Back to Journals » Journal of Inflammation Research » Volume 15
Elevated TNF-α Induces Thrombophagocytosis by Mononuclear Cells in ex vivo Whole-Blood Co-Culture with Dengue Virus
Authors Satria RD, Jhan MK, Chen CL, Tseng PC, Wang YT, Lin CF
Received 1 January 2022
Accepted for publication 24 February 2022
Published 5 March 2022 Volume 2022:15 Pages 1717—1728
DOI https://doi.org/10.2147/JIR.S356742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Rahmat Dani Satria,1– 4,* Ming-Kai Jhan,4,5,* Chia-Ling Chen,6 Po-Chun Tseng,4,7 Yung-Ting Wang,4 Chiou-Feng Lin4,5,7
1International Ph.D. Program in Medicine, College of Medicine, Taipei Medical University, Taipei, 110, Taiwan; 2Department of Clinical Pathology and Laboratory Medicine, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia; 3Clinical Laboratory Installation, Dr. Sardjito Central General Hospital, Yogyakarta, 55281, Indonesia; 4Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, 110, Taiwan; 5Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, 110, Taiwan; 6School of Respiratory Therapy, College of Medicine, Taipei Medical University, Taipei, 110, Taiwan; 7Core Laboratory of Immune Monitoring, Office of Research & Development, Taipei Medical University, Taipei, 110, Taiwan
*These authors contributed equally to this work
Correspondence: Chiou-Feng Lin, Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, 110, Taiwan, Tel +886 2 27361661 ext. 7156, Email [email protected]
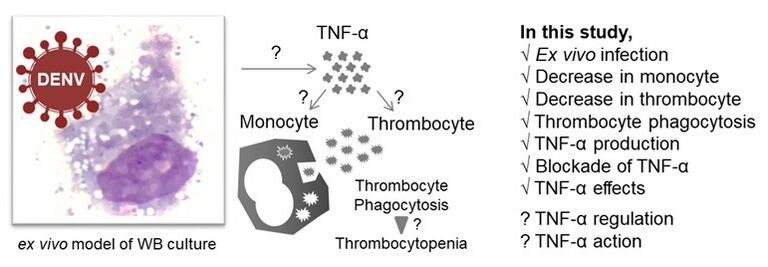
Background: Infection with dengue virus (DENV) causes hematological complications in dengue diseases characterized by thrombocytopenia accompanied by macrophage activation syndrome and hemophagocytosis in fatal patients.
Methods: In this study, we investigate the undefined mechanisms underlying the progression of thrombocytopenia caused by thrombophagocytosis based on an ex vivo whole-blood co-culture model of DENV infection for mimicking the acute febrile phase of infection.
Results: In this model, complete blood count test showed a decrease in monocytes (p < 0.01), but not neutrophils nor other white blood cells, accompanied by a low thrombocyte count (p < 0.01) in DENV infection with a positive correlation (r = 0.636, p < 0.05). Furthermore, DENV exposure caused significant thrombophagocytosis in mononuclear cells (p < 0.05). Abnormal production of tumor necrosis factor (TNF)-α was highly associated with induction of thrombophagocytosis (r = 0.758, p < 0.01), decreased monocytes (r = − 0.758, p < 0.01), and decreased thrombocyte (r = − 0.728, p < 0.01). Neutralizing TNF-α considerably (p < 0.05) reversed such DENV-induced effects and was further validated by immunostaining-based flow cytometry analysis on mononuclear CD14 positive monocytes. Exogenous administration of TNF-α effectively caused thrombophagocytosis accompanied by decreased monocytes and thrombocytes, probably causing monocyte activation.
Conclusion: These results demonstrate the potential pathogenesis of thrombocytopenia caused by TNF-α-induced thrombophagocytosis in monocytes during DENV infection.
Keywords: dengue virus, monocyte, thrombophagocytosis, ex vivo, TNF-α
Graphical Abstract:
Introduction
Thrombocytopenia and leukopenia are the most common hematological changes in patients with dengue virus (DENV) infection.1 Thrombocytopenia has always been one of the criteria used by WHO guidelines as a potential indicator of clinical severity in DENV infection.2 The mechanisms involved in thrombocytopenia and bleeding during DENV infection are not fully understood. Several hypotheses have been proposed to explain the mechanisms involved. Some theories suggest that DENV can directly or indirectly affect megakaryocytes in the bone marrow by inhibiting their function and differentiation.3–5 Moreover, DENV infection induces thrombocyte destruction due to direct interaction with virus itself, FcγRII-mediated interaction with immuno-complexes, increased apoptosis, lysis by the complement system, and binding of viral-induced anti-thrombocyte antibodies in the peripheral circulation.6,7
Thrombocytes have an essential role in inflammation. Thrombocytes store and release many biologically active substances, including growth factors and cytokines.8,9 Thrombocytes also have a role in their interaction with leukocytes. Thrombocytes can form thrombocyte-leukocyte aggregates that immobilize pathogens and prevent their spread. The presence of surface receptors such as cytokine receptors and pattern recognition receptors on the thrombocyte surface also influences their initiation and activity in immunological responses.10–12 Monocytes are the central phagocytic cells. In acute DENV infection, monocytes can engulf the virus to control blood viremia. However, these phagocytes also have a role in the aggravation of the disease. Several studies have reported that monocytes are target cells for the DENV.13–15 Monocytes promote dengue pathogenesis by being the main reservoir of viral replication.15,16 During acute DENV infection, these cells are believed to induce tissue damage. Monocytes produce a variety of different pro-inflammatory cytokines in response to infection, thereby mediating endothelial damage. These cytokines disrupt the integrity of the endothelial cell layer, possibly causing vascular leakage, which is a sign of severe hemorrhagic disease.9,17
Excessive activation of monocytes in response to inflammatory cytokines can be hazardous for certain people. Macrophages activation syndrome (MAS) is an example of a disease caused by the activation of monocytes that are hyperresponsive to cytokines.18,19 These monocytes that turn into macrophages can engulf everything, including erythrocytes, leukocytes, or thrombocytes.20 It has been proposed that the increased production of cytokines, interferon-γ, and tumor necrosis factor-α (TNF-α) plays a role in the pathogenesis of MAS. Several reports have shown that TNF-α acts as a regulator of MAS events.21–23 Therapy with TNF-α is said to cure MAS,24,25 indicating the importance of this cytokine in the occurrence of MAS. Recently, it has been suggested that MAS is involved in exacerbation of coronavirus disease 2019 (COVID-19) in lung due to cytokine storm.26 In this study, we identified the phenomenon of thrombophagocytosis with high levels of TNF-α and its association with thrombocytopenia in an experimental model of DENV infected whole blood ex vivo.
Methods
Reagents and Antibodies
The following were the antibodies (Abs) and reagents used in the experiment: recombinant human TNF-α (hTNF-α, PeproTech, USA); crystal violet (Merck, Sigma-Aldrich, USA); Anti-TNF-α antibody (Abcam, Cambridge); CD14 monoclonal antibody, APC (Catalog# 17–0149-42); Alexa Fluor 488- anti-human CD16 antibody (Catalog# 302019); and CD 163 monoclonal antibody, APC (Catalog# 17–1639-42).
Cell and Virus Culture
Dulbecco’s Modified Eagle Medium (DMEM, ThermoFisher Scientific) with 10% fetal bovine serum (FBS, Merck, Sigma-Aldrich, USA) was used to cultivate BHK21 [C-13] cells (ATCC CCL-10) and Aedes albopictus clone C6/36 (ATCC CRL-1660) cells. To retain dengue virus (DENV) serotype 2 (PL046), the Aedes albopictus clone cells were exposed to DENV2. DENV was incubated at 28°C with 5% CO2 for five days at a multiplicity of infection (MOI) of 0.01 on C6/36 cell monolayers. Concentrated and filtered viral supernatant was kept in the freezer at −80°C before use, using Millipore Amicon Ultra centrifugal filters (Billerica, MA, USA).
Whole Blood Sample (Venipuncture)
The research on humans was conducted in accordance with the Declaration of Helsinki following the Taipei Medical University-Joint Institutional Review Board (TMU-JIRB N201801042) protocol. All subjects (N=6) given informed consent, as required by the TMU-JIRB. They were all adults >18 years, healthy, non-medicated volunteers with no current infectious illness. They were in physically healthy and had a confirmed COVID-19 free. Using sodium heparin vacutainer collection tubes, all samples were taken simultaneously (5 mL; Becton Drive Vacutainer, Franklin Lakes, USA). In order to mix the blood with the additives, all blood collection tubes were gently flipped five times immediately after blood draw, then each blood sample is separated by 2, one for mock group and one for treatment group.
Ex vivo DENV Inoculation
An ex vivo model of DENV infection in the whole blood (WB) was conducted accordingly.27 In brief, 100 microliters RPMI medium containing DENV (MOI = 1) was added to 100 microliters of heparin-contained WB seeded in a 24-well plate. In the DENV+Anti-TNF-α group, 50 g/mL neutralizing anti-TNF-α antibody was added into solution. This was done using a hematology analyzer (XN-450, Sysmex Corporation, Kobe, Japan) to determine the total number of white blood cells. The process took 24 hours to incubate the WB at 37°C with the DENV plaque-forming units that were calculated. In addition, the culture supernatants were collected in order to measure cytokine production in the cell culture supernatants.
Exogenous Treatment of TNF-α
One hundred ng/mL human TNF-α was added into WB ex vivo. The process took 24 hours to incubate the WB at 37°C. After 24 hours of treatment, we checked the CBC data and blood smear staining.
Blood Smear Staining
Following DENV ex vivo infection for 24 h, a drop of WB approximately 3 mm in diameter on each sample was placed at one end of the slide and then spread across the width of the slide. After air-drying, all smears were stained with the Wright-Giemsa stain (Tonyar Biotech, Taipei, Taiwan). Then, cells were photographed and counted (Olympus CX23; Olympus, Tokyo, Japan).
Complete Blood Counts (CBC)
Counting the changes in cell expression in WB used gently mixed WB culture 24 h post-inoculation. According to the manufacturer’s recommendation, the CBC examination was performed using a hematology analyzer XN-450 (Sysmex). The parameters tested included the proportion of different white blood cell (WBC) components, including lymphocytes, monocytes, neutrophils, eosinophils, and basophils, and thrombocyte count.
Leucocyte Antigen Profile
According to the manufacturer’s protocol, an immune profile analysis was performed using no-wash no-lyse detection (Thermo Fisher Scientific). In brief, the diluted WB samples were immunoblocked (Mouse BD Fc Block™) at 4°C. After 30 minutes, the cells were immunohybridized with specific antibodies against cell surface immune markers, including CD14, CD16, and CD163, at 4°C for 1 hour. The samples were analyzed using flow cytometry (Attune NxT, Thermo Fisher Scientific).
ELISA
The human TNF-α concentration in the culture supernatant was quantified under manufacturer directions using DuoSet ELISA kits (R&D Systems). In this process, the microwells are covered with TNF-α capture antibodies. Samples are loaded, and the immobilized antibody bonds every analyte (TNF-α) present. Unbound materials are removed and then blocked with 1% bovine serum albumin in PBS. After loading with a human TNF-α detection antibody, streptavidin-horseradish peroxidase (HRP) is added to bind to the detection antibody. Unbound streptavidin-HRP is washed away, and then tetramethylbenzidine (TMB) substrate solution is added to the wells, and a blue color develops in proportion to the amount of analyte present in the sample. The color development is stopped with stop solution then the absorbance of the color was determined using a microplate reader at 450 nm.
Statistical Analysis
Groups were compared using the student’s paired t-tests, and the results expressed as the mean ± standard deviation (SD) values. Statistical significance was determined by setting the threshold at the p-value of 0.05. Analysis was done with Prism 8 from GraphPad (GraphPad Software).
Results
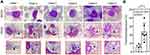
Decrease in Monocytes is Associated with a Decrease in Thrombocytes in DENV-Infected Blood ex vivo
To determine whether there was a change in complete blood count (CBC), we examined DENV-WB coculture after 24 and referred to our current established ex vivo model.27 We found a decrease in the number of monocytes in the DENV group (DENV, 1.14 ± 0.55%; MOCK, 4.94 ± 1.80%; p = 0.002) (Figure 1A) and a decrease in the number of thrombocytes in the DENV group (DENV, 28.61 ± 8.54 × 103; MOCK, 41.75 ± 9.12 × 103; p = 0.003) (Figure 1B). It revealed a reduction in thrombocytes closely associated with monocyte numbers (r = 0.636, p = 0.026) (Figure 1C). The results indicate a high association with decreased monocytes and decreased thrombocytes in the ex vivo model of DENV-WB coculture.
DENV Inoculation Causes Thrombophagocytosis in WB ex vivo
After DENV inoculation, we performed Giemsa staining to prove that thrombocyte reduction may have resulted from phagocytoses. Following DENV (MOI = 1) coculture in 100 μL of WB ex vivo for 24 h, Wright-Giemsa staining showed a thrombophagocytosis phenomenon (Figure 2A). In the cytoplasm of the mononuclear leucocytes, we detected thrombocyte engulfment within the detected cells (arrows). There was an increase in the percentage of thrombophagocytosed cells after inoculation with DENV at 24 h (DENV, 23.62 ± 10.03%; MOCK, 5.6 ± 3.25%; p = 0.003) (Figure 2B). These results indicate that the decline in thrombocyte counts is probably caused by thrombophagocytosis in mononuclear cells.
TNF-α is the Principal Cause of Thrombophagocytosis Followed by a Decreasing Number of Monocytes and Thrombocytes
Our results have shown that the drop in change in monocytes is consistent with the increase in thrombophagocytosis in ex vivo DENV-WB coculture. It is believed that the pro-inflammatory cytokines induced by DENV may be involved in triggering thrombophagocytosis. Regarding this hypothesis, we previously identified that DENV-activated TNF-α production facilitates vacuolization in monocytes.27 TNF-α production was evaluated by ELISA. In a 24 h post-incubation, we identified aberrant TNF-α production (DENV, 238.19 ± 186.76 pg/mL, p = 0.026) in the DENV-inoculation groups (Figure 3A). A strong association was identified between TNF-α production and the amount of thrombophagocytosis (r = 0.758; p = 0.004) (Figure 3B). We also found a significant association between monocytes and TNF-α (r = – 0.758; p = 0.004) (Figure 3C) and thrombocytes and TNF-α (r = –0.728; p = 0.007) (Figure 3D). These results indicate that the occurrence of thrombophagocytosis is highly correlated with DENV-induced TNF-α.
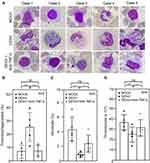
Anti-TNF-α Has a Neutralizing Effect on the Thrombophagocytosis Process
To further substantiate the role of TNF-α as a regulator of the thrombophagocytosis phenomenon, we isolated WB from healthy donors under TNF-α blocking with DENV inoculation, as described previously. Remarkably, blocking TNF-α before exposure to DENV infection abolished the thrombophagocytosis phenomenon (Figure 4A). After quantification, Wright-Giemsa staining showed a significant decrease in the number of thrombophagocytosed cells after cotreatment with anti-TNF-α (DENV, 25.56 ± 10.87%; DENV+anti-TNF-α, 7.88 ± 4.35%; MOCK, 6.82 ± 5.03%) (Figure 4B). Moreover, the decreased thrombophagocytosed cell numbers was followed by the rise in the number of monocytes (DENV, 0.90 ± 0.30%; DENV+ anti-TNF-α, 2.34 ± 1.17%; MOCK, 4.37 ± 1.67%) (Figure 4C) and thrombocytes (DENV, 25.08 ± 7.49 × 103; DENV+anti-TNF-α; 31.68 ± 8.07 × 103, MOCK, 36.84 ± 7.22×103) (Figure 4D). These data indicate that the induction of thrombophagocytosis and decrease in monocytes and thrombocytes are regulated by TNF-α in the ex vivo model of DENV-WB coculture.
Immune Profiling Shows CD14 Positive Cells Changes in the WB Coculture with DENV ex vivo
Since the CBCs demonstrated an alteration in the monocyte population, the immunological profiling approach was used to analyze WB cells to verify our CBC data. The CD14 positive cell subpopulations were assessed with a multi-parameter flow cytometric analysis (Figure 5A). In this analysis, DENV infection reduced the frequency of mononuclear CD14 positive monocytes (CD14+), whereas anti-TNF-α rescued the number of these cells (DENV, 4.26 ± 1.65%; DENV+ anti-TNF-α, 10.7 ± 0.57%; MOCK, 11.88 ± 1.95%) (Figure 5B). Thus, the findings suggest that TNF-α causes an effect in reducing mononuclear CD14 positive monocytes in the ex vivo model of DENV-WB coculture.
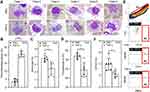
Exogenous Treatment of TNF-α Determines Thrombophagocytosis in WB Culture
To ensure that thrombophagocytosis was due to TNF-α excreted by leucocytes rather than the presence of the virus itself, we administered human TNF-α (hTNF-α, 100 ng/mL) to WB ex vivo. After 24 h of treatment, Wright-Giemsa staining showed a thrombophagocytosis phenomenon (Figure 6A). After quantification, there was an increase in thrombophagocytosis after co-treatment with hTNF-α for 24 h (TNF-α, 20.58 ± 1.83%; PBS, 3.55 ± 1.48%; p < 0.0001) (Figure 6B) followed by decreased monocytes (TNF-α, 2.45 ± 0.84%; PBS, 3.75 ± 0.38%; p = 0.01) (Figure 6C) and thrombocytes (TNF-α, 44.33 ± 3.88 × 103; PBS, 52 ± 1.41 × 103; p = 0.01) (Figure 6D). To investigate the activation of monocytes ex vivo, decreased expression of CD16328 was assessed with a multi-parameter flow cytometric analysis (Figure 6E), TNF-α treatment induced the frequency of CD163-downregulating CD14 positive monocytes (TNF-α, 2.25 ± 0.55%; PBS, 3.967 ± 1.04%; p = 0.035) (Figure 6F). These results demonstrated that the TNF-α could cause thrombophagocytosis following the induction of monocyte activation ex vivo in WB.
Discussion
This study uses a blood culture model to explore the possibility that DENV infection may cause hematopathological features ex vivo. As a result, it was found that the DENV may cause the activation of monocytes and thrombophagocytosis through the induction of increased levels of the cytokine TNF-α. This discovery means that the virus may activate monocytes through inflammatory responses and then undergo thrombophagocytosis, related to the clinical findings of thrombocytopenia and hemophagocytic syndrome. Since previous studies have only demonstrated the occurrence of thrombocytopenia caused by DENV infection in patients and mice in vivo,29–31 this research is the first to develop these two ex vivo experimental models for exploring the occurrence of hematopathological features. Thus, it may present infeasible strategies for reducing thrombocytopenia and hemophagocytic syndrome.
Dengue diseases often have abnormal manifestations of blood cells in hematopathology, including the proportion of lymphocytes, leukopenia, and thrombocytopenia.32–38 Hemophagocytic syndrome can also be found in patients with severe dengue hemorrhagic bleeding.39–41 Therefore, transient thrombocytopenia has always been a critical clinical blood parameter to determine dengue disorders. Also, severe and prolonged thrombocytopenia is a laboratory diagnostic criterion of severe dengue disease. In the ex vivo model of this study, the down-regulation of monocytes and thrombocytes can be observed under the co-culture conditions of blood with DENV. The down-regulation of monocytes should be caused by cell activation after infection and may adhere to the culture plate. At the same time, it is accompanied by thrombophagocytosis, which leads to the down-regulation of thrombocytes in the blood. There is no official report on the down-regulation of mononuclear cells in the hematological examination of dengue patients, and it is speculated that it is related to its potential occurrence in acute leukopenia. This part of the disease pathogenesis requires further immune profiling data from the dengue patients’ hematological examination to verify the findings of this study.
In the past, our Laboratory confirmed the occurrence of down-regulation of the number of monocytes in the blood27 by using the ex vivo virus-whole blood co-culture model. This experiment found for the first time that the vacuolization of mononuclear cells after activation was related to the abnormal increase of cytokine TNF-α caused by DENV infection. This study further found that the activation of monocytes also promotes the occurrence of thrombophagocytosis. Thus, cell vacuolization and thrombophagocytosis should be the consequence after monocyte activation, but the mechanism of this phenomenon is still unclear. Thrombophagocytosis and hemophagocytic syndrome are related to infections associated with severe hematopathological disorders.20,42–44 The causes include the direct action of pathogens, the response of cytokines, and the complex results of the host’s autoimmunity.45–47 Since this cytopathology revealed that it was found in blood tissue specimens of severe cases or even death cases, there is currently no appropriate research model to verify the theory, which is quite challenging for scientific research.
Cytokine storm is one of the immunopathogenesis of severe dengue disease.48–51 TNF-α is a typical pro-inflammatory cytokine and has a significant pathological relationship with the hemorrhagic group in severe dengue disease.52–56 Therefore, the immunotherapeutic strategy to target its aberrant expression is considered one of the feasible options for treating moderate diseases. Remarkably, this study also confirmed that the overproduction of TNF-α is associated with down-regulation of monocyte number, thrombocytopenia, and thrombophagocytosis. In clinical studies, it has been reported that some cytokines in dengue patients were increased but only TNF-α levels were statistically significant, and negatively correlated with thrombocyte counts. This suggests that TNF really does have a role in DENV pathogenesis by contributing to the thrombocytopenia associated with this disease.57 Although the possible regulatory mechanism is still unclear, TNF-α can directly act on the immune activation of monocytes and thrombocytes that continually express TNF-α receptors, which partially explains their possible regulation.58–63 The antibody neutralization test and exogenous TNF-α stimulation mode in this study verified this hypothesis. Under the stimulation of TNF-α, this study reproduced the results of monocyte activation as demonstrated by the decreased expression of CD163.28 Furthermore, the blood test in the stimulation mode verified that TNF-α could cause the down-regulation of monocytes and thrombocytes, accompanied by the occurrence of thrombophagocytosis. The molecular regulation that activates monocytes and thrombocytes by aberrant expression of TNF-α and its receptors and then causes thrombophagocytosis is worth continuing to explore in the future. The related factors, including viral proteins and other potential endogenous pyrogens in patients, and host cells involved in thrombophagocytosis may be used to treat the incidence of hematological diseases.
In summary, the ex vivo model of DENV and blood co-culture provides a study on the mechanisms that may lead to hematopathological features when the acute phase of viremia occurs, including the manifestation of clinically relevant cytokine storms in thrombocytopenia, and hemophagocytic syndrome. DENV infection is very likely to activate monocytes and thrombocytes through the induction of the cytokine TNF-α and initiate the incidence of monocyte-mediated thrombophagocytosis.
Funding
This work was supported by the Ministry of Science and Technology (MOST109-2320-B-038-050, 109-2327-B-006-010, 110-2327-B-006 -005, and 110-2320-B-038-064-MY3), Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20(2):97–113. doi:10.1016/j.cytogfr.2009.02.004
2. World Health Organization. Comprehensive guideline for prevention and control of dengue and dengue haemorrhagic fever; 2011.
3. Vogt MB, Lahon A, Arya RP, Spencer Clinton JL, Rico-Hesse R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl Trop Dis. 2019;13(11):e0007837. doi:10.1371/journal.pntd.0007837
4. Basu A, Jain P, Gangodkar SV, Shetty S, Ghosh K. Dengue 2 virus inhibits in vitro megakaryocytic colony formation and induces apoptosis in thrombopoietin-inducible megakaryocytic differentiation from cord blood CD34+ cells. FEMS Immunol Med Microbiol. 2008;53:46–51. doi:10.1111/j.1574-695X.2008.00399.x
5. Sridharan A, Chen Q, Tang KF, Ooi EE, Hibberd ML, Chen J. Inhibition of megakaryocyte development in the bone marrow underlies dengue virus-induced thrombocytopenia in humanized mice. J Virol. 2013;87(21):11648–11658. doi:10.1128/JVI.01156-13
6. Huang KJ, Lin YS, Liu HS, Yeh TM, Liu CC, Lei HY. Generation of anti-platelet autoantibody during dengue virus infection. Am J Infect Dis. 2008;4(1):50–59. doi:10.3844/ajidsp.2008.50.59
7. Assinger A. Platelets and infection – an emerging role of platelets in viral infection. Front Immunol. 2014;5. doi:10.3389/fimmu.2014.00649
8. Mannaioni P, Di Bello M, Masini E. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res. 1997;46(1):4–18. doi:10.1007/PL00000158
9. Hottz ED, Medeiros-de-moraes IM, Vieira-de-abreu A, et al. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J Immunol. 2014;193(4):1864–1872. doi:10.4049/jimmunol.1400091
10. Gawaz M, Fateh‐Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest. 1995;25(11):843–851. doi:10.1111/j.1365-2362.1995.tb01694.x
11. Dib PRB, Quirino‐Teixeira AC, Merij LB, et al. Innate immune receptors in platelets and platelet‐leukocyte interactions. J Leukoc Biol. 2020;108(4):1157–1182. doi:10.1002/JLB.4MR0620-701R
12. Cognasse F, Nguyen KA, Damien P, et al. The inflammatory role of platelets via their TLRs and Siglec receptors. Front Immunol. 2015;6:83. doi:10.3389/fimmu.2015.00083
13. Kou Z, Quinn M, Chen H, et al. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J Med Virol. 2008;80(1):134–146. doi:10.1002/jmv.21051
14. Durbin AP, Vargas MJ, Wanionek K, et al. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology. 2008;376(2):429–435. doi:10.1016/j.virol.2008.03.028
15. Alhoot MA, Wang SM, Sekaran SD. Inhibition of dengue virus entry and multiplication into monocytes using RNA interference. PLoS Negl Trop Dis. 2011;5(11):e1410. doi:10.1371/journal.pntd.0001410
16. Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci. 2018;19(9):2821. doi:10.3390/ijms19092821
17. Ong SP, Lee LM, Leong YFI, Ng ML, Chu JJH. Dengue virus infection mediates HMGB1 release from monocytes involving PCAF acetylase complex and induces vascular leakage in endothelial cells. PLoS One. 2012;7(7):e41932. doi:10.1371/journal.pone.0041932
18. Remy S, Gossez M, Belot A, et al. Massive increase in monocyte HLA-DR expression can be used to discriminate between septic shock and hemophagocytic lymphohistiocytosis-induced shock. Critl Care. 2018;22(1):1–2. doi:10.1186/s13054-018-2146-2
19. Pascarella A, Bracaglia C, Caiello I, et al. Monocytes from patients with macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis are hyperresponsive to interferon gamma. Front Immunol. 2021;12:801. doi:10.3389/fimmu.2021.663329
20. Ma T, Hudgins JP, Vergara‐Lluri M, Pham H, Brynes R, Shulman IA. Platelet transfusion refractoriness and thrombophagocytosis in an HIV/AIDS patient with hemophagocytic lymphohistiocytosis. Transfusion. 2020;60:2176–2177. doi:10.1111/trf.15955
21. Akashi K, Hayashi S, Gondo H, et al. Involvement of interferon‐γ and macrophage colony‐stimulating factor in pathogenesis of haemophagocytic lymphohistiocytosis in adults. Br J Haematol. 1994;87(2):243–250. doi:10.1111/j.1365-2141.1994.tb04905.x
22. Shimizu M, Inoue N, Mizuta M, Nakagishi Y, Yachie A. Characteristic elevation of soluble TNF receptor II: i ratio in macrophage activation syndrome with systemic juvenile idiopathic arthritis. Clin Exp Immunol. 2018;191(3):349–355. doi:10.1111/cei.13026
23. Billiau AD, Roskams T, Van Damme-lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6-and TNF-α-producing macrophages. Blood. 2005;105(4):1648–1651. doi:10.1182/blood-2004-08-2997
24. Henzan T, Nagafuji K, Tsukamoto H, et al. Success with infliximab in treating refractory hemophagocytic lymphohistiocytosis. Am J Hematol. 2006;81(1):59–61. doi:10.1002/ajh.20462
25. Ideguchi H, Ohno S, Takase K, et al. Successful treatment of refractory lupus-associated haemophagocytic lymphohistiocytosis with infliximab. Rheumatology. 2007;46(10):1621–1622. doi:10.1093/rheumatology/kem205
26. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi:10.1016/j.autrev.2020.102537
27. Satria RD, Huang T-W, Jhan M-K, et al. Increased TNF-α initiates cytoplasmic vacuolization in whole blood coculture with dengue virus. J Immunol Res. 2021;2021. doi:10.1155/2021/6654617
28. Nunes PR, Romão-Veiga M, Peraçoli JC, et al. Downregulation of CD163 in monocytes and its soluble form in the plasma is associated with a pro-inflammatory profile in pregnant women with preeclampsia. Immunol Res. 2019;67(2–3):194–201. doi:10.1007/s12026-019-09078-8
29. Masri MFB, Mantri CK, Rathore AP, John ALS. Peripheral serotonin causes dengue virus–induced thrombocytopenia through 5HT2 receptors. Blood. 2019;133:2325–2337.
30. Sun DS, King CC, Huang HS, et al. Antiplatelet autoantibodies elicited by dengue virus non‐structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost. 2007;5(11):2291–2299. doi:10.1111/j.1538-7836.2007.02754.x
31. Chao C-H, Wu W-C, Lai Y-C, et al. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019;15(4):e1007625. doi:10.1371/journal.ppat.1007625
32. Clarice CSH, Abeysuriya V, de Mel S, et al. Atypical lymphocyte count correlates with the severity of dengue infection. PLoS One. 2019;14(5):e0215061. doi:10.1371/journal.pone.0215061
33. Fadilah S, Sahrir S, Raymond A, Cheong S, Aziz JA, Sivagengei K. Quantitation of T lymphocyte subsets helps to distinguish dengue hemorrhagic fever from classic dengue fever during the acute febrile stage. Southeast Asian J Trop Med Public Health. 1999;30(4):710–717.
34. Joshi AA, Muneer F. Dynamics of differential count in dengue; 2018.
35. Ho T-S, Wang S-M, Lin Y-S, Liu -C-C. Clinical and laboratory predictive markers for acute dengue infection. J Biomed Sci. 2013;20(1):1–8. doi:10.1186/1423-0127-20-75
36. Achalkar GV. Dengue: a clinico-pathological study of 50 cases. J Evol Med Dent Sci. 2013;2(48):9380–9386. doi:10.14260/jemds/1626
37. Patel K, Patel D, Das V. Hematological parameters and its utility in dengue fever: a prospective study. Int J Sci Res. 2016;5:1077–1079.
38. Azeredo ELD, Monteiro RQ, De-oliveira Pinto LM. Thrombocytopenia in dengue: interrelationship between virus and the imbalance between coagulation and fibrinolysis and inflammatory mediators. Mediators Inflamm. 2015;2015:1–16. doi:10.1155/2015/313842
39. Giang HTN, Banno K, Minh LHN, et al. Dengue hemophagocytic syndrome: a systematic review and meta‐analysis on epidemiology, clinical signs, outcomes, and risk factors. Rev Med Virol. 2018;28(6):e2005. doi:10.1002/rmv.2005
40. Srichaikul T, Punyagupta S, Kanchanapoom T, Chanokovat C, Likittanasombat K, Leelasiri A. Hemophagocytic syndrome in Dengue hemorrhagic fever with severe multiorgan complications. J Med Assoc Thai. 2008;91(1):104–109.
41. Lu P-L, Hsiao -H-H, Tsai -J-J, et al. Dengue virus-associated hemophagocytic syndrome and dyserythropoiesis: a case report. Kaohsiung J Med Sci. 2005;21(1):34–39. doi:10.1016/S1607-551X(09)70274-8
42. Ansuini V, Rigante D, Esposito S. Debate around infection-dependent hemophagocytic syndrome in paediatrics. BMC Infect Dis. 2013;13(1):1–8. doi:10.1186/1471-2334-13-15
43. Mroczek E, Weisenburger D, Grierson HL, Markin R, Purtilo D. Fatal infectious mononucleosis and virus-associated hemophagocytic syndrome. Arch Pathol Lab Med. 1987;111:530–535.
44. Janka G, Imashuku S, Elinder G, Schneider M, Henter J-I. Infection-and malignancy-associated hemophagocytic syndromes: secondary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am. 1998;12(2):435–444. doi:10.1016/S0889-8588(05)70521-9
45. Crayne CB, Albeituni S, Nichols KE, Cron RQ. The Immunology of macrophage activation syndrome. Front Immunol. 2019;10:119. doi:10.3389/fimmu.2019.00119
46. Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10(3):387–392. doi:10.1097/PCC.0b013e3181a1ae08
47. Deane S, Selmi C, Teuber SS, Gershwin ME. Macrophage activation syndrome in autoimmune disease. Int Arch Allergy Immunol. 2010;153(2):109–120. doi:10.1159/000312628
48. Srikiatkhachorn A, Mathew A, Rothman AL. Immune-mediated cytokine storm and its role in severe dengue. Semin Immunopathol. 2017;39(5):563–574. doi:10.1007/s00281-017-0625-1
49. Mangione JN, Huy NT, Lan NTP, et al. The association of cytokines with severe dengue in children. Trop Med Health. 2014;42(4):137–144. doi:10.2149/tmh.2014-09
50. Sun Y, Jin C, Zhan F, et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis. 2012;206(7):1085–1094. doi:10.1093/infdis/jis452
51. Patro A, Mohanty S, Prusty BK, et al. Cytokine signature associated with disease severity in dengue. Viruses. 2019;11(1):34. doi:10.3390/v11010034
52. Fernandez‐Mestre M, Gendzekhadze K, Rivas‐Vetencourt P, Layrisse Z. TNF‐α‐308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens. 2004;64(4):469–472. doi:10.1111/j.1399-0039.2004.00304.x
53. Vejbaesya S, Luangtrakool P, Luangtrakool K, et al. TNF and LTA gene, allele, and extended HLA haplotype associations with severe dengue virus infection in ethnic Thais. J Infect Dis. 2009;199:1442–1448. doi:10.1086/597422
54. Alagarasu K, Mulay A, Singh R, Gavade V, Shah P, Cecilia D. Association of HLA-DRB1 and TNF genotypes with dengue hemorrhagic fever. Hum Immunol. 2013;74(5):610–617. doi:10.1016/j.humimm.2013.01.027
55. Wang L, Chen R, Liu J, Kuo H, Kuo H, Yang KD. Implications of dynamic changes among tumor necrosis factor-alpha (TNF-alpha), membrane TNF receptor, and soluble TNF receptor levels in regard to the severity of dengue infection. Am J Trop Med Hyg. 2007;77(2):297. doi:10.4269/ajtmh.2007.77.297
56. Cardier JE, Mariño E, Romano E, et al. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-α in endothelial cell damage in dengue. Cytokine. 2005;30(6):359–365. doi:10.1016/j.cyto.2005.01.021
57. Meena AA, Murugesan A, Sopnajothi S, et al. Increase of plasma TNF-α is associated with decreased levels of blood platelets in clinical dengue infection. Viral Immunol. 2020;33:54–60. doi:10.1089/vim.2019.0100
58. Arthur J, Shen Y, Gardiner E, et al. TNF receptor‐associated factor 4 (TRAF4) is a novel binding partner of glycoprotein Ib and glycoprotein VI in human platelets. J Thromb Haemost. 2011;9(1):163–172. doi:10.1111/j.1538-7836.2010.04091.x
59. Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92(9):1041–1048. doi:10.1161/01.RES.0000070111.98158.6C
60. Piguet PF, Vesin C, Da Kan C. Activation of platelet caspases by TNF and its consequences for kinetics. Cytokine. 2002;18(4):222–230. doi:10.1006/cyto.2002.0889
61. Hart PH, Hunt EK, Bonder CS, Watson CJ, Finlay-Jones JJ. Regulation of surface and soluble TNF receptor expression on human monocytes and synovial fluid macrophages by IL-4 and IL-10. J Immunol. 1996;157(8):3672–3680.
62. Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol. 1998;160(5):2488–2494.
63. Leeuwenberg J, Dentener MA, Buurman WA. Lipopolysaccharide LPS-mediated soluble TNF receptor release and TNF receptor expression by monocytes. Role of CD14, LPS binding protein, and bactericidal/permeability-increasing protein. The. J Immunol. 1994;152:5070–5076.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.