further exploration is needed for nanotechnology specifically designed for small molecules of anesthetics.
- The adverse effects of general anesthetics, including their toxicity, necessitate research on reducing their side effects. Addressing this issue is a crucial direction for scientific research and development, as well as for the clinical application of anesthetics.
Therefore, immediate improvement in both anesthetics and anesthetic nanomedicine research is necessary. To address the challenges and opportunities in this field, several potential research directions and challenges can be explored.
- Continued exploration of the effects of general anesthetics is necessary. The mechanism of general anesthetics remains uncertain, and it is a crucial issue in the field of anesthesia research.97 This theoretical matter could lead to the development of novel anesthetics, which would help to identify the most suitable drugs for general anesthesia and enable accurate drug administration to patients.
- To further advance the field of anesthetics, it is essential to investigate the combined effect of anesthetics nanomedicines and other approaches. General anesthetics act on the brain, but the BBB poses a challenge for most nanomedicines to pass through. To overcome this, researchers can explore a combination of biochemical or physical methods in addition to nanomedicines modifications. For example, incorporating biological targeting molecules such as lactoferrin on the surface of nanomedicines can facilitate more effective targeting of the BBB by anesthetics.98 Alternatively, researchers can investigate the use of ultrasound and other techniques to safely and effectively open the BBB, allowing large quantities of anesthetics to enter the brain.99 This approach can significantly reduce the required dosage of anesthetics, thereby mitigating their toxicity.
- Exploring different nanocarriers is crucial in the development of effective anesthetic nanomedicines. Most anesthetics are small molecules with few functional groups, making it difficult to coat or combine them with nanocarriers. Currently, the available types of nanocarriers for local or general anesthesia are limited, and research on nanocarriers for general anesthesia is even more scarce. Moreover, the study of nanocarriers should focus on their compatibility, modification, adaptability, and efficacy within the brain environment. For instance, some studies have shown that starch carriers are released after contact with the BBB.84 It can also bind to active targeted molecules to the BBB, including glucose, L-dopa, L-carnitine, monoclonal antibodies (Mabs), peptides, transferrin receptors, insulin receptors, nicotinic acetylcholine receptors and many other biomolecules.100 Additionally, nanocarriers can be used for synergistic therapy, opening up new avenues for research and development of nanomedicines. Therefore, future research should prioritize the development of new nanocarriers, new technologies, new theories, and mechanisms of action for more effective brain delivery of general anesthetic nanomedicines.
- To enhance the development of anesthetic nanomedicines, it is crucial to integrate them with modern advanced technologies. Studies have shown that using smart materials such as sound-controlled, light-controlled, and heat-controlled release materials can improve nanomedicines administration by enabling on-demand drug delivery. However, the current research in this area is limited and needs to be further developed to meet therapeutic requirements and achieve personalized anesthetics administration goals. Moreover, an integrated anesthesia medical system that combines diagnosis and therapy is necessary. Such a system can effectively monitor the level of anesthesia and the amount of anesthetics released, providing clinicians with real-time feedback and improving patient outcomes.
- Reducing toxicity and improving the performance of anesthetics is crucial, especially with the increasing demand for anesthetics in aging populations. Precise targeting of the brain is a promising approach for achieving this goal. For example, propofol is known to cause respiratory depression, which is a significant side effect. By administering propofol through nanomedicines, it can be targeted precisely to the site of action in the central nervous system, while avoiding the potential damage to other systems. Each group of general anesthetics has its own toxicity level, dosage capability, and potency, and further research is needed to explore ways to reduce toxicity and increase efficacy of these anesthetics.
In conclusion, the emerging field of research on anesthetic nanomedicines is full of challenges and opportunities. By fostering collaboration among researchers from diverse areas. Meeting opportunities and overcome challenges, we can minimize the toxicity, increase the diversity, and enhance the efficacy of anesthetic nanomedicines, leading to a reduction in medical errors and improved surgical outcomes, which will make new contributions to the development of anesthetics. Ultimately, these efforts will benefit humanity by advancing the field of anesthesia and improving patient care.
Abbreviations
BBB, blood-brain barrier; GABA, γ-aminobutyric acid; PABA, produce para-aminobenzoic acid; LD50, lethal dose 50; SLNs, solid lipid nanoparticles; LNPs, lipid nanoparticles; NLCs, nanostructured lipid carriers; GNRs, gold nanorods; NIR, near-infrared; LBL, layer-by-layer; LBL-LA/NLCs, lidocaine loaded nanostructured lipid carriers; FDA, Food and Drug Administration; PEG, polyethylene glycol; PLA, polylactic acid; PGA, polyglutamic acid; PLGA, poly lactic glycolic acid; PCL, poly Ɛ-caprolactone; M-Bup, micelle formulation of bupivacaine; L-Bup, liposomal bupivacaine; TTX, tetrodotoxin; TDP, [poly(triol dicarboxylic acid)-co-poly(ethylene glycol)]; LPHNPs, Lipid-polymer hybrid nanoparticles; PEG DSPE, poly real (ethylene glycol)-distearoylphosphatidylethanolamine; RPV, Ropivacaine; LDC, lidocaine; LA, local anesthetic; Lipo-PS-TTX, liposomes co-loaded with PS and TTX; Lipo-DMED, dexmedetomidine loaded liposomes; P407-CM-T, Poloxamer 407-couramin-tetracaine; LTSL-GNR-TTX, low temperature sensitive liposomes-gold nanorods-tetrodotoxin; Lip-DMED, dexmedetomidine loaded liposomes; PLA NPs, poly(L-lactide) nanoparticles; EMLA, Eriskeutectic Mixture of Local Anesthet; LDC LPHNs, lidocaine hybrid lipid-polymer nanoparticles; RPV-LPNs, Ropivacaine entrapped lipid-polymer hybrid nanoparticles; Lip-GNR-TD, Lip-GNRs containing the site 1 sodium channel blocker tetrodotoxin and the α2-adrenergic agonist dexmedetomidine; SNEDDS, selfnanoemulsifying drug delivery systems; HAAs, hydrophobic amino acids; HLPAH, propionylated amylose helix; CNS, central nervous system; SO, soybean oil; GQY, Gly-Gln-Tyr; PROP, propofol; ET, etomidate; ET26, an analog of etomidate; LCNP, Liquid crystalline nanoparticles; Eto-SOPM, etomidate-loaded poloxamer micelles; ETM-ILE, etomidate intravenous lipid emulsion.
Funding
This work was sponsored in part by the National Natural Science Foundation of China (Grant NO.81971290 and 81871476); Young Top-notch Talents of “High-level Talents Special Support Program” of Shaanxi Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Patterson R. Dr Charles Thomas Jackson’s (1805–80) life after death: the 20th century mythology. J Med Biogr. 2007;15(3):147–152. doi:10.1258/j.jmb.2007.06-17
2. Barttfelda P, Uhriga L, Sitta JD, et al. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A. 2015;112(3):887–892. doi:10.1073/pnas.1418031112
3. Kamel I, Ahmed M, Sethi A. Regional anesthesia for orthopedic procedures: what orthopedic surgeons need to know. World J Orthop. 2022;13(1):11–35. doi:10.5312/wjo.v13.i1.11
4. Izaki S, Hayama K, Fujita H. A case of contact dermatitis due to dental surface anaesthetic. Contact Dermatitis. 2021;84(3):210–212. doi:10.1111/cod.13716
5. Flanagan DF. The effectiveness of articaine in mandibular facial infiltrations. Local Reg Anesth. 2015;9:1–6. doi:10.2147/LRA.S94647
6. Atanassoff PG, Lobato A, Aguilar JL. Intravenous regional anesthesia with long-acting local anesthetics. An update. Rev Esp Anestesiol Reanim. 2014;61(2):87–93. doi:10.1016/j.redar.2013.08.006
7. Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89(1):52–61. doi:10.1093/bja/aef163
8. Hussain N, Brull R, Sheehy B, et al. Perineural liposomal bupivacaine is not superior to nonliposomal bupivacaine for peripheral nerve block analgesia: a systematic review and meta-analysis. Anesthesiology. 2021;134(2):147–164. doi:10.1097/ALN.0000000000003651
9. Yanagidate F, Strichartz GR. Local anesthetics. Handb Exp Pharmacol. 2007;177:95–127. doi:10.1007/978-3-540-33823-9_4
10. Neuman MD, Feng R, Carson JL, et al. Spinal anesthesia or general anesthesia for hip surgery in older adults. N Engl J Med. 2021;385(22):2025–2035. doi:10.1056/nejmoa2113514
11. Hemmings HC, Riegelhaupt PM, Kelz MB, et al. Towards a comprehensive understanding of anesthetic mechanisms of action: a decade of discovery. Trends Pharmacol Sci. 2019;40(7):464–481. doi:10.1016/j.tips.2019.05.001
12. Kim JJ, Gharpure A, Teng J, et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature. 2020;585(7824):303–308. doi:10.1038/s41586-020-2654-5
13. Brown EN, Pavone KJ, Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg. 2018;127(5):1246–1258. doi:10.1213/ANE.0000000000003668
14. He Y, Qin L, Huang Y, Ma C. Advances of nano-structured extended-release local anesthetics. Nanoscale Res Lett. 2020;15(1):13. doi:10.1186/s11671-019-3241-2
15. Weil AR. Precision medicine. Health Aff. 2018;37(5):687. doi:10.1377/hlthaff.2018.0520
16. Mi P, Cabral H, Kataoka K. Ligand-installed nanocarriers toward precision therapy. Adv Mater. 2020;32(13):e1902604. doi:10.1002/adma.201902604
17. Qin M, Du G, Sun X. Biomimetic cell-derived nanocarriers for modulating immune responses. Biomater Sci. 2020;8(2):530–543. doi:10.1039/c9bm01444f
18. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85. doi:10.1186/s13045-021-01096-0
19. Wang J, Zhang L, Chi H, Wang S. An alternative choice of lidocaine-loaded liposomes: lidocaine-loaded lipid–polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016;23(4):1254–1260. doi:10.3109/10717544.2016.1141259
20. Babaie S, Taghvimi A, Hong JH, et al. Recent advances in pain management based on nanoparticle technologies. J Nanobiotechnology. 2022;20(1):290. doi:10.1186/s12951-022-01473-y
21. Turner-Lawrence DE, Kerns W. Intravenous fat emulsion: a potential novel antidote. J Med Toxicol. 2008;4(2):109–114. doi:10.1007/BF03160965
22. Ruetsch Y, Boni T, Borgeat A. From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem. 2005;1(3):175–182. doi:10.2174/1568026013395335
23. Geddes IC. Chemical structure of local anaesthetics. Br J Anaesth. 1962;34(4):229–239. doi:10.1093/bja/34.4.229
24. Arthur GR. Pharmacokinetics of local anesthetics. Local Anesthetics. 1986;165–186. doi:10.2199/jjsca.6.44
25. Taylor A, McLeod G. Basic pharmacology of local anaesthetics. BJA Educ. 2020;20(2):34–41. doi:10.1016/j.bjae.2019.10.002
26. Weiniger CF, Golovanevski M, Sokolsky-Papkov M, Domb AJ. Review of prolonged local anesthetic action. Expert Opin Drug Deliv. 2010;7(6):737–752. doi:10.1517/17425241003767383
27. Eggleston ST, Lush LW. Understanding allergic reactions to local anesthetics. Ann Pharmacother. 1996;30(7–8):851–857. doi:10.1177/106002809603000724
28. Sobanko JF, Miller CJ, Alster TS. Topical anesthetics for dermatologic procedures: a review. Dermatol Surg. 2012;38(5):709–721. doi:10.1111/j.1524-4725.2011.02271.x
29. de Araújo DR, de Ribeiro LN, de Paula E. Lipid-based carriers for the delivery of local anesthetics. Expert Opin Drug Deliv. 2019;16(7):701–714. doi:10.1080/17425247.2019.1629415
30. Zhang LY, Li WY, Ji M, et al. Efficacy and safety of using premedication with simethicone/Pronase during upper gastrointestinal endoscopy examination with sedation: a single center, prospective, single blinded, randomized controlled trial. Dig Endosc. 2018;30(1):57–64. doi:10.1111/den.12952
31. Gur A, Tekin E. 10% Lidocaine spray as a local anesthetic in blood gas sampling: a randomized, double-blind, placebo-controlled study. Am J Emerg Med. 2021;49:89–93. doi:10.1016/j.ajem.2021.05.060
32. Ben-David B, Solomon E, Levin H, et al. Intrathecal fentanyl with small-dose dilute bupivacaine: better anesthesia without prolonging recovery. Anesth Analg. 1997;85(3):560–565. doi:10.1097/00000539-199709000-00014
33. Kuzma PJ, Kline MD, Calkins MD, Staats PS. Progress in the development of ultra-long-acting local anesthetics. Reg Anesth. 1997;22(6):543–551. PMID: 9425971.
34. Gazerani P, Cairns BE. Venom-based biotoxins as potential analgesics. Expert Rev Neurother. 2014;14(11):1261–1274. doi:10.1586/14737175.2014.962518
35. Makarova M, Rycek L, Hajicek J, et al. Tetrodotoxin: history, biology, and synthesis. Angew Chem Int Ed Engl. 2019;58(51):18338–18387. doi:10.1002/anie.201901564
36. Moldovan M, Alvarez S, Rothe C, et al. An in vivo mouse model to investigate the effect of local anesthetic nanomedicines on axonal conduction and excitability. Front Neurosci. 2018;12:494. doi:10.3389/fnins.2018.00494
37. Zhan C, Wang W, Santamaria C, et al. Ultrasensitive phototriggered local anesthesia. Nano Lett. 2017;17(2):660–665. doi:10.1021/acs.nanolett.6b03588
38. Lea-Banks H, O’Reilly MA, Hamani C, Hynynen K. Localized anesthesia of a specific brain region using ultrasound-responsive barbiturate nanodroplets. Theranostics. 2020;10(6):2849–2858. doi:10.7150/thno.41566
39. Liu X, Zhao Q. Long-term anesthetic analgesic effects: comparison of tetracaine loaded polymeric nanoparticles, solid lipid nanoparticles, and nanostructured lipid carriers in vitro and in vivo. Biomed Pharmacother. 2019;117:109057. doi:10.1016/j.biopha.2019.109057
40. Lalatsa A, Patel PV, Sun Y, et al. Transcutaneous anaesthetic nano-enabled hydrogels for eyelid surgery. Int J Pharm. 2020;577:119003. doi:10.1016/j.ijpharm.2019.119003
41. Weldon C, Ji T, Nguyen MT, et al. Nanoscale bupivacaine formulations to enhance the duration and safety of intravenous regional anesthesia. ACS Nano. 2019;13(1):18–25. doi:10.1021/acsnano.8b05408
42. Wang Y, Qin M, Hou J, Chen Y. In vitro and in vivo evaluation of a lidocaine loaded polymer nanoparticle formulation co-loaded with lidocaine for local anesthetics effect. Process Biochem. 2021;102:333–340. doi:10.1016/j.procbio.2021.01.010
43. Li A, Yang F, Xin J, Bai X. An efficient and long-acting local anesthetic: ropivacaine-loaded lipid-polymer hybrid nanoparticles for the control of pain. Int J Nanomedicine. 2019;14:913–920. doi:10.2147/IJN.S190164
44. Lalatsa A, Emeriewen K, Protopsalti V, et al. Developing transcutaneous nanoenabled anaesthetics for eyelid surgery. Br J Ophthalmol. 2016;100(6):871–876. doi:10.1136/bjophthalmol-2015-308250
45. Han H, Li S, Xu M, et al. Polymer- and lipid-based nanocarriers for ocular drug delivery: current status and future perspectives. Adv Drug Deliv Rev. 2023;196:114770. doi:10.1016/j.addr.2023.114770
46. Mishra V, Bansal KK, Verma A, et al. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics. 2018;10(4):191. doi:10.3390/pharmaceutics10040191
47. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
48. Salvi VR, Pawar P. Nanostructured lipid carriers (NLC) system: a novel drug targeting carrier. J Drug Deliv Sci Technol. 2019;51:255–267. doi:10.1016/j.jddst.2019.02.017
49. Bhardwaj P, Tripathi P, Gupta R, Pandey S. Niosomes: a review on niosomal research in the last decade. J Drug Deliv Sci Technol. 2020;56:101581. doi:10.1016/j.jddst.2020.101581
50. Zhan C, Wang W, McAlvin JB, et al. Phototriggered local anesthesia. Nano Lett. 2016;16(1):177–181. doi:10.1021/acs.nanolett.5b03440
51. Zhang W, Ji T, Li Y, et al. Light-triggered release of conventional local anesthetics from a macromolecular prodrug for on-demand local anesthesia. Nat Commun. 2020;11(1):2323. doi:10.1038/s41467-020-16177-w
52. Garcês A, Amaral MH, Sousa Lobo JM, Silva AC. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharm Sci. 2018;112:159–167. doi:10.1016/j.ejps.2017.11.023
53. Zhang L, Wang J, Chi H, Wang S. Local anesthetic lidocaine delivery system: chitosan and hyaluronic acid-modified layer-by-layer lipid nanoparticles. Drug Deliv. 2016;23(9):3529–3537. doi:10.1080/10717544.2016.1204569
54. Ilfeld BM, Eisenach JC, Gabriel RA. Clinical effectiveness of liposomal bupivacaine administered by infiltration or peripheral nerve block to treat postoperative pain: a narrative review. Anesthesiology. 2021;134(2):283–344. doi:10.1097/ALN.0000000000003630
55. Zielinska A, Carreiró F, Oliveira AM, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16):3731. doi:10.3390/molecules25163731
56. Li K, Liu B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem Soc Rev. 2014;43(18):6570–6597. doi:10.1039/c4cs00014e
57. Zhao C, Liu A, Santamaria CM, et al. Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity. Nat Commun. 2019;10(1):2566. doi:10.1038/s41467-019-10296-9
58. Mukherjee A, Waters AK, Kalyan P, et al. Lipid-polymer hybrid nanoparticles as a next generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomedicine. 2019;14:1937–1952. doi:10.2147/IJN.S198353
59. Lirk P, Picardi S, Hollmann MW. Local anaesthetics: 10 essentials. Eur J Anaesthesiol. 2014;31(11):575–585. doi:10.1097/EJA.0000000000000137
60. Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;349(9):909–910. doi:10.1056/nejmra021261
61. Whalen FX, Bacon DR, Smith HM. Inhaled anesthetics: an historical overview. Best Pract Res Clin Anaesthesiol. 2005;19(3):323–330. doi:10.1016/j.bpa.2005.02.001
62. Ishizawa Y. General anesthetic gases and the global environment. Surv Anesthesiol. 2012;56(4):173. doi:10.1097/sa.0b013e31825c1ed3
63. Salehi B, Mohammadbeigi A, Kamali AR, et al. Impact comparison of ketamine and sodium thiopental on anesthesia during electroconvulsive therapy in major depression patients with drug-resistant; A double-blind randomized clinical trial. Ann Card Anaesth. 2015;18(4):486–490. doi:10.4103/0971-9784.166444
64. Colombo G, Lobina C, Agabio R, et al. Selective breeding of two rat lines differing in sensitivity to GHB and baclofen. Brain Res. 2001;902(1):127–130. doi:10.1016/S0006-8993(01)02389-7
65. Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–1560. doi:10.1001/jama.2012.304
66. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. doi:10.1124/pr.117.015198
67. Ebert TJ, Muzi M, Berens R, et al. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76(5):725–733. doi:10.1097/00000542-199205000-00010
68. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327–335. doi:10.1176/appi.ajp.2017.17060647
69. Kumar D, Rub MA. Effect of anionic surfactant and temperature on micellization behavior of promethazine hydrochloride drug in absence and presence of urea. J Mol Liq. 2017;238:389–396. doi:10.1016/j.molliq.2017.05.027
70. van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium the REDUCE randomized clinical trial. JAMA. 2018;319(7):680–690. doi:10.1001/jama.2018.0160
71. Borgeat A, Fuchs T, Wilder-Smith O, Tassonyi E. The effect of nalbuphine and droperidol on spontaneous movements during induction of anesthesia with propofol in children. J Clin Anesth. 1993;5(1):12–15. doi:10.1016/0952-8180(93)90081-O
72. Wang Y, Ma L, Wang X, Qin L. Differential modulation of the auditory steady state response and inhibitory gating by chloral hydrate anesthesia. Sci Rep. 2018;8(1):3683. doi:10.1038/s41598-018-21920-x
73. Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery. Anesthesiology. 2016;124(1):69–79. doi:10.1097/01.sa.0000508187.35407.3f
74. Landoni G, Lomivorotov V, Nigro Neto C, et al. Volatile anesthetics versus total intravenous anesthesia for cardiac surgery. N Engl J Med. 2019;380(13):1214–1225. doi:10.1056/nejmoa1816476
75. Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9(Suppl 1):S3. doi:10.1186/1471-2377-9-S1-S3
76. Jiron JM, Calle JLM, Castillo EJ, et al. Comparison of isoflurane, ketamine-dexmedetomidine, and ketamine-xylazine for general anesthesia during oral procedures in rice rats (Oryzomys palustris). J Am Assoc Lab Anim Sci. 2019;58(1):40–49. doi:10.30802/AALAS-JAALAS-18-000032
77. Dutta S, Ebling WF, Matsumoto Y, et al. Steady-state propofol brain: plasma and brain: blood partition coefficients and the effect-site equilibration paradox. Br J Anaesth. 1998;81(3):422–424. doi:10.1093/bja/81.3.422
78. Mayer M, Doenicke A, Nebauer AE, Hepting L. Induction of anaesthesia with etomidate in lipid emulsion and propofol: haemodynamics and patient comfort. Anaesthesist. 1996;45:1082–1084. doi:10.1007/s001010050343
79. 360 Research Resports. Global general anesthesia drugs market insights and forecast to 2028; 2022. Available from: https://www.360researchreports.com/global-general-anesthesia-drugs-market-19925302.
80. Potoćnik I, Hostnik A, Božič JM. Do inhalational anesthetic agents still hold their place in modern anesthesia practice? Signa Vitae. 2019;15(2):14–17. doi:10.22514/SV152.092019.1
81. Liu H, Zhou X, Wang Y, et al. Mixed micelle as nanocarrier for etomidate: development, in vitro characterizations, and in vivo study on toxicity and anesthetic effects. J Drug Deliv Sci Technol. 2019;49:123–131. doi:10.1016/j.jddst.2018.10.038
82. Assani NAH, Azodi-Deilami S, Abdouss M, et al. Synthesis and evaluation of hydroponically alginate nanoparticles as novel carrier for intravenous delivery of propofol. J Mater Sci Mater Med. 2015;26(3):145. doi:10.1007/s10856-015-5452-0
83. Airan RD, Meyer RA, Ellens NPK, et al. Noninvasive targeted transcranial neuromodulation via focused ultrasound gated drug release from nanoemulsions. Nano Lett. 2017;17(2):652–659. doi:10.1021/acs.nanolett.6b03517
84. Gao W, Liu Y, Jing G, et al. Rapid and efficient crossing blood-brain barrier: hydrophobic drug delivery system based on propionylated amylose helix nanoclusters. Biomaterials. 2017;113:133–144. doi:10.1016/j.biomaterials.2016.10.045
85. Liu J, Peng F, Kang Y, et al. High-loading self-assembling peptide nanoparticles as a lipid-free carrier for hydrophobic general anesthetics. Int J Nanomedicine. 2021;16:5317–5331. doi:10.2147/IJN.S315310
86. Han FY, Liu Y, Kumar V, et al. Sustained-release ketamine-loaded nanoparticles fabricated by sequential nanoprecipitation. Int J Pharm. 2020;581:119291. doi:10.1016/j.ijpharm.2020.119291
87. Jee JP, Parlato MC, Perkins MG, et al. Exceptionally stable fluorous emulsions for the intravenous delivery of volatile general anesthetics. Anesthesiology. 2012;116(3):580–585. doi:10.1097/ALN.0b013e3182475d4d
88. Wu A, Wang Y, Min S, et al. Etomidate-loaded micelles for short-acting general anesthesia: preparation, characterizations, and in vivo studies. J Drug Deliv Sci Technol. 2018;46:156–161. doi:10.1016/j.jddst.2018.05.013
89. Geng D, Li Y, Wang C, et al. Optimization, and in vitro and in vivo evaluation of etomidate intravenous lipid emulsion. Drug Deliv. 2021;28(1):873–883. doi:10.1080/10717544.2021.1917729
90. Nyman Y, von Hofsten K, Palm C, et al. Etomidate-®Lipuro is associated with considerably less injection pain in children compared with propofol with added lidocaine. Br J Anaesth. 2006;97(4):536–539. doi:10.1093/bja/ael187
91. Macario A, Weinger M, Truong P, Lee M. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg. 1999;88(5):1085–1091. doi:10.1097/00000539-199905000-00023
92. Cicero M, Graneto J. Etomidate for procedural sedation in the elderly: a retrospective comparison between age groups. Am J Emerg Med. 2011;29(9):1111–1116. doi:10.1016/j.ajem.2010.08.004
93. Keleş GT, Kurutepe S, Tok D, et al. Comparison of antimicrobial effects of dexmedetomidine and etomidate-lipuro with those of propofol and midazolam. Eur J Anaesthesiol. 2006;23(12):1037–1040. doi:10.1017/S0265021506000949
94. Shi Y, Jiang Y, Cao J, et al. Boosting RNAi therapy for orthotopic glioblastoma with nontoxic brain-targeting chimaeric polymersomes. J Control Release. 2018;292:163–171. doi:10.1016/j.jconrel.2018.10.034
95. Wei Y, Sun Y, Wei J, et al. Selective transferrin coating as a facile strategy to fabricate BBB-permeable and targeted vesicles for potent RNAi therapy of brain metastatic breast cancer in vivo. J Control Release. 2021;337:521–529. doi:10.1016/j.jconrel.2021.07.048
96. Wei J, Wu D, Shao Y, et al. ApoE-mediated systemic nanodelivery of granzyme B and CpG for enhanced glioma immunotherapy. J Control Release. 2022;347:68–77. doi:10.1016/j.jconrel.2022.04.048
97. Hemmings HC, Akabas MH, Goldstein PA, et al. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26(10):503–510. doi:10.1016/j.tips.2005.08.006
98. Qiao R, Jia Q, Hüwel S, et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano. 2012;6(4):3304–3310. doi:10.1021/nn300240p
99. Zhang S, Zhang S, Luo S, et al. Ultrasound-assisted brain delivery of nanomedicines for brain tumor therapy: advance and prospect. J Nanobiotechnology. 2022;20(1):287. doi:10.1186/s12951-022-01464-z
100. Mitusova K, Peltek OO, Karpov TE, Muslimov AR, Zyuzin MV, Timin AS. Overcoming the blood-brain barrier for the therapy of malignant brain tumor: current status and prospects of drug delivery approaches. J Nanobiotechnology. 2022;20(1):412. doi:10.1186/s12951-022-01610-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
