Back to Journals » Journal of Hepatocellular Carcinoma » Volume 7
Elevated Platelet Count is Associated with Poor Survival After Transarterial Chemoembolization Treatment in Patients with Hepatocellular Carcinoma: A Cohort Study
Authors Lu L , Zhang Y, Zheng P , Wu Z, Wang X, Chen Y , Chen X
Received 30 July 2020
Accepted for publication 17 September 2020
Published 15 October 2020 Volume 2020:7 Pages 191—199
DOI https://doi.org/10.2147/JHC.S274349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
linbin Lu,1,* Yan Zhang,1,* Peichan Zheng,2,* Zhixian Wu,3 Xuewen Wang,1 Yaying Chen,1 Xiong Chen1
1Department of Oncology, The 900th Hospital of Joint Logistic Support Force, PLA, Fuzong Clinical College of Fujian Medical University, Fuzhou, Fujian 350025, People’s Republic of China; 2Fujian Center for Safety Evaluation of New Drug, Fujian Medical University, Fuzhou, Fujian 350122, People’s Republic of China; 3Department of Hepatobiliary, The 900th Hospital of Joint Logistic Support Force, PLA, Fuzong Clinical College of Fujian Medical University, Fuzhou, Fujian 350025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiong Chen
Department of Oncology, The 900th Hospital of Joint Logistic Support Force, PLA, Fuzong Clinical College of Fujian Medical University, 156 Xierhuan Northern Road, Fuzhou, Fujian 350025, People’s Republic of China
Tel +86 1-216-612-2438
Fax +86-591-24937089
Email [email protected]
Background: Platelet count (PLT) has been proved as an essential biomarker for the survival of hepatocellular carcinoma (HCC). However, the prognostic value of PLT change (ΔPLT) is still uncertain. The aim of this study was to explore the relationship between ΔPLT and HCC survival after transarterial chemoembolization (TACE) treatment.
Methods: Cox proportional hazard regression models were used to calculate multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for HCC. The non-linear relationship between ΔPLT and OS was estimated through a restricted cubic spline regression analysis, and a two-piece-wise Cox proportional hazard model was further performed to calculate the threshold effect.
Results: A total of 597 HCC patients treated with TACE were selected for the secondary analysis. Compared with the ΔPLT within ± 20 (× 109/L), ΔPLT≥ 20 (× 109/L) was significantly associated with an 64% increase in risk of death (HR, 1.64; 95% CI: 1.21 to 2.22) after adjustment for confounding variables, but the association was not significant in the group of ΔPLT≤-20 (HR, 1.23; 95% CI: 0.92 to 1.63). We also found a U-shape relationship between ΔPLT and HCC survival at the turning point of ΔPLT as 0 (20× 109/L). The HR for the death was 1.12 (95% CI: 1.06, 1.18) with ΔPLT≥ 0 (20× 109/L) while 0.95 (95% CI: 0.92, 0.98) with ΔPLT< 0 (20× 109/L). After potential confounding factors were adjusted, the non-linear relationship between ΔPLT and OS was still significant (P=0.013). Besides, ΔPLT≥ 20 (× 109/L) was associated with new lesions (OR, 2.74; 95% CI: 1.38 to 5.45).
Conclusion: Elevated PLT was associated with poor overall survival of HCC patients after TACE treatment.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, platelet count, prognosis, real-world study
Introduction
Hepatocellular carcinoma (HCC) is the second cancer-related death cause worldwide1 and the fifth major cause of death in China.2 The majority of HCC patients are diagnosed at an advanced stage, with a median overall survival (OS) no more than one year.3 Due to its poor prognosis, reliable biomarkers are in urgent need for improving the curative effect and reducing the mortality of HCC.
Recently, platelet has been proved as a critical factor in HCC proliferation,4 and its adhesion receptors, such as GPIIb/IIIa, GPIb-IX-V and P-selectin, probably contribute to distant metastasis.5 For the patients with early-stage HCC, predictive models based on platelet count (PLT) are effective in evaluating the recurrence after curative resection.6–8 While for huge HCC (diameter over 10 cm), a tumour subgroup of intermediate-stage (BCLC-B) HCC, high PLT is associated with an increased risk of distant metastasis after transarterial chemoembolization (TACE).9 In line with this finding, another large retrospective study showed that thrombocytopenia is associated with favourable outcomes in patients with unresectable HCC.10
In clinical practice, TACE plays a critical role in unresectable HCC, especially in intermediate-stage HCC. However, the responses to TACE among HCC patients are highly heterogeneous, leading to a great challenge to predict clinical benefit. Although some predictive models, such as the model based on aspartate aminotransferase-to-platelet ratio, have been established to predict the clinical outcomes of the HCC patients undergoing postoperative adjuvant TACE,11 the PLT-based predictive model is eager to be further explored. The great interest has aroused whether the change value of PLT (ΔPLT) is a promising biomarker for HCC after TACE treatment. To address this issue, we retrospectively investigated the association between ΔPLT and OS in HCC patients after treatment with TACE.
Patients and Methods
Patient Selection
Clinical and haematological data were obtained from the patients enrolled in a large-scale multicentre cohort study about the predictive model for intermediate-stage HCC.12 In this retrospective cohort study, we mainly analysed the derivation cohort with 979 newly diagnosed consecutive HCC patients at Sun Yat-sen University Cancer Center (SYSUCC). Of the 979 participants, 262 patients were excluded firstly, including 37 patients refusing to receive treatment (3.8%) and 225 patients undergoing surgical resection (23.0%) as their first-line treatment. Besides, 120 patients who had only one follow-up record were excluded, and finally, totally 597 patients were included into final analysis and treated with TACE as their first-line treatment. See Figure 1 for detailed information of inclusion/exclusion criteria.
 |
Figure 1 Flowchart for the patients with HCC after TACE treatment. |
The study protocol (2017-FXY-129) had been approved by the department of clinical research at SYSUCC. The requirement for informed consent was waived for this was a secondary analysis study and the data were anonymous. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Definition and Measurements
OS, which was defined as the time from diagnosis to death by any cause, was regarded as the primary outcome of this study, while the new lesion at second follow-up record was noted to be the secondary outcome. Vascular invasion was only defined as macroscopic vascular invasion, and its diagnosis was based on the standard radiological imaging prior to treatment at first and second follow-up visits.13,14 Regarding to the second follow-up record, the new lesions included vascular invasion (including hepatic vein, vena cava, right atrium, portal vein or its branch), lymph node or distant metastasis (LN/DM), and new intrahepatic lesions but not the residual lesions of main lesion within 6 months.ΔPLT was defined as the difference value between the baseline PLT at first and the second follow-up record before any treatment. Moreover, both lobes with lesions were defined as lesions in both left and right lobes of the liver. Only the second follow-up data on the clinical record, serum tumour markers, medical imaging, and biochemical indices were included in the analysis.
Statistical Analysis
In this study, we firstly divided the study population into three groups based on the turning point of ΔPLT. Among the three subgroups, the Kruskal–Wallis test was used to compare continuous variables while the chi-square test or Fisher exact test was applied for analysis on the categorical data. To explore the relationship between ΔPLT and OS of HCC, univariate and multivariate analyses were performed based on the Cox proportional hazards model. Also, the logistic regression analysis was performed to explore the relationship between ΔPLT and new lesions.
To identify a non-linear relationship, a smooth curve was then drawn to estimate the shape between ΔPLT and hazard risk through a restricted cubic spline regression analysis. A two-piece-wise Cox proportional hazards model was further performed to calculate the threshold effect of ΔPLT on OS using a smoothing plot. The inflexion point was determined using the recursive method, where the maximum model likelihood was used. Furthermore, a log-likelihood ratio test was conducted to compare the one-line linear regression.
A 2-tailed P-value<0.05 was considered statistically significant. All analyses were performed using Empower (R) (www.empowerstats.com, X&Y solutions, Inc. Boston, MA).
Results
Patients and Baseline Characteristics at the Second Follow-Up Visit
From January 2007 to May 2012, a total of 979 consecutive HCC participants at SYSUCC were retrospectively reviewed, and 597 patients were selected for the final analysis. The majority of the patients (517/597, 86.6%) were with HBV infection. By the deadline of the study, 351 (58.8%) patients died. The median follow-up time was 13.9 months (range, 0.1 to 112.5) and the median cumulated time from second to first follow-up visit is 2.1 months (range, 0.4 to 8.0). After TACE treatment, 131 patients developed new lesions, including 45 patients with vascular invasion and 96 patients with LN/DM.
As shown in Table 1, the baseline characteristics of patients in three groups were presented, which presented significant differences in age, diameter of the main tumour, location of lesions, alpha-fetoprotein (AFP) and C-reactive protein (CRP) among the three groups.
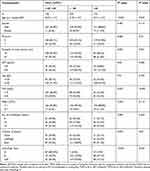 |
Table 1 Baseline Characteristics of Patients After TACE Treatment at the Second Follow-Up Visit |
Association Between ΔPLT and OS After TACE Treatment
In this part, we firstly evaluated the median OS of the three groups by the Kaplan–Meier method with Log-rank test applied for statistical analysis. In Figure 2, the median OS time was 28.9 months (95% CI: 20.4 to 39.8) for the group of ΔPLT within±20 (×109/L), 16.7 months (95% CI: 15.0 to 23.7) for the group of ΔPLT≤-20 (×109/L) and 15.1 months (95% CI: 11.0 to 20.7) for the group of ΔPLT≥20 (×109/L). The differences among the three groups were significant (P=0.002).
 |
Figure 2 Kaplan–Meier curves of OS in patients with HCC after TACE treatment. Differences among the three groups are significant (P=0.002). |
As reported in Table 2, in the univariate analysis, comparing with the group of ΔPLT within±20 (×109/L), we found that the PS score (1: HR, 3.17; 95% CI: 2.24, 4.48), main tumour (≥5 cm: HR, 2.76; 95% CI: 2.08 to 3.68), intrahepatic lesion (>3: HR, 3.06; 95% CI: 2.11 to 4.44; ≤3: HR, 2.02; 95% CI: 1.33 to 3.05), location of lesions (left/right: HR, 2.01; 95% CI: 1.34 to 3.02; both: HR, 3.17; 95% CI: 2.18, 4.62), AFP (≥25 ng/mL: HR, 2.58; 95% CI: 1.90 to 3.50), CRP (≥10 mg/L: HR, 1.97; 95% CI: 1.46 to 2.65) and white blood cell (WBC) (≥11×109/L: HR,1.64; 95% CI: 1.04 to 2.60) were associated with a higher death risk in the group of ΔPLT≥20 (×109/L), but Hgb≥120 g/L was associated with a lower risk. The similar results were also found in the group of ΔPLT≤-20 (×109/L).
 |
Table 2 Univariate Analysis for Risk Factors of OS After TACE Treatment |
Based on clinical and statistical reasons, confounding factors including age, PS score, Child-Pugh class, diameter of main tumour, location of lesions, No. of intrahepatic lesions, CRP and AFP levels were selected. In Table 3, ΔPLT≥20 (×109/L) was associated with a 61% increase in the risk of death (unadjusted HR, 1.61; 95% CI: 1.21 to 2.13). After the confounding factors in the Model II were adjusted, the positive relationship was still robust (HR, 1.64; 95% CI: 1.21 to 2.22). However, in the group of ΔPLT≤-20 (×109/L), ΔPLT was not significantly associated with the increase in death (HR, 1.23; 95% CI: 0.92, 1.63). Based on the confounding variables in the Model II, Model III was further adjusted for PLT (smooth), which was applied by restricted cubic spline. The results of Model III revealed that the ΔPLT was independent of PLT as a biomarker for HCC survival after TACE treatment.
 |
Table 3 The ΔPLT and Multivariate HRs of OS with 95% CIs in HCC After TACE |
Non-Linear Relationship and Threshold Effect of ΔPLT on OS
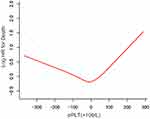
In Figure 3, a U-shape relationship was observed between the ΔPLT and OS. The latter increased with PLT decreasing after the turning point (ΔPLT=0×109/L). The threshold effect of ΔPLT on OS was significant (P for non-linearity<0.001). The HR was 1.12 (95% CI: 1.06 to 1.18) for ΔPLT≥0 (20×109/L) and 0.95 (95% CI: 0.92 to 0.98) for ΔPLT<0 (20×109/L). After potential confounders, including age, PS score, Child-Pugh class, diameter of main tumour, location of lesions, No. of intrahepatic lesions, CRP and AFP, were adjusted, the threshold effect of ΔPLT on OS was still significant (P for non-linearity=0.013, Table 4).
 |
Table 4 Threshold Effect Analysis for the ΔPLT in the Derivation Cohort Using a Two-Piece-Wise Cox Proportional Hazards Model |
Relationship Between ΔPLT and New Lesions
In comparison with ΔPLT within±20 (×109/L), ΔPLT≥20 (×109/L) was significantly associated with new lesions (OR, 2.74; 95% CI: 1.38 to 5.45), but not markedly related to vascular invasion (OR, 1.02; 95% CI:0.43 to 2.42) and LN/DM (OR, 1.02; 95% CI: 0.43, 2.42). The results in the group of ΔPLT≤-20 (×109/L) were consistent and shown in Figure 4.
Sensitivity Analysis
To further explore the relationship between the ΔPLT and survival of patients after TACE, stratified analysis was performed in the subgroups of second-line treatment (HR/ablation, TACE, none) and liver function (Child-Pugh class A, B, NA). Based on the univariate logistic regression model, we also evaluated the relationship between the ΔPLT and the mortality of patients at 6 months, 1 year, 2 years and 3 years. Details of the method were showed in Supplementary Materials, and the core results were consistent with the initial cohort (All P for interaction>0.05, see Tables S1 and S2).
Discussion
In this large-scale, hospital-based, retrospective cohort study, we found that elevated PLT was associated with worse OS in HCC patients after TACE treatment, which was consistent with the findings of previous studies.9,13,14 We further revealed a U-shape relationship between ΔPLT and OS of HCC at the turning point of ΔPLT as 0×109/L. The PLT response to TACE increasing per 20 (×109/L) was associated with 8% death risk in HCC patients after adjustment for confounding factors. Interestingly, a non-linear relationship was also found between PLT and OS in HCC populations.15 Additionally, we identified a high-risk subgroup for new lesions after TACE, with ΔPLT over 20 (×109/L). To the best of our knowledge, this clinical study firstly demonstrated that ΔPLT may be a promising biomarker for HCC patients after TACE treatment.
We also found that ΔPLT over20 (×109/L) was significant associated with the new lesions after TACE treatment, which was consistent with the previous study.15 It was revealed that elevated PLT might be a biomarker for HCC patients treated with antiplatelet therapy. In secondary prevention, the inverse relationship reported between antiplatelet therapy and HCC recurrence was further confirmed by liver resection, transarterial embolization (TAE) and sorafenib.16–18 A large retrospective study revealed that use of aspirin/clopidogrel can reduce the risk of HCC recurrence to 27% and the risk of overall mortality to 43% in patients with HBV-related HCC after liver resection. Moreover, a small-scale cohort study suggested that aspirin administration can improve liver function test results and survival after TACE for HCC but not response or time to progression.17
Our study has some strengths, including the secondary analysis of a large, well-characterised HCC population with retrospective clinical data and more than 115.3 months of follow-up. Besides, in this study, a U-shape relationship between ΔPLT and OS of HCC was calculated through restricted cubic spline regression, and the threshold effect of ΔPLT on OS was calculated through a two-piece wise Cox proportional hazards model. We thus identified a high-risk subgroup of ΔPLT for HCC patients with TACE treatment.
Some limitations still exist in our study. First, the data used here were collected from a single centre in China. Therefore, our results might not be generalizable to other geographic areas. Besides, bias could be caused by residual and unmeasured confounders. Although patients with blood system malignancies and significant decreased value of blood cell count were excluded before TACE treatment, we indeed could not evaluate the impact from other diseases. Second, in this secondary analysis, the inherent limitations of raw data were unavoidable. We lacked information about platelet size to evaluate its implication on survival. Additionally, the medication regimes were not available from the raw data. Third, we indeed could not exclude the lesions previously missed in diagnosis but found at the second follow-up visit. However, the data were retrospectively collected, which made the bias toward the null among the subgroups.
In conclusion, our study showed that elevated PLT was associated with worse OS for HCC patients after TACE treatment.
Abbreviations
PLT, platelet count; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; TAE, transarterial embolization; BCLC, Barcelona clinic liver cancer; SYSUCC, Sun Yat-sen University Cancer Center.
Data Sharing Statement
The raw data were freely obtained from the Dryad Digital Repository database (www.Datadryad.org; Dryad data package: Shen, Lujun et al (2019), Data from: Dynamically prognosticating patients with hepatocellular carcinoma through survival paths mapping based on time-series data, Dryad, Dataset, https://doi.org/10.5061/dryad.pd44k8r).
Ethics Approval and Informed Consent
The study protocol (2017-FXY-129) was approved by the department of clinical research at SYSUCC. Because this was a secondary analysis study, and the data were anonymous, the requirement for informed consent was waived.
Consent for Publication
All the authors confirm that the details of any images, videos, recordings, etc. can be published.
Acknowledgments
We gratefully thank for the raw data from Prof. Peihong Wu (E-mail: [email protected]) and the statistical support from Empower U team of the Department of Epidemiology and Biostatistics, X&Y solutions Inc. in Boston.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received financial support from the Natural Science Foundation of Fujian Province (Nos 2018J01352 and 2016J01576 and 2016J01586); the Science and Technology Innovation Joint Foundation of Fujian Province (Nos 2017Y9125); the Natural Science Foundation of 900th hospital of PLA (Nos 2016Q05 and 2017Q02 and 2017L17 and 2016L03 and 2017J03).
Disclosure
The authors have no conflicts of interest for this work.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi:10.3322/caac.20107
2. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2019;394:1145–1158. doi:10.1016/S0140-6736(19)30427-1
3. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462.
4. Pavlovic N, Rani B, Gerwins P, Heindryckx F. Platelets as key factors in hepatocellular carcinoma. Cancers. 2019;11(7):1022. doi:10.3390/cancers11071022
5. Wu CY, Chen YJ, Ho HJ. Platelet integrins in tumor metastasis: do they represent a therapeutic target? Cancers. 2017;9.
6. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi:10.1158/1078-0432.CCR-14-0442
7. Chan AW, Chan SL, Wong GL, et al. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. 2015;22:4138–4148. doi:10.1245/s10434-015-4516-1
8. Huang PY, Wang CC, Lin CC, et al. Predictive effects of inflammatory scores in patients with BCLC 0-A hepatocellular carcinoma after hepatectomy. J Clin Med. 2019;8:1676. doi:10.3390/jcm8101676
9. Xue TC, Ge NL, Xu X, et al. High platelet counts increase metastatic risk in huge hepatocellular carcinoma undergoing transarterial chemoembolization. Hepatol Res. 2016;46:1028–1036. doi:10.1111/hepr.12651
10. Scheiner B, Kirstein M, Popp S, et al. Association of platelet count and mean platelet volume with overall survival in patients with cirrhosis and unresectable hepatocellular carcinoma. Liver Cancer. 2019;8:203–217. doi:10.1159/000489833
11. Zhu GQ, Wang K, Wang B, et al. Aspartate aminotransferase-to-platelet ratio index predicts prognosis of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization. Cancer Manag Res. 2019;11:63–79. doi:10.2147/CMAR.S186150
12. Shen L, Zeng Q, Guo P, et al. Dynamically prognosticating patients with hepatocellular carcinoma through survival paths mapping based on time-series data. Nat Commun. 2018;9:2230. doi:10.1038/s41467-018-04633-7
13. Lee CH, Lin YJ, Lin CC, et al. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015;35:2327–2336. doi:10.1111/liv.12817
14. Han S, Lee S, Yang JD, et al. Risk of posttransplant hepatocellular carcinoma recurrence is greater in recipients with higher platelet counts in living donor liver transplantation. Liver Transpl. 2018;24:44–55. doi:10.1002/lt.24961
15. Liu PH, Hsu CY, Su CW, et al. Thrombocytosis is associated with worse survival in patients with hepatocellular carcinoma. Liver Int. 2020;40(10):2522–2534. doi:10.1111/liv.14560
16. Lee PC, Yeh CM, Hu YW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23:874–883. doi:10.1245/s10434-016-5520-9
17. Boas FE, Brown KT, Ziv E, et al. Aspirin is associated with improved liver function after embolization of hepatocellular carcinoma. Am J Roentgenol. 2019;213:1–7. doi:10.2214/AJR.18.20846
18. Li S, Dai W, Mo W, et al. By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma. Int J Cancer. 2017;141:2571–2584. doi:10.1002/ijc.31022
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.