Back to Journals » Journal of Pain Research » Volume 12
Electroacupuncture treatment upregulates α7nAChR and inhibits JAK2/STAT3 in dorsal root ganglion of rat with spared nerve injury
Authors Wang Y , Xue M, Xia Y, Jiang Q, Huang Z , Huang C
Received 1 February 2019
Accepted for publication 27 May 2019
Published 2 July 2019 Volume 2019:12 Pages 1947—1955
DOI https://doi.org/10.2147/JPR.S203867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Ying Wang,1 Meng Xue,1 Yangyang Xia,1 Qian Jiang,1 Zhihua Huang,1,2 Cheng Huang1,2
1Department of Physiology, Gannan Medical University, Ganzhou 341000, People’s Republic of China; 2Pain Medicine Research Institute, Gannan Medical University, Ganzhou 341000, People’s Republic of China
Background: Neuropathic pain with complicated mechanism severely disrupts patient quality of life. The novel approaches and more effective management should be further investigated. It was reported that alpha-7 nicotinic acetylcholine receptor (α7nAChR) and janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling in dorsal root ganglion (DRG) contributed to the pathogenesis of neuropathic pain. Our previous study has shown that electroacupuncture (EA) alleviated neuropathic pain via activating α7nAChR in the spinal cord. However, whether the effect of 2 Hz EA on spared nerve injury (SNI)-induced neuropathic pain is mediated through modulation of α7nAChR and JAK2/STAT3 pathway in the DRG remains unclear.
Materials and methods: The SNI-induced neuropathic pain rat model was used in this study. After application of 2 Hz EA treatment to SNI rats on day 3, 7, 14 and 21 post-surgery, the expression levels of α7nAChR, JAK2/STAT3 and some cytokines in DRG were determined by qRT-PCR and Western blot analysis.
Results: We found that SNI induced significant down-regulation of α7nAChR mRNA and protein expression. SNI also obviously elicited the decrease in anti-inflammatory cytokine IL-10 protein expression. The enhancement of p-JAK2, p-STAT3, pro-inflammatory cytokines IL-1β and IL-6 protein levels induced by SNI were also observed. However, 2 Hz EA treatment to SNI rats distinctly improved α7nAChR and IL-10 levels and reduced p-JAK2, p-STAT3, IL-1β and IL-6 expression in the DRG.
Conclusion: Our present study suggested that 2 Hz EA treatment indeed activated α7nAChR, suppressed JAK2/STAT3 signaling and re-balanced the relationship between pro-inflammatory and anti-inflammatory cytokines in DRG of SNI rat, which provided insight into our understanding of the mechanism for 2 Hz EA to attenuate neuropathic pain.
Keywords: neuropathic pain, electroacupuncture, α7nAChR, JAK2/STAT3, dorsal root ganglion
Introduction
Neuropathic pain is a complex chronic condition resulting from peripheral nerve injury. Evidence shows a role of neuro-inflammation in the pathogenesis of neuropathic pain.1 The pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α participate in the initiation and maintenance of neuropathic pain.2 In contrast, IL-10, a powerful anti-inflammatory cytokine, exerts its anti-inflammatory effects in neuropathic pain. These findings revealed that an imbalance between the pro-inflammatory and anti-inflammatory cytokines mediated the modulation of neuropathic pain.3–5 Currently, cholinergic anti-inflammatory pathway may provide a new attempt to explore novel treatments against neuropathic pain. The cholinergic anti-inflammatory pathway modulates the nervous systems via acetylcholine (ACh) acting on the alpha-7 nicotinic acetylcholine receptor (α7nAChR, encoded by the cholinergic receptor nicotinic alpha 7 subunit [CHRNA7] gene).6 α7nAChR was revealed to modulate chronic pain and be widely distributed in spinal cord and dorsal root ganglion (DRG).7,8 The down-regulation of α7nAChR expression in DRG was observed in chronic constriction injury (CCI)-induced neuropathic pain rats.9,10 Activation of α7nAChR attenuates neuropathic pain via reducing the production of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α and increasing the anti-inflammatory cytokine IL-10.11–13 Our previous results also confirmed the down-regulation of spinal α7nAChR expression level in spared nerve injury (SNI) rats,14 indicating that α7nAChR played a key role in the modulation of neuropathic pain. Accumulating studies showed that α7nAChR inhibited its downstream molecules janus kinase 2 (JAK2, encoded by the Janus kinase 2 [Jak2] gene)/signal transducer and activator of transcription 3 (STAT3, encoded by the signal transducer and activator of transcription 3 [Stat3] gene) phosphorylation and pro-inflammatory cytokines IL-1β, IL-6 and TNF-α release.12,13,15 The activation of JAK2/STAT3-signaling-induced-neuropathic pain was attenuated by intrathecal injection of JAK2/STAT3 inhibitor.16–18 Collectively, the data indicated that the suppression of JAK/STAT3 signaling via activating α7nAChR implicated in controlling neuropathic pain.
It is reported that neuropathic pain involves some pathophysiological alterations that occur within the peripheral and CNS.19 The DRG is well known to be a critical position for integration and transmission of nociceptive signaling from the peripheral nerve to CNS.20 The injury site in DRG leads to peripheral and central sensitization and then elicits neuropathic pain.21,22 The results demonstrated that DRG plays a pivotal role in the transmission and modulation of chronic pain.
The mechanism underlying neuropathic pain is complicated and it has ineffective treatment.23,24 Thus, neuropathic pain remains a major public health problem that affects millions of individuals.25 Electroacupuncture (EA) has been identified as an effective management for neuropathic pain.26,27 Our recent study has revealed that 2 Hz EA could alleviate SNI-induced neuropathic pain via activating α7nAChR in the spinal cord.14 The α7nAChR was reported to be associated with JAK2/STAT3 signaling to participate in modulating neuropathic pain.13 Moreover, EA treatment was shown to attenuate visceral hypersensitivity through inhibiting spinal JAK2/STAT3 signaling.28 Taken together, the activation of α7nAChR or JAK2/STAT3 signaling in spinal cord may mediate EA stimulation suppressing pain hypersensitivity. However, no corresponding report has been found in DRG. Based on these previous results, we focus on investigating whether α7nAChR and JAK2/STAT3 signaling in DRG mediates the effect of 2 Hz EA on SNI-evoked neuropathic pain.
In this experiment, we aim to explore the effect of 2 Hz EA on the expression levels of α7nAChR and JAK2/STAT3 signaling in DRG of SNI rat. Additionally, we further assess the effect of 2 Hz EA treatment on the relationship between pro-inflammatory cytokines IL-1β, IL-6 and anti-inflammatory cytokine IL-10. Findings from this study may provide evidence that α7nAChR and JAK2/STAT3 signaling pathway in DRG serves as new targets for 2 Hz EA treatment to neuropathic pain.
Materials and methods
Experimental animals
Healthy adult male Sprague–Dawley (SD) rats weighing 160–180 g (seven to eight weeks old) were obtained from Hunan SLAC Laboratory Animal Co., Ltd. (Changsha, China). The rats were housed five per cage with free access to food and water, and they were kept at a temperature-controlled (22–24°C) room and under a 12/12 hrs light/dark cycle. The rats were acclimatized to the surrounding for 5 days before the initiation of study. This experiment was performed in a quiet environment and conducted in a double-blind way. Rats were randomly divided into three groups of sham, SNI and SNI+EA. All animal experimental procedures were approved by the Animal Use and Protection Committee of Gannan Medical University in China and carried out in accordance with the guidelines of the International Association for the Study of Pain. A total of 70 rats were used in this study.
Electroacupuncture stimulation
Rats were restrained in specially designed holders with their hind legs and tails exposed as described in our previous studies.14,29 Briefly, the skin of rat hind legs was sterilized with 75% alcohol. Two stainless-steel needles (4 mm length, 0.4 mm diameter) were inserted into each leg of rat. One needle was inserted at the Zusanli acupoint (ST36), which was 5 mm lateral to the anterior tubercle of the tibia marked by a notch, and the other needle was inserted at the Sanyinjiao acupoint (SP6), which was 3 mm proximal to the medial malleolus and at the posterior border of the tibia. The stimulation square waves of EA generated from a Han’s Acupoint Nerve Stimulator (HANS, LH202, Beijing Huawei Industrial Developing Company, China) were applied to both legs of rat simultaneously. Rats received EA stimulation for 30 mins once every other day lasting for 21 days. The EA stimulation frequency was an application of 2 Hz with pulse width of 0.6 ms, and the stimulation intensity was increased in a stepwise way at 1-2-3 mA, each intensity lasting for 10 mins. Rats were always kept awaken in the process of EA treatment.
Establishment of spared nerve injury model
Following a five day surrounding adaptation, the SNI-induced neuropathic pain rat model was established according to the method described by Decosterd and Woolf.30 Briefly, rats were anesthetized by 2–3% isoflurane, and then exposing the left lateral sciatic nerve and its three terminal branches (the sural, common peroneal and tibial nerves). The left lateral common peroneal and the tibial nerves were tight-ligated with 5–0 silk suture and sectioned distal to the ligation, removing 2–4 mm of the distal nerve stump, and leaving the sural nerve intact. Muscle and skin were closed in two layers under sterile operation. Only rats that developed mechanical hypersensitivity were used in the study. The mechanical hypersensitivity was examined by PWT as described in previous study.14 Sham-surgery rats underwent all identical surgical procedures except that the tibial and common peroneal nerves were left intact.
Quantitative RT-PCR analysis
The mRNA expression of α7nAChR was detected by quantitative reverse transcription and real-time polymerase chain reaction (qRT-PCR). Under deep anesthesia with 2–3% isoflurane, the fresh L4-L6 segments DRG of rat were rapidly collected in 1.5 ml centrifuge tube and stored in liquid nitrogen. Total RNA was isolated from the L4-L6 segments DRG and prepared for qRT-PCR as described in previous report.31 The primer sequences for qRT-PCR were provided by Invitrogen (USA), the primer sequences used in this experiment were as the follows: α7nAChR, sense CCCTGGCTCTGCTGGTATTC, antisense TGGTGCTGGCGAAGTATTGT; GAPDH, sense CAGCCGCATCTTCTTGTGC, antisense GGTAACCAGGCGTCCGATA. Quantitative RT-PCR data were normalized with GAPDH mRNA level which was used as a control, and relative mRNA level was expressed as 2-∆∆Ct values.
Western blot analysis
Following deep anesthesia with 2–3% isoflurane, the DRG of rat was dissected and isolated immediately and rinsed in ice-cold PBS. The grounded L4-L6 segments DRG were rapidly removed for the extraction of protein. This procedure was performed as described in previous study.31 Thirty micrograms of protein per sample were denatured and then separated with 10% SDS-PAGE and western-blotted on a PVDF (Millipore, CA) membrane using a minigel and mini transblot apparatus (Bio-Rad, Hercules, CA). The membrane was blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 for 60 mins at room temperature, and subsequently the membrane respectively immuno-labelled overnight at 4°C with antibodies of rabbit anti-α7nAChR (1:200, ab10096, Abcam, UK), anti-phospho-STAT3 (Y785) XP® Rabbit mAb (1:200, D3A7, CST, UK), anti-STAT3 mAb (1:400, 124H6, CST, UK), anti-phospho-JAK2 (Tyr1007/1008) (1:200, 3771, CST, UK) or anti-JAK2 (D2E12) XP® Rabbit mAb (1:400, 3230, CST, UK), anti-IL-1β (1:200, ab6722, Abcam, UK), anti-IL-6 (1:200, ab6722, Abcam, UK), anti-IL-10 (1:200, SC-365858, Santa Cruz,UK), β-actin (1:1,000, Solarbio, China) or β-tubulin (1:1,000, Solarbio, China). The blots were washed with Tris-buffered saline and Tween and then incubated with the horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:1,000, Cell Signaling Technology, USA) for 60 mins at 4°C. The blots site of the antigen–antibody complex was visualized with the Immobilon Western Chemiluminescent HRP Substrate (Millipore, MA). The bands were analyzed using Quantity One software (Bio-Rad). β-actin or β-tubulin was used as the internal control. The values of α7nAChR, phospho-JAK2, phospho-STAT3, IL-1β, IL-6 and IL-10 were represented as the ratio of the optical density of band to the density of the related β-actin or β-tubulin band.
Statistical analysis
Statistical analyses were performed using Prism 5.0 software. All experimental data were expressed as the mean ± SEM. The data differences between groups were analyzed using one-way ANOVA followed by Newman–Keuls post-hoc tests. P<0.05 was considered to represent statistically significant.
Results
Effect of 2 Hz EA on α7nAChR mRNA and protein expression in DRG
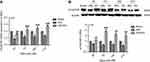
After 2 Hz EA treatment to rats, the expression levels of α7nAChR in DRG were detected on day 3, 7, 14 and 21 post-surgery. In comparison with the sham group, α7nAChR mRNA expression in the SNI group was significantly decreased (P<0.05, P<0.01, P<0.001) on day 3, 7, 14 and 21, respectively. Compared with the SNI group, α7nAChR mRNA expression in the SNI+EA group was obviously increased (P<0.01, P<0.001) on day 3, 7, 14 and 21, respectively. Similarly, in comparison with the sham group, α7nAChR protein expression in the SNI group was significantly decreased (P<0.05) on day 3, 7, 14 and 21, respectively. α7nAChR protein expression in the SNI+EA group was markedly increased compared to the SNI group (P<0.01, P<0.001) on day 3, 7, 14 and 21, respectively (Figure 1). The results indicated that 2 Hz EA stimulation increased the expression level of α7nAChR which is down-regulated by SNI in DRG.
Effect of 2 Hz EA on JAK2 and STAT3 protein expression in DRG
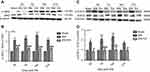
Following administration of 2 Hz EA treatment to rats, the protein expression of JAK2 and STAT3 in DRG were determined on day 3, 7, 14 and 21 post-surgery. The p-JAK2 protein expression in the SNI group was significantly increased compared to the sham group (P<0.001) on day 3, 7, 14 and 21, respectively. However, in comparison with the SNI group, the p-JAK2 protein expression in the SNI+EA group was markedly decreased (P<0.001) on day 3, 7, 14 and 21, respectively (Figure 2A and B). Similarly, in comparison with the sham group, the p-STAT3 protein expression in the SNI group was obviously up-regulated (P<0.05, P<0.001) on day 3, 7, 14 and 21, respectively. While in comparison with the SNI group, the p-STAT3 protein expression in the SNI+EA group was markedly down-regulated (P<0.05, P<0.01, P<0.001) on day 3, 7, 14 and 21, respectively (Figure 2C and D). These data revealed that SNI-evoked up-regulation of JAK2 and STAT3 protein expression was inhibited by 2 Hz EA stimulation in DRG.
Effect of 2 Hz EA on pro-inflammatory cytokines IL-1β and IL-6 protein expression in DRG
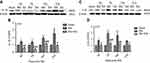
After rats received 2 Hz EA stimulation, the pro-inflammatory cytokines IL-1β and IL-6 protein expression levels in DRG were determined on day 3, 7, 14 and 21 after SNI surgery. In comparison with the sham group, IL-1β protein expression in the SNI group was significantly increased on day 3, 7, 14 and 21 (P<0.05, P<0.01, P<0.001), respectively. However, compared with the SNI group, IL-1β protein expression in the SNI+EA group was obviously decreased on day 3, 7, 14, 21 (P<0.05, P<0.01, P<0.001), respectively (Figure 3A and B). Similarly, IL-6 protein expression in the SNI group was significantly increased compared to the sham group (P<0.05, P<0.001) on day 3, 7, 14 and 21, respectively. Meanwhile, in comparison with the SNI group, IL-6 protein expression in the SNI+EA group was significantly decreased (P<0.05, P<0.01, P<0.001) on day 3, 7, 14 and 21, respectively (Figure 3C and D). The finding showed that 2 Hz EA suppressed pro-inflammatory cytokines IL-1β and IL-6 expression which was induced by nerve injury in DRG.
Effect of 2 Hz EA on anti-inflammatory cytokine IL-10 protein expression in DRG
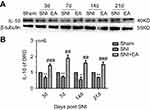
After rats were given 2 Hz EA treatment, the anti-inflammatory cytokine IL-10 protein expression in DRG was determined on day 3, 7, 14 and 21 post-surgery. As shown in Figure 4, IL-10 protein expression in the SNI group was significantly decreased compared to the sham group (P<0.05, P<0.01) on day 3, 7, 14 and 21, respectively. However, in comparison with the SNI group, IL-10 protein expression in the SNI+EA group was significantly enhanced (P<0.01, P<0.001) on day 3, 7, 14 and 21, respectively, which indicated that 2 Hz EA treatment exerted an anti-neuroinflammation effect through the up-regulation of anti-inflammatory cytokine IL-10 protein expression in DRG.
Discussion
The mechanism underlying neuropathic pain induced by peripheral nerve injury is extremely complex, and has no effective management. Our previous results have revealed that the activation of α7nAChR in spinal cord mediated 2 Hz EA alleviating mechanical hypersensitivity induced by neuropathic pain.14 DRG is indicated to be a pivotal region for detecting, processing and conveying primary sensory information such as painful stimuli to CNS.20–22 Based on these data, we have focused on investigating the effect of α7nAChR and JAK2/STAT3 signaling on 2 Hz EA relieving SNI-induced neuropathic pain in the DRG. This present study showed that SNI elicited the down-regulation of α7nAChR and anti-inflammatory cytokine IL-10 expression, as well as the enhancement of JAK2/STAT3, pro-inflammatory cytokines IL-1β and IL-6 levels in the DRG. Meanwhile, 2 Hz EA treatment significantly increased α7nAChR and IL-10 and reduced the expression of JAK2/STAT3, IL-1β and IL-6 in DRG of SNI rats. Finding from this study provided novel insight into understanding the molecular mechanism underlying 2 Hz EA ameliorating SNI-induced neuropathic pain.
Cholinergic anti-inflammatory pathway provides a novel strategy against chronic pain and modulates the immune and nervous systems through α7nAChR activation.32 The α7nAChR was displayed to mainly express on pain transmission pathways such as spinal cord and DRG, and mediated anti-inflammatory effects via down-regulating pro-inflammatory cytokines.32,33 Similarly, our previous experiment has shown that α7nAChR mRNA and protein expression were markedly decreased in the spinal cord of SNI rats.14 In this study, we also found that SNI elicited significant down-regulation of α7nAChR mRNA and protein expression in the DRG. Collectively, the results suggested that α7nAChR has taken part in modulating neuropathic pain in the spinal cord and DRG.
Neuropathic pain greatly disrupts patient quality of life and leads to a wide variety of problems worldwide. However, there are some limitations in the current pharmacological treatment for neuropathic pain. Therefore, another management for neuropathic pain should be taken into consideration. EA stimulation with few side effects has possessed action to alleviate chronic pain.34,35 Evidence also showed that EA treatment distinctly ameliorated mechanical hypersensitivity evoked by neuropathic pain.22,29,34,36 2 Hz EA-induced analgesic effect to neuropathic pain was better than 100 Hz EA did,35 and the application of 2 Hz EA for once every other day produced the better cumulative analgesic effect.37 Thus, here 2 Hz EA stimulation was selected to treat the SNI-induced neuropathic pain as described in our previous study.14 Our recent study has indicated that 2 Hz EA could attenuate mechanical hypersensitivity and increase spinal α7nAChR mRNA and protein expression in SNI rats.14 In addition, immunofluorescence staining also revealed that 2 Hz EA treatment up-regulated α7nAChR expression in the spinal cord of SNI rats.14 However, whether involvement of α7nAChR in 2 Hz EA alleviating SNI-induced neuropathic pain in DRG remains poorly understood. In agreement with our previous studies, the up-regulation of α7nAChR evoked by 2 Hz EA treatment to SNI rats was also observed in the DRG, which suggested that α7nAChR in DRG also implicated in 2 Hz EA-induced analgesia in SNI rats.
The formation of neuropathic pain involved various cellular transduction of signaling pathways, such as JAK2/STAT3 signaling which was one of the most important cascades, in response to both pro-inflammatory (IL-1β, IL-6 and TNF-α) and anti-inflammatory (IL-10) cytokines.38 The JAK/STAT3 signaling was mainly expressed in the spinal cord and DRG.17 The up-regulation of JAK/STAT3 expression was also found in neuropathic pain.3 The studies indicated that the JAK/STAT3 signaling pathway contributed to the genesis of neuropathic pain. Our present results displayed that SNI induced the enhancement of p-JAK2 and p-STAT3 expression levels in the DRG, which implied that JAK2/STAT3 signaling in DRG may be involved in the modulation of SNI-induced neuropathic pain. Our further investigation revealed that 2 Hz EA treatment could inhibit the phosphorylation of JAK2 and STAT3 in DRG of SNI rats, suggesting that 2 Hz EA relieved SNI-evoked neuropathic pain maybe via suppression of JAK2/STAT3 signaling in the DRG.
Neuro–immune interaction may be crucial for controlling chronic pain.39 Several inflammatory mediators participated in the neuro-immune response. Among them, the pro-inflammatory cytokines play critical role in the neuro-immune response induced by SNI.4 The release of pro-inflammatory cytokines and the loss of anti-inflammatory cytokines contributed to the process of pain sensitization.4 Indicating that an imbalance in the cytokine micro-environment mediated the modulation of neuropathic pain.3,5 Some pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α were showed to implicate in the genesis of neuropathic pain.34 Conversely, IL-10 was regarded as a powerful anti-inflammatory cytokine which exerted its anti-inflammatory effects in neuropathic pain. Such as repeated intrathecal injections of IL-10 could reverse mechanical hypersensitivity induced by neuropathic pain,40 IL-10 obviously reduced the inflammatory response via suppressing pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) at the site of nerve injury, IL-10 protein expression in DRG was significantly down-regulated in lumbar ventral root transection and CCI-induced neuropathic pain.41,42 Consistently, our present data revealed that SNI distinctly induced the up-regulation of IL-1β and IL-6 protein expression in the DRG, and along with the significant down-regulation of IL-10 level, which suggested that the imbalance between pro-inflammatory cytokines IL-1β, IL-6 and anti-inflammatory cytokine IL-10 in DRG contributed to SNI-induced neuropathic pain.
Based on the relationship between pro-inflammatory and anti-inflammatory cytokines in SNI-evoked neuropathic pain, our further study was required to elucidate the mechanism for 2 Hz EA attenuating neuropathic pain in DRG. Presently, we found that 2 Hz EA treatment markedly suppressed the up-regulation of pro-inflammatory cytokines (IL-1β and IL-6) expression and the down-regulation of anti-inflammatory cytokine (IL-10) level elicited by SNI in DRG. Thus, 2 Hz EA alleviated SNI-induced neuropathic pain maybe via re-balancing the cytokines micro-environment in the DRG.
There are some limitations in this study. First, the SNI rat model being used in this experiment elicited distinct phenotypic variations, but which did not represent all neuropathic pain conditions. Second, based on our previous studies, the activation of α7nAChR, suppression of JAK2/STAT3 signaling and re-balance in cytokines micro-environment by 2 Hz EA were taken into consideration in this study, but there was no further investigation for the link between the alterations of α7nAChR, JAK2/STAT3 and some cytokines protein expression and the analgesic effect of 2 Hz EA with inhibitors or antagonists.
Conclusion
The present study suggested that 2 Hz EA treatment activated α7nAChR, suppressed JAK2/STAT3 signaling and re-balanced the cytokines micro-environment relationship between pro-inflammatory cytokines (IL-1β and IL-6) and anti-inflammatory cytokine (IL-10) in DRG of SNI rat, which provided evidence that α7nAChR and JAK2/STAT3 signaling may be potential therapeutic targets for 2 Hz EA treatment to neuropathic pain.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (No. 31060144), and the Talent Project of Department of Scientific and Technology, Jiangxi Province, China (No. 20142BCBC22008). The authors thank Doctor Tao Gan in the Department of Biochemistry, Gannan Medical University, China, for his help in preparing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6(4):713–737. doi:10.1016/j.nurt.2009.08.002
2. Reichling DB, Green PG, Levine JD. The fundamental unit of pain is the cell. Pain. 2013;154(Suppl 1):S2–S9. doi:10.1016/j.pain.2013.05.037
3. Popiolek-Barczyk K, Mika J. Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem. 2016;23(26):2908–2928.
4. Burke NN, Geoghegan E, Kerr DM, Moriarty O, Finn DP, Roche M. Altered neuropathic pain behaviour in a rat model of depression is associated with changes in inflammatory gene expression in the amygdala. Genes Brain Behav. 2013;12(7):705–713.
5. Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169(2):386–391. doi:10.1006/exnr.2001.7677
6. Egea J, Buendia I, Parada E, Navarro E, Leon R, Lopez MG. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol. 2015;97(4):463–472. doi:10.1016/j.bcp.2015.07.032
7. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi:10.1038/nature01339
8. Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi:10.1146/annurev.pharmtox.40.1.431
9. Hs X, Qh H, Fx Z, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99(12):8360–8365. doi:10.1073/pnas.122231899
10. Freitas K, Ghosh S, Ivy CF, Lichtman AH, Imad DM. Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology. 2013;65(2):156–164. doi:10.1016/j.neuropharm.2012.08.022
11. Zhang Q, Lu Y, Bian H, Guo L, Zhu H. Activation of the alpha7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am J Transl Res. 2017;9(3):971–985.
12. Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. Faseb J. 2006;20(12):2093–2101. doi:10.1096/fj.06-6191com
13. de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–851. doi:10.1038/ni1229
14. Wang Y, Jiang Q, Xia YY, Huang ZH, Huang C. Involvement of alpha7nAChR in electroacupuncture relieving neuropathic pain in the spinal cord of rat with spared nerve injury. Brain Res Bull. 2018;137:257–264. doi:10.1016/j.brainresbull.2018.01.002
15. Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15(7–8):195–202. doi:10.2119/molmed.2009.00039
16. Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci. 2010;30(16):5754–5766. doi:10.1523/JNEUROSCI.5007-09.2010
17. Tsuda M, Kohro Y, Yano T, et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134(Pt 4):1127–1139. doi:10.1093/brain/awr025
18. Liu S, Li Q, Zhang MT, et al. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1beta via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep. 2016;6:28956. doi:10.1038/srep28956
19. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi:10.1146/annurev.neuro.051508.135531
20. Liem L, van Dongen E, Huygen FJ, Staats P, Kramer J. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med. 2016;41(4):511–519. doi:10.1097/AAP.0000000000000408
21. Sommer C. [Neuropathic pain: pathophysiology, assessment, and therapy]. Schmerz. 2013;27(6):619–632; quiz 633–614. doi:10.1007/s00482-013-1344-8
22. Zhu M, Sun X, Chen X, Xiao H, Duan M, Xu J. Impact of gabapentin on neuronal high voltage-activated Ca(2+) channel properties of injured-side axotomized and adjacent uninjured dorsal root ganglions in a rat model of spinal nerve ligation. Exp Ther Med. 2017;13(3):851–860. doi:10.3892/etm.2017.4071
23. Colvin LA, Dougherty PM. Peripheral neuropathic pain: signs, symptoms, mechanisms, and causes: are they linked? Br J Anaesth. 2015;114(3):361. doi:10.1093/bja/aeu323
24. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2009;72(14):1282–1283. doi:10.1212/01.wnl.0000346325.50431.5f
25. Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 2009;10(5):918–929. doi:10.1111/j.1526-4637.2009.00655.x
26. Gim GT, Lee JH, Park E, et al. Electroacupuncture attenuates mechanical and warm allodynia through suppression of spinal glial activation in a rat model of neuropathic pain. Brain Res Bull. 2011;86(5–6):403–411. doi:10.1016/j.brainresbull.2011.09.010
27. Yu J, Zhao C, Luo X. The effects of electroacupuncture on the extracellular signal-regulated kinase 1/2/P2X3 signal pathway in the spinal cord of rats with chronic constriction injury. Anesth Analg. 2013;116(1):239–246. doi:10.1213/ANE.0b013e31826f0a4a
28. Wan J, Ding Y, Tahir AH, et al. Electroacupuncture attenuates visceral hypersensitivity by inhibiting JAK2/STAT3 signaling pathway in the descending pain modulation system. Front Neurosci. 2017;11:644. doi:10.3389/fnins.2017.00644
29. Huang C, Li HT, Shi YS, Han JS, Wan Y. Ketamine potentiates the effect of electroacupuncture on mechanical allodynia in a rat model of neuropathic pain. Neurosci Lett. 2004;368(3):327–331. doi:10.1016/j.neulet.2004.07.073
30. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158.
31. Zeng J, Ly C, Feng Y, Ding MX. Electroacupuncture relieves neuropathic pain via upregulation of glutamate transporters in the spinal cord of rats. Neurosci Lett. 2016;620:38–42. doi:10.1016/j.neulet.2016.03.041
32. Alsharari SD, Freitas K, Damaj MI. Functional role of alpha7 nicotinic receptor in chronic neuropathic and inflammatory pain: studies in transgenic mice. Biochem Pharmacol. 2013;86(8):1201–1207. doi:10.1016/j.bcp.2013.06.018
33. Cordero-Erausquin M, Pons S, Faure P, Changeux JP. Nicotine differentially activates inhibitory and excitatory neurons in the dorsal spinal cord. Pain. 2004;109(3):308–318. doi:10.1016/j.pain.2004.01.034
34. Xu J, Chen XM, Zheng BJ, Wang XR. Electroacupuncture relieves nerve injury-induced pain hypersensitivity via the inhibition of spinal P2X7 receptor-positive microglia. Anesth Analg. 2016;122(3):882–892. doi:10.1213/ANE.0000000000001097
35. Meng X, Zhang Y, Li A, et al. The effects of opioid receptor antagonists on electroacupuncture-produced anti-allodynia/hyperalgesia in rats with paclitaxel-evoked peripheral neuropathy. Brain Res. 2011;1414:58–65. doi:10.1016/j.brainres.2011.08.004
36. Chen SP, Kan Y, Zhang JL, et al. Involvement of hippocampal acetylcholinergic receptors in electroacupuncture analgesia in neuropathic pain rats. Behav Brain Funct. 2016;12(1):13. doi:10.1186/s12993-016-0096-x
37. Wang H, Wan Y, Yao L, Han J. Comparison of the therapeutic effects of electroacupuncture with different interval for treatment of chronic neuropathic pain in rats. Acupuncture Res. 200227(2):112–118.
38. Li H, Rong T, Jing W, Xia L. Upregulation of miR-375 level ameliorates morphine analgesic tolerance in mouse dorsal root ganglia by inhibiting the JAK2/STAT3 pathway. J Pain Res. 2017;10:1279–1287. doi:10.2147/JPR.S125264
39. Ellis A, Bennett DLH. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111(1):26–37. doi:10.1093/bja/aet128
40. Milligan ED, Sloane EM, Langer SJ, et al. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126(1):294–308. doi:10.1016/j.pain.2006.07.009
41. Jančálek R, Dubový P, Svíženská I, Klusáková I. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation. 2010;7(1):1–9.
42. Ouyang H, Nie B, Wang P, et al. Ulinastatin attenuates neuropathic pain induced by L5-VRT via the calcineurin/IL-10 pathway. Mol Pain. 2016;12:1744806916646785.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.