Back to Journals » Cancer Management and Research » Volume 11
Elevated expression of CXCL16 correlates with poor prognosis in patients with colorectal cancer
Authors Chen Z, Dai W, Yang L, Yang H, Ding L, He Y , Song X , Cui J
Received 6 December 2018
Accepted for publication 29 April 2019
Published 23 May 2019 Volume 2019:11 Pages 4691—4697
DOI https://doi.org/10.2147/CMAR.S197354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Zhihui Chen,1,* Weigang Dai,1,* Liang Yang,1,* Hong Yang,2 Li Ding,3 Yulong He,1 Xinming Song,1 Ji Cui1
1Department of Gastrointestinal Surgery Center, First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, People’s Republic of China; 2Operating Department, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China; 3Department of Pathology, First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, People’s Republic of China
*These authors contributed equally to this work
Aims: To examine the expression of CXCL16 in colorectal cancer (CRC) tissue and to clarify the relationships between CXCL16 and clinicopathological features and survival in CRC.
Methods: A total of 142 consecutive CRC patients undergoing colorectal surgery at the Department of Gastrointestinal Center, First Affiliated Hospital, Sun Yat-sen University, between January 2010 and December 2010 were enrolled in this study. CXCL16 was measured by immunohistochemical staining in CRC tissue. Association between CXCL16 expression and clinicopathologic parameters was analyzed with a chi-square test. Survival curves were calculated by the Kaplan–Meier method, and the differences between CXCL16 high- and low-expression groups were analyzed using the log-rank test. Cox univariate and multivariate analyses were used to determine risk factors for overall survival (OS).
Results: CXCL16 expression was elevated in CRC. CXCL16-positive expression was significantly related to tumor size (P=0.043), tumor differentiation (P=0.046) and distant metastasis (P=0.038), and there was a trend toward lymph node metastasis (P=0.070). CXCL16 expression, together with differentiation, depth of invasion, lymph node metastasis, and distant metastasis, was a significant independent prognostic factor for OS of patients with CRC (HR 2.026, 95% CI 1.128–3.640, P=0.018).
Conclusion: CXCL16 expression was enhanced in CRC tissue and was negatively correlated with survival in CRC patients. Furthermore, CXCL16-positive expression was an independent prognostic factor for CRC patients, whilst the underlying mechanisms remain unclear; thus, further studies are needed.
Keywords: colorectal neoplasms, chemokine, CXCL16, prognosis
Introduction
Colorectal cancer (CRC) is the third most prevalent malignancy in the world. In 2018, it was estimated that approximately 140,250 new CRC cases occurred in the United States.1 Many biomarkers, such as XRCC1, ZG16, SMAD4 and MYO5B, have been proposed as candidate prognostic factors in CRC.2–5 However, few of these have been employed in clinical practice. Considering that CRC is of great heterogeneity, it is necessary to identify additional molecular prognostic factors.
Chemokine CXCL16 is expressed in soluble form across the cell membrane. Its interaction with CXCR6 receptor on the surface of T lymphocytes directs the migration of activated T lymphocytes in rheumatoid arthritis, systemic lupus erythematosus and coronary atherosclerotic heart disease.6,7 A recent study demonstrated that CXCL16 was involved in tumor progression and metastasis in lung cancer,8 breast cancer,9 meningioma10 and hepatocellular carcinoma.11 However, few studies have examined the expression of CXCL16 in CRC or the relationships between CXCL16 expression and the prognosis and clinical characteristics of patients with CRC.
Materials and methods
Patients
A total of 142 consecutive CRC patients undergoing colorectal surgery at the Department of Gastrointestinal Surgery Center, First Affiliated Hospital, Sun Yat-sen University, between January 2010 and December 2010 were enrolled in this study. Clinicopathological data and tissue samples were obtained from these CRC patients according to the following inclusion criteria: (1) the patient received primary colorectal resection and had histologically proven adenocarcinoma, (2) there were no synchronous cancers or other history of malignancy and (3) there was no preoperative chemotherapy and/or radiotherapy. These patients included 86 males and 56 females with a median age of 58.9 years (range: 22–87 years). The patients’ demographic and clinicopathological features are shown in Table 1. Tumor locations are gathered into right (ascending plus 2/3 transverse) and left (1/3 rest of transverse, descending, sigmoid and rectum). The tumor node Metastasis (TNM) status of CRC was applied according to the guidelines of the 2017 American Joint Committee on Cancer Staging Manual (AJCC 8th edition). All patients were followed up by phone or letter as well as at the outpatient clinic every 3–6 months for the first two years, every 6 months for the third to fifth years and every 12 months thereafter. The last follow-up was in December 2017. Written informed consent was obtained from each patient which was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the First Affiliated Hospital, Sun Yat-Sen University Institutional Review Board.
 | Table 1 Correlations between CXCL16 expression and clinicopathological features |
Immunohistochemical staining and assessment
A total of 142 primary CRC paraffin-embedded tissues and 15 adjacent normal tissues that were more than 5 cm from the primary tumor sites were obtained for immunohistochemical staining and assessment. The immunohistochemical staining procedure was performed according to our previous study.12 Briefly, consecutive sections (4 µm thick) were cut from each block and used for H&E staining and immunohistochemical staining. Samples were deparaffinized with xylene, followed by dehydration using a diluted alcohol series. Then, the sections were subjected to high-temperature and high-pressure EDTA to retrieve antigenicity before incubation in 3% hydrogen peroxide to block endogenous peroxidase activity. The sections were then incubated with a polyclonal primary antibody against CXCL16 (1:400 dilution; R&D Systems, Minneapolis, MN) at 4°C overnight. After incubation with a goat anti-mouse/rabbit secondary antibody (Gene Tech Co Ltd, GTVisionTM III Detection System/Mo&Rb, Shanghai, China) and 3, 3-diaminobenzidine, the slides were counterstained with hematoxylin decolored by hydrochloric alcohol solution. PBS buffer was used to replace the primary antibodies in negative control staining.
The CXCL16 staining results for each slide were scored independently by LD and WD in a blinded manner based on both intensity of staining and the proportion of positively stained tumor tissue. The scoring based on the percentage of positive cells per tumor was as follows: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). Staining intensity scoring was as follows: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). For each sample, the scores from the two scoring systems were multiplied to obtain a final point score: 0 points, negative staining (-); 1–3 points, weak positive staining (+); 3–6 points, medium positive staining (++) and >6 points, strong positive staining (+++). Then, the results were finally dichotomized into negative (the former) and positive (the latter three).
Statistical analysis
Statistical analysis was performed with the Statistical Product and Service Solutions statistical software package (version 19.0). Associations between CXCL16 expression and clinicopathologic parameters were analyzed with a chi-square test. Survival curves were calculated by the Kaplan–Meier method, and the differences between CXCL16 high- and low-expression groups were analyzed using the log-rank test. Cox univariate and multivariate analyses were used to determine risk factors for overall survival (OS). P<0.05 was considered statistically significant.
Results
Expression of CXCL16 in primary CRC and adjacent normal tissues
CXCL16 was distributed in the membrane and cytoplasm of CRC tumor cells (Figure 1). Positive staining of CXCL16 was detected in 53.5% (76 out of 142) of primary CRC patients, which is significantly higher than that of adjacent normal tissues (2 out of 15, P<0.001).
 | Figure 1 Immunohistochemical staining of CXCL16 expression in CRC. |
Correlations between CXCL16 expression and clinicopathological features of patients with CXCL16
Table 1 shows the correlations between CXCL16 expression and clinicopathological features in CRC, including gender, age, tumor size, differentiation, tumor location, depth of invasion, lymph node metastasis, distant metastasis, TNM stage and chemotherapy regimen. The data revealed that CXCL16-positive expression was significantly related to tumor size (P=0.043), tumor differentiation (P=0.046) and distant metastasis (P=0.038), and it was a trend toward lymph node metastasis (P=0.070).
Survival analysis and prognostic significance of CXCL16 expression
The total median OS time of 42 patients was 64 months. The median OS time of patients with positive CXCL16 in primary tumors was 59 months, while the median OS time of patients with negative CXCL16 was 65 months. The 5-year OS rates of CXCL16-positive patients were significantly lower than those of CXCL16-negative patients (Figure 2, P=0.002). In subgroup analysis, the 5-year OS rates were not significantly different between CXCL16-positive and CXCL16-negative patients with stage I/II CRC (P=0.181, Figure 3), while the 5-year OS rates of CXCL16-positive patients were significantly lower than those of CXCL16-negative patients with stage III/IV CRC (P=0.011, Figure 4). Univariate analysis revealed that the OS of CRC patients significantly correlated with CXCL16, differentiation, depth of invasion, lymph node metastasis and distant metastasis (all P<0.05, Table 2). Multivariate analysis was used to evaluate all the statistically (including marginally) significant variables revealed by univariate analysis. CXCL16 expression, together with differentiation, depth of invasion, lymph node metastasis and distant metastasis, was a significant independent prognostic factor for OS of patients with CRC (HR 2.026, 95% CI 1.128–3.640, P=0.018).
 | Table 2 Cox proportional hazard regression analysis for overall survival (OS) |
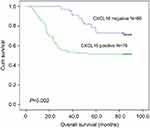 | Figure 2 The 5-year overall survival rates of CXCL16-positive patients were significantly lower than those of CXCL16-negative patients (P=0.002). |
 | Figure 3 The 5-year overall survival rates were not significantly different between CXCL16-positive and CXCL16-negative patients with stage I/II CRC (P=0.181). |
 | Figure 4 The 5-year overall survival rates of CXCL16-positive patients were significantly lower than those of CXCL16-negative patients with stage III/IV CRC (P=0.011). |
Discussion
In the present study, we found that CXCL16 expression was enhanced in CRC tissue and negatively correlated with survival in CRC patients. Furthermore, CXCL16-positive expression was significantly related to several known clinicopathological features that correlated with outcomes in CRC, including tumor size, tumor differentiation and distant metastasis, and it was a trend toward lymph node metastasis. These findings demonstrated that enhanced CXCL16 expression may be a common phenomenon in CRC, suggesting that CXCL16 was associated not only with tumorigenesis but also with progression.
CXCL16 was proved to be involved in tumor proliferation and metastasis. For example, studies reported that elevated CXCL16 expression in lung cancer or hepatocellular carcinoma tissue promoted the proliferation and invasion of lung cancer cells via the NF-κB pathway by regulating expressions of c-Rel, Rel-B, p10513 or MMP2, MMP9.14 Chung et al15 proved that cancer-associated fibroblasts derived from human breast cancer brain metastasis expressed significantly higher levels of chemokine CXCL16 than did fibroblasts from primary breast tumors or normal breast cells and that human brain metastasis cancer-associated fibroblasts potently attracted breast cancer cells via chemokine CXCL16. Richardsen et al16 found that high protein expression of CXCL16 and high protein coexpression of CXCL16/CXCR6 in prostate cancer were independent predictors of a worse clinical outcome. Whether these mechanisms work in CRC remains unclear and further studies are needed. And previous results of CXCL16 in CRC were inconsistent.17–19 Wågsäter et al17 found that expression of CXCL16, examined by eitherWestern blot (n=23) or H&E (n=8), was down-regulated in human rectal tumor tissue. While the study by Hojo et al18 found that up-regulated expression of CXCL16 was found in CRC tissues (n=58), which was in line with the present study. Studies by Hojo S team18,19 also showed that CXCL16 inhibited CRC liver metastasis by recruitment of CD4+ and CD8+ tumor-infiltrating lymphocytes and M1 macrophages, leading to a good prognosis for CRC patients, by contrast to our speculations. The underlying exploration could be that there is a discrepancy of CXCL16 expression profile between colon cancer and rectal cancer. To obtain a definite conclusion, colon and rectal cancer should be studied separately in future.
In this study, we found that there was a significant correlation between positive CXCL16 expression and poor survival in CRC patients. Furthermore, subgroup analysis illustrated that CRC patients with positive CXCL16 expression had lower OS rates than those with negative CXCL16 expression in TNM stage III/IV but not stage I/II, possibly due to the small sample size. Finally, we identified CXCL16 as an independent prognostic factor for OS of CRC patients in Cox multivariate analysis. All these results implied that CXCL16 served as an oncogene in CRC, in accordance with the research findings from other human cancers mentioned above.
Conclusions
We found that CXCL16 expression was enhanced in CRC tissue and was negatively correlated with survival in CRC patients. Furthermore, CXCL16-positive expression was an independent prognostic factor for CRC patients, while the underlying mechanisms remain unclear; thus, further studies are needed.
Abbreviations list
CRC, colorectal cancer; TNM, tumor node metastasis; AJCC Staging, American Joint Committee on Cancer Staging; DAB, 3, 3-diaminobenzidine; OS, overall survival.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of Guangdong Province, People's Republic of China (2017A030310194), the Science and Technology Planning Project of Guangdong Province, People's Republic of China (2016A020216008) and the Administration of Traditional Chinese Medicine of Guangdong Province, People's Republic of China (20151162).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Zhang L, Zhao J, Yu B, et al. Correlations between microsatellite instability, ERCC1/XRCC1 polymorphism and clinical characteristics, and FOLFOX adjuvant chemotherapy effect of colorectal cancer patients. Cancer Genet. 2017;218–219:51–57. doi:10.1016/j.cancergen.2017.09.004
3. Meng H, Li W, Boardman LA, Wang L. Loss of ZG16 is associated with molecular and clinicopathological phenotypes of colorectal cancer. BMC Cancer. 2018;18(1):433. doi:10.1186/s12885-018-4242-8
4. Mizuno T, Cloyd JM, Vicente D, et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. Eur J Surg Oncol. 2018;44(5):684–692. doi:10.1016/j.ejso.2018.02.247
5. Letellier E, Schmitz M, Ginolhac A, et al. Loss of Myosin Vb in colorectal cancer is a strong prognostic factor for disease recurrence. Br J Cancer. 2017;117(11):1689–1701. doi:10.1038/bjc.2017.352
6. Abel S, Hundhausen C, Mentlein R, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–6372.
7. Xing J, Liu Y, Chen T. Correlations of chemokine CXCL16 and TNF-α with coronary atherosclerotic heart disease. Exp Ther Med. 2018;15(1):773–776. doi:10.3892/etm.2017.5450
8. Ke C, Ren Y, Lv L, Hu W, Zhou W. Association between CXCL16/CXCR6 expression and the clinicopathological features of patients with non-small cell lung cancer. Oncol Lett. 2017;13(6):4661–4668. doi:10.3892/ol.2017.6088
9. Xiao G, Wang X, Wang J, et al. CXCL16/CXCR6 chemokine signaling mediates breast cancer progression by pERK1/2-dependent mechanisms. Oncotarget. 2015;6(16):14165–14178. doi:10.18632/oncotarget.3690
10. Hattermann K, Bartsch K, Gebhardt HH, et al. “Inverse signaling” of the transmembrane chemokine CXCL16 contributes to proliferative and anti-apoptotic effects in cultured human meningioma cells. Cell Commun Signal. 2016;14(1):26. doi:10.1186/s12964-016-0149-7
11. Liu J, Chen S, Wang W, et al. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Lett. 2016;379(1):49–59.
12. Xu K, Song X, Chen Z, Qin C, He Y, Zhan W. XRCC2 promotes colorectal cancer cell growth, regulates cell cycle progression, and apoptosis. Medicine (Baltimore). 2014;93(28):e294. doi:10.1097/MD.0000000000000294
13. Liang K, Liu Y, Eer D, Liu J, Yang F, Hu K. High CXC chemokine ligand 16 (CXCL16) expression promotes proliferation and metastasis of lung cancer via regulating the NF-κB pathway. Med Sci Monit. 2018;24:405–411.
14. Wang YH, Dong YY, Wang WM, et al. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J Exp Clin Cancer Res. 2013;32(1):51. doi:10.1186/1756-9966-32-51
15. Chung B, Esmaeili AA, Gopalakrishna-Pillai S, et al. Human brain metastatic stroma attracts breast cancer cells via chemokines CXCL16 and CXCL12. NPJ Breast Cancer. 2017;3:6. doi:10.1038/s41523-017-0008-8
16. Richardsen E, Ness N, Melbø-Jørgensen C, et al. The prognostic significance of CXCL16 and its receptor C-X-C chemokine receptor 6 in prostate cancer. Am J Pathol. 2015;185(10):2722–2730. doi:10.1016/j.ajpath.2015.06.013
17. Wågsäter D, Hugander A, Dimberg J. Expression of CXCL16 in human rectal cancer. Int J Mol Med. 2004;14(1):65–69.
18. Hojo S, Koizumi K, Tsuneyama K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67(10):4725–4731. doi:10.1158/0008-5472.CAN-06-3424
19. Kee JY, Ito A, Hojo S, et al. CXCL16 suppresses liver metastasis of colorectal cancer by promoting TNF-α-induced apoptosis by tumor-associated macrophages. BMC Cancer. 2014;14:949. doi:10.1186/1471-2407-14-949
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
