Back to Journals » Journal of Inflammation Research » Volume 14
Electroacupuncture at ST36 Relieves Visceral Hypersensitivity via the NGF/TrkA/TRPV1 Peripheral Afferent Pathway in a Rodent Model of Post-Inflammation Rectal Hypersensitivity
Authors Chen Y , Cheng J, Zhang Y , Chen JDZ, Seralu FM
Received 3 October 2020
Accepted for publication 31 December 2020
Published 5 February 2021 Volume 2021:14 Pages 325—339
DOI https://doi.org/10.2147/JIR.S285146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yan Chen, 1, 2 Jiafei Cheng, 1 Yiling Zhang, 1 Jiande DZ Chen, 1, 3 Florin M Seralu 1
1Division of Gastroenterology and Hepatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 2Division of Gastroenterology, Binzhou Medical University Hospital, Binzhou, Shandong, People’s Republic of China; 3Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, MI, USA
Correspondence: Florin M Seralu
Division of Gastroenterology and Hepatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Tel/Fax +1 4105023147
Email [email protected]
Jiande DZ Chen
Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, MI, USA
Tel +1 7346475749
Email [email protected]
Purpose: The aim of the study was to investigate the effects of electroacupuncture (EA) at ST36 on rectal hypersensitivity and compliance in DSS-treated post-inflammation rats. In addition, we explored the involvement of mast cells-triggered NGF/TrkA/TRPV1 peripheral afferent pathway.
Methods: Rats were provided water with 5% dextran sulphate sodium (DSS) for 7 days. Two weeks after the DSS treatment they were subjected to initial and repetitive EA. Different sets of parameters were compared in the initial test and then EA with the selected parameters were performed for 2 weeks. Rectal compliance was assessed by colorectal distension while visceral sensitivity was evaluated by abdominal withdraw reflexes (AWR) and electromyogram (EMG). Masson’s trichrome staining was performed to stain collagen and toluidine blue staining was applied to assess the degranulation of mast cells. Nerve growth factor (NGF), tryptase, TrkA and TRPV1 were measured by Western blot or immunofluorescence staining.
Results: EA at 100 Hz was more effective in improving rectal compliance and visceral hypersensitivity. Daily EA improved visceral hypersensitivity but not rectal compliance. Five weeks after DSS treatment, fibrosis was noted in both sham-EA and EA groups. The expression and activation of mast cells were significantly reduced after the 2-week EA treatment with a concurrent decrease in the expression of colonic NGF/TrkA and TRPV1 in both colon and dorsal root ganglions.
Conclusion: EA at ST36 with a special set of parameters has no effect on reduced rectal compliance but relieves visceral hypersensitivity via the mast cells-triggered NGF/TrkA/TRPV1 peripheral afferent pathway in DSS-treated post-inflammation rats.
Keywords: electroacupuncture, inflammation remission, ulcerative colitis, visceral hypersensitivity, mast cells
Erratum for this paper has been published
Introduction
Patients with inflammatory bowel disease (IBD) display symptoms of fecal urgency, fecal incontinence and pain, typically associated with active mucosal inflammation and flares. However, both research and clinical experience have suggested that some of these symptoms can continue after inflammation has subsided.1 Subsequently, these symptoms are often interpreted as insufficient response to anti-inflammatory treatments, with resulting escalation of immunosuppressive treatments. A potential explanation for the persistence of these symptoms in spite of improvement in inflammation is that these patients develop colonic motility dysfunction and visceral hypersensitivity.1 As a matter of fact, one study reported the presence of at least one functional gastrointestinal disorder in 81.9% of 361 patients with IBD.2 Of interest, functional anorectal disorders were most prevalent, affecting 53.7% of IBD patients.2 In spite of these data, little attention has been paid to treat colorectal dysmotility or visceral hypersensitivity in patients with inactive inflammation.
Current treatment options for colorectal dysmotility and rectal hypersensitivity in patients who do not have IBD include dietary modifications, medications (such as loperamide or tricyclic antidepressants) and biofeedback training.3 Unfortunately, if same symptoms occur in patients with IBD, they are typically thought to be attributed to inflammation and therefore, adjustment is made in the anti-inflammation therapy rather than in treating colorectal dysmotility or visceral hypersensitivity. Accordingly, there is a need to appropriately treat colorectal dysmotility and visceral hypersensitivity in IBD patients in remission.
Acupuncture is a common treatment in traditional Chinese medicine and performed via thin needles inserted into acupoints. Electroacupuncture (EA) is a modification of traditional acupuncture by replacing manual manipulation with electrical stimulation. The acupoint, Zusanli or ST36, has been consistently shown to be an effective acupoint for regulating gastrointestinal motility, protecting gastric mucosa, and promoting glandular secretion.4 EA at ST36 was reported to improve visceral hypersensitivity in a rodent model of chronic visceral hypersensitivity.5 Furthermore, EA was also shown to suppress colonic inflammation by inhibiting pro-inflammatory cytokines via the autonomic mechanism.6 Based on these previous findings, we hypothesized that EA might be effective in improving post-inflammation rectal hypersensitivity and rectal compliance.
Visceral hypersensitivity was reported to be triggered by mast cell degranulation that activated the nerve growth factor (NGF)/tropomyosin receptor kinase (Trk) A/transient receptor ion channel 1 (TRPV1) pathway.7,8 Several mediators, including tryptase, serotonin and NGF, were released by mast cell degranulation in the mucosa of the gut. Numerous studies have revealed that the NGF binding to its high-affinity receptor, TrkA, leads to peripheral sensitization and visceral hypersensitivity.7 TRPV1 expressed in peripheral sensory neurons was reported to be activated by NGF to induce visceral hypersensitivity depending on mucosal mast cell-to-nociceptor signaling.8 It is however unknown whether the hypothesized suppressive effect of EA on visceral hypersensitivity is also mediated via the mast cell-triggered NGF/TrkA/TRPV1 peripheral afferent pathway.
Accordingly, the aims of this study were 1) to derive an effective stimulation method for EA to treat rectal hypersensitivity and compliance, 2) to investigate the chronic effect of daily EA on rectal hypersensitivity and compliance and 3) to explore the NGF/TrkA/TRPV1 peripheral afferent pathway involved in the possible ameliorating effect of EA in a rodent model of post-inflammation rectal hypersensitivity.
Materials and Methods
Animal Experiments
Animal Preparation
Thirty-two male Sprague Dawley rats (160–200 g, 6–8 week) were bought from Charles River Laboratories (MD, USA) and housed under constant laboratory conditions (22±1°C, 12/12 h light-dark cycle). After one week of acclimation, the rats were randomly divided into four groups: normal group (n=6, no treatment at all), model group (n=10, treated with DSS only), sham-EA group (n=8, treated with DSS and sham-EA) and EA group (n=8, treated with DSS and EA). The study was conducted in accordance with the recommendations given by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of the Johns Hopkins University (RA17M292).
Model of Colonic Inflammation
The rats in the model, sham-EA and EA group were provided drinking water with 5% dextran sulphate sodium (DSS, molecular weight 40 kD, Alfa Aesar, CA) for 7 consecutive days to induce intestinal inflammation. The rats in the normal group were given normal drinking water. Seven days later, all rats were provided normal drinking water without DSS.
Surgery for Electrode Implantation
The rat was anesthetized with isoflurane inhalation (1–1.5%, Abbott Laboratories, North Chicago, IL, USA). For the implantation of stimulation electrodes, a surgical incision was made on the right leg below the knee in the sham-EA and EA groups. One stainless steel cardiac pacing wire (A&E Medical, Farmingdale, NJ) was inserted at acupoint ST36 and fixed with sutures and another same electrode wire was inserted into the muscular layer 5 mm vertically below ST36.9 The electrode connecting wires were tunneled underneath the skin and externalized at the back of the neck. For the recording of electromyogram (EMG), a 2 cm incision was made in the right upper abdomen and another pair of cardiac pacing wires was sutured in the external oblique muscles of the abdomen.10 Similarly, the other ends of the electrode wires were subcutaneously tunneled and externalized at the back of the neck. Afterwards, the rat was put into a cage individually to avoid the damage of the wires (possibly chewing off by other rats) and allowed to recover for 7 days before any experiment began.
Experimental Protocols of EA
Before the initiation of the experiment, the rat was placed in a transparent restrainer for 1 h daily for 3 days to eliminate the influence of potential stress. The experimental flow chart is shown in Figure 1. The animals were subjected to an initial EA for a comparison among three sets of stimulation parameters and a repetitive daily EA lasing 2 weeks for studying its effects on visceral hypersensitivity and rectal compliance.
Protocol of Initial EA
The initial EA was performed in the 16 rats with chronically implanted electrodes at ST36 and treated with DSS for one week. Each of three sets of parameters was tested in 8 rats (randomly selected from the 16 rats) for the evaluation of the effects of acute EA on rectal compliance and rectal hypersensitivity. Since the rats treated with DSS might have impaired anorectal motility, visceral hypersensitivity and low-grade inflammation, the following three sets of parameters previously reported to improve several hypersensitivity, gastrointestinal motility and intestinal inflammation, respectively, were tested: 1) Parameter 1 (P1: 100 Hz, 0.1 s-on, 0.4 s-off, 0.5 ms and 0.5 mA), previously used to inhibit visceral pain in a rodent model of gastric hyperalgesia;11 2) Parameter 2 (P2: 25 Hz, 2 s-on, 3 s-off, 0.5 ms and 0.5 mA), previously used to improve gastric motility in rats, dogs and humans;12–14 3) Parameter 3 (P3: 5 Hz, 10 s-on, 90 s-off, 0.5 ms and 0.5 mA), previously shown to improve intestinal inflammation in rats.15 In each testing session, the abdominal EMG responses to CRD were recorded at baseline in the fasting state and after 30 min of EA with one set of parameters. EA was performed via the externalized stimulation electrode wires at ST36 by an external multi-channel electrical stimulator (Digital Stimulator DS8000, World Precision Instruments, USA).
Similarly, each of the three sets of parameters was tested in 8 rats of the 16 rats (randomly selected). Rectal compliance was measured at baseline without EA and during EA with one set of parameters (see below for the method of rectal compliance test).
Protocol of Repetitive EA
Based on the results of the initial EA, the set of parameters that was most effective in improving rectal hypersensitivity and rectal compliance was selected for the following repetitive EA. The 16 rats treated with DSS and with chronic stimulation electrodes were randomly divided into two groups (8 in each group): EA and sham-EA. The rats in the EA group were treated with EA one hour daily for a period of 2 weeks starting on D28, whereas, the rats in the sham-EA group were treated with sham-EA (stimulation electrodes connected to the stimulator but the output current was set at 0 mA).
Measurements
Measurement and Analysis of Rectal Compliance
Rectal compliance was reflected by the relationship between the pressure and volume during isobaric phasic distention of an intrarectal balloon. The balloon (polyethylene, max volume of 10 mL when fully inflated, diameter 2 cm) was lubricated with glycerin enema and inserted into the colorectum 5 cm from the anus without air under anesthesia with isoflurane inhalation (1–1.5%, Abbott Laboratories, North Chicago, IL, USA). The distention of the intrarectal balloon was accomplished by an external barostat device (Distender IIR, G&J Electronics Inc, Toronto, Ontario, Canada). The rectum was distended via the balloon from a pressure of 0 to 20 mmHg with a stepwise increment of 2 mmHg.16 Each distention was kept for 20 s with 1 min intervals. The slop of the pressure-volume curve was used to represent rectal compliance.
Measurement of Abdominal Withdrawal Reflex (AWR) and EMG
The assessment of AWR was adopted to reflect the degree of visceral discomfort/pain in response to colorectal distention (CRD) at different pressures. The AWR was scored as: 0: normal behavior without response; 1: slight head movement; 2: contraction of abdominal muscles; 3: lifting of abdominal wall; and 4: body arching and lifting of pelvic structures.17
The EMG of the external oblique muscle was recorded in response to CRD at different pressures (20, 30, 40, 50 and 60 mmHg). These distention pressures were applied sequentially and each distention maintained for 20s with a 4-min interval between two consecutive distention pressures. An EMG amplifier (EMG 100C; Biopac systems, Inc, Santa Barbara, CA, USA) was used to record the EMG signal with a sampling frequency of 5000 Hz. The area under the curve (AUC) of the EMG was calculated by special software (Acknowledge; Biopac System, Inc., Santa Barbara CA). The EMG response to rectal distention was assessed by the AUC of the EMG during the 20s distention divided by the AUC of EMG during the 20s baseline recording before each distention.18
Evaluation of Inflammation
The disease activity index (DAI) was employed to assess the inflammation in the process and evaluated for body weight, stool consistency, and the presence of gross blood in feces according to a previous study.19 The score was the sum of the three: (1) weight: 0, no loss; 1, 5–10% weight loss; 2, 10–15% weight loss; 3, 15–20% weight loss; 4, 20% weight loss; (2) stool: 0, normal; 2, loose stool; 4, diarrhea; (3) bleeding: 0, no blood; 2, presence; 4, gross blood.
At the completion of the study, the rat was anesthetized with isoflurane inhalation (1–1.5%, Abbott Laboratories, North Chicago, IL, USA) and the blood sample was obtained from the aorta ventralis and placed in a test tube containing EDTA. After the collection, the blood sample was centrifuged at 3000 rpm for 15 min to extract and store the plasma. The plasma level of TNF-α was assessed by an ELISA kit (Abcam, Cambridge, UK) according to the manufacturer’s protocol.
At the end of the study, the distal colon was isolated from the anus to a third of the entire colon length, and cut opened longitudinally and flattened on a white board. The colon was carefully checked and evaluated by two independent observers. The colonic mucosal damage index (CMDI) was scored according to the previous study20 as follows: (1) ulcer and inflammation: 0, normal; 1, focal congestion, no ulcer; 2, ulcer without congestion or thickening of the intestinal wall; 3, one inflammatory ulcer; 4, two ulcers and inflammatory sites; 5, the main part of the damage along the colon extension ≥1 cm; 6–10, injury along the length of the colon extended ≥2 cm; for each increase of 1 cm damage, the score increased by 1 point; (2) adhesion: 0, normal; 1, slight adhesion; 2, the main adhesion.
The distal colon tissue fixed in paraformaldehyde was dehydrated using graded ethanol and embedded in paraffin wax blocks, and slices were made to stain with hematoxylin and eosin. The histological scores were evaluated by architectural derangements, inflammatory cell infiltration, goblet cell depletion and ulceration.20
Masson’s Trichrome Staining for Fibrosis
At the completion of the experiment, distal colon tissues were harvested for Masson’s trichrome staining. The staining was performed using a special commercial kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions. The Image J software (National Institutes of Health, Bethesda, MD) was used to calculate the semi-quantitative expression of the collagen which was displayed in blue.
Toluidine Blue Staining of Mast Cells
To differentiate mast cells from other inflammatory cells and further to identify the degranulation of mast cells, toluidine blue staining was employed in harvested distal colon tissues. After deparaffinized and rehydrated, the distal colon sections were stained with 0.5% toluidine blue (Sigma-Aldrich, Saint Louis, MO). As a result, mast cells were revealed in violet/red purple with blue background, and the degranulation of mast cells was assessed based on extruded granules.
Immune Staining for the Assessment of Tryptase, TrkA and TRPV1
For the immunofluorescence staining of the distal colon and dorsal root ganglions (DRG, from right S2 to S4), deparaffinized and hydrated sections were incubated with normal serum to block the non-specific reaction and then added with a primary antibody of tryptase (1:100; ab2378, Abcam, Cambridge, UK), PGP9.5 (1:100; ab109261, Abcam, Cambridge, UK), TrkA (1:100; cat 06–574, Sigma-Aldrich, Saint Louis, MO), TRPV1 (1:100; ab6166, Abcam, Cambridge, UK) overnight for 4°C. Secondary antibodies were incubated for 1 h at room temperature and DAPI for 5 min. The sections were sealed with anti-fade mounting medium and then observed under an Olympus fluorescence microscope.
Quantifications of tryptase, TrkA and TRPV1 were performed by the Image J software (National Institutes of Health, Bethesda, MD). Immunopositive areas of tryptase or TrkA in the distal colon and TRPV1 in the S2-S4 DRGs, and double-labeled neurons (TRPV1 and PGP9.5) in the distal colon were measured to compare the difference among the groups. Each slide contained 2 to 4 nonconsecutive sections and random microfields were taken from each slide. The average value per each animal was used for statistical analysis.
Western Blot for NGF and TrkA
Frozen distal colon tissues were grinded with the RIPA Buffer and protease inhibitor. After centrifuged, the supernatant was taken for the assessment of total protein. The protein concentration was measured through the Bradford protein assay kit. The whole protein (20 μg) was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). After blocked in 5% skim milk, the membranes were incubated by the primary anti-NGF antibodies (1:1000; ab6199, Abcam, Cambridge, UK), anti-TrkA (1:1000; cat 06–574, Sigma-Aldrich, Saint Louis, MO) or mouse anti-β-actin (1:20,000; Abcam, Cambridge, UK) and then incubated with HRP-linked secondary antibody, the immunoreactive bands were displayed by enhanced chemiluminescence (ECL). Finally, the blotting images were analyzed by Image J (National Institutes of Health, Bethesda, MD).
Statistical Analyses
All data are presented as mean±SEM. Student’s t-test was used to compare the mean between two groups. One-way analysis of variance (ANOVA) was used to determine the difference among three or more groups. LSD test or Dunnett’s T3 test was applied for multiple comparisons on the basis of normality and homogeneity of variance. Statistical analyses were performed using SPSS 24.0 (Chicago, IL, USA). P<0.05 was regarded as statistically significant.
Results
Effects of EA with Different Parameters on Rectal Compliance and Hypersensitivity
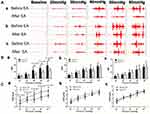
Rectal compliance was significantly increased by EA with parameter P1 (0.145±0.009 vs 0.126±0.011, P=0.002) and parameter P2 (0.135±0.009 vs 0.125±0.005, P=0.019) but not parameter P3 (0.133±0.009 vs 0.136±0.014, P=0.534).
Rectal hypersensitivity was reduced with EA assessed by both abdominal EMG and AWR (Figure 2). The AUC of the EMG was remarkably decreased by EA with parameter P1 (40 mmHg: 12.016±4.866, P=0.015; 50 mmHg: 15.336±4.226, P=0.008; 60 mmHg: 19.230±6.407, P=0.022) but not with parameter P2 or P3 (all distention pressure: P>0.05). Similarly, the AWR score was also decreased by EA with parameter P1 at all pressures (from 20 mmHg to 60 mmHg: P<0.023) but not with parameter P2 or P3 at any pressures (all P>0.05).
Parameter P1 was chosen for the subsequent repetitive EA treatment since it was effective in improving both rectal compliance and rectal hypersensitivity.
Effects of Repetitive EA on Rectal Compliance and Hypersensitivity
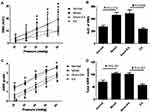
As expected, rectal compliance was significantly decreased from 0.157±0.008 in the normal group (no DSS treatment) to 0.130±0.003 in the model group (DSS treated but without EA or sham-EA (P=0.002)). Rectal compliance in the EA and sham-EA groups was also significantly lower than the normal group (P=0.014 and P=0.002, vs the normal group). At the end of the 2-week treatment, no statistical difference was noted in rectal compliance between the EA group and the sham-EA group (EA: 0.139±0.005 vs sham-EA: 0.136±0.012, P=0.461), suggesting that the ameliorating effect of acute EA on rectal compliance was not sustainable.
Rectal hypersensitivity was noted in the post-inflammation period but normalized after 2 weeks of daily EA. As shown in Figure 3A, at the end of the study (Day 42 or 35 days after the DSS treatment), the EMG response to CRD was increased in the model group in comparison with that in the normal group (from distention pressure of 40 mmHg to 60 mmHg, all P<0.011). The 2-week treatment of EA down-regulated the EMG response to CRD, compared with the sham-EA (from 40 mmHg to 60 mmHg: P<0.002). As displayed in Figure 3B, the total AUC of the EMG under all distention pressures was markedly increased in the model group in comparison with that in the normal group (46.07±4.56 vs 28.25±3.18, P=0.018) but reduced in the TEA group to a level that was comparable to that in the normal group (P>0.05, EA vs Normal) and significantly lower than that in the sham-EA group (P=0.002, EA vs sham-EA).
Similar findings were noted in the AWR score. The AWR score was increased from CRD pressure of 20 mmHg to 60 mmHg in the model group (vs normal rats, all P<0.011, Figure 3C). The total AWR score under all distention pressures was also increased in the model group in comparison with the normal rats but reduced in the EA group to a level that was comparable to the normal group (Figure 3D).
Effects of Repetitive EA on Inflammation
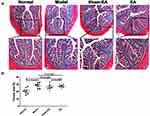
At the end of the study (35 days after the termination of 7-day DSS treatment), inflammation was spontaneously recovered. According to the macroscopic assessment, no obvious edema, hyperemia, mucosal erosions or ulcers were observed on the surface of the distal colon in any of the groups. There was no significant difference in the CMDI score between the normal group and the model group (DSS treated) (0.25±0.16 vs 0.50±0.19, P=0.322) or between the sham-EA group and the EA group (0.38±0.18 vs 0.25±0.16, P=0.618). Histopathological assessment showed that there was no distinct inflammatory infiltration, ulceration, or goblet cell depletion in any of the groups. The histological scores were not different between the normal group and the model group (0.38±0.18 vs 0.63±0.18, P=0.346) or between the sham-EA group and the EA group (0.50±0.19 vs 0.38±0.18, P=0.636). Similarly, there was no significant difference in the DAI score or the levels of plasma TNF-α between the normal group and the model group (DAI: 0.50±0.13 vs 1.00±0.22, P=0.071; TNF-α: 149.26±16.26 vs 243.60±45.48, P=0.142) or between the sham-EA group and the EA group (DAI: 0.88±0.30 vs 0.88±0.30, P=1; TNF-α: 197.2±37.4 pg/mL vs 234.4±57.2 pg/mL, P=0.527).
Increased Fibrosis/Collagen Fiber Deposition Post-Inflammation
As shown in Figure 4, the expression of collagen fiber in the model group was remarkably increased in comparison with the normal group (P=0.003). Neither sham-EA nor EA was able to decrease the expression of the collagen fiber; an increase in the collagen fiber was also noted in the EA group at the end of the study, suggesting that fibrosis was developed in the distal colon after the recovery of DSS-induced inflammation, leading to a reduced rectal compliance.
Mechanism of Repetitive EA on Visceral Hypersensitivity
Effects of EA on Mast Cells
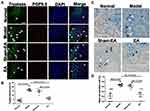
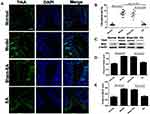
The number of mast cells was elevated in the DSS-treated rats and EA reduced the DSS-induced increase in mast cells in the post-inflammation stage. Figure 5A shows the co-localization of mast cells (tryptase+ cells) and enteric nerve endings (PGP9.5+ cells). In the model group, ample tryptase+ cells were present in the mucosa compared with the normal group. Additionally, the connections between mast cells and nerve endings were closer. Concurrently, there were increased tryptase+ cells in the sham-EA group, closely associated with nerve fibers. However, the number of mast cells in EA group was decreased compared with the model group and the sham-EA group. In Figure 5B, the tryptase positive areas were significantly increased in the model and sham-EA groups (16.83±0.95% and 14.83±1.19%), while it was reduced in EA group compared with the sham-EA group (6.33±0.67% vs 14.83±1.19%, P<0.001).
The activated mast cells presented with granule compounds in the surroundings (Figure 5C). A mast cell was recognized by dark violet stained in the mucosa of the normal group. There were ample degranulated mast cells noted in the model group and the sham-EA group with many granules scattered around the mast cells. However, few of mast cells were found in a blue background in the distal colon tissue in the EA group. As shown in Figure 5D, the mast cell activation rate was increased in the model group compared with the normal group (P=0.027), but it was lower in the EA group than the sham-EA group (P=0.049).
Effects of EA on TrkA and NGF
The expressions of TrkA and NGF proteins in the distal colon are shown in Figure 6. The TrkA immunoreactivity was observed mainly in the mucosa and abundant of TrkA+ cells were found in the model and sham-EA groups; the number of the TrkA+ cells was dramatically reduced in the EA group (Figure 6A and B). As shown in Figure 6C, the protein expressions of TrkA and NGF in the model group were substantially increased in comparison with the normal group (both P<0.001). EA but not sham-EA down-regulated the protein expressions of TrkA and NGF (both P<0.001).
Effects of EA on the Expression of Sensory Neurons in the Colon
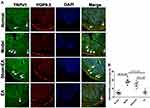
TRPV1 and PGP9.5 were double-labeled to accurately manifest the position of sensory neurons and nerve fibers in the distal colon of DSS-induced colitis rats (Figure 7). TRPV1+ and PGP9.5+ neurons were primarily distributed in the submucosal layer and myenteric plexus of the wall of the colon. TRPV1+ and PGP9.5+ nerve fibers were detected in each layer of the colon, including the mucosal, submucosal, muscle layer and myenteric plexus. The expressions of TRPV1+/PGP9.5+ neurons/nerve fibers were increased in the model and sham-EA groups (18.33±1.38% and 14.83±1.66%), but decreased to 5.67±1.45% in the EA group (P<0.001, vs sham-EA).
Effects of EA on the Expression of TRPV1 in the DRGs
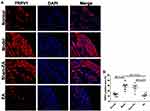
As shown in Figure 8, a large amount of TRPV1+ ganglion cells were recognized in the model group in comparison with the normal group (29.33±3.03% vs 13.17±1.38%, P<0.001). However, compared with the sham-EA group, EA reduced the number of TRPV1+ cells in the DRGs (11.33±1.76% vs 27.00±3.37%, P<0.001).
Discussion
The present study revealed that after the recovery of inflammation in an acute colitis, rectal compliance and visceral sensitivity remained abnormal. We found that EA at a pulse frequency of 100 Hz and a pulse train frequency of 20 Hz was effective in improving both rectal compliance and rectal hypersensitivity. Further, repetitive EA with this special set of parameters was found to relieve visceral hypersensitivity but not rectal compliance. The mechanism of reduced rectal compliance was probably attributed to the formation of colon fibrosis but repetitive EA was ineffective in treating colon fibrosis. Finally, the EA-induced ameliorating effect on post-inflammation rectal hypersensitivity was found to be attributed to the down-regulation of mast cells, NGF/TrkA and TRPV1 in the distal colon and S2-S4 DRGs.
DSS-induced intestinal inflammatory is a commonly used rodent model for studying the clinical and histological features of IBD, especially ulcerative colitis.7 According to our previous study, it took about two to three weeks for the inflammation to fully recover after a 7-day DSS treatment. Others also reported that deoxycholic acid-induced mild, transient colonic inflammation was resolved within 3 weeks.21 Though symptoms of abdominal pain, rectal urgency, diarrhea and tenesmus in IBD patients are thought to be attributed to inflammation, patients in remission are still agitated by these symptoms.1 Accordingly, treatment of post-inflammation symptoms in IBD patients is of great clinical significance.
Rectal compliance and colorectal sensation play an important role in the pathophysiological mechanisms of bowel dysfunctions.22 Symptoms of fecal urgency and frequent defecation suggest reduced rectal compliance and colorectal hypersensitivity among other things.23 Rectal compliance reflects the viscoelastic properties of the rectal wall in the form of the pressure-volume relationship through the isobaric phasic distention of the rectum; colorectal sensitivity in rats is typically assessed by abdominal EMG responses to CRD. In this study, we found that acute EA with a set of parameters previously reported to improve gastric hypersensitivity in rats11,24,25 was effective in improving both rectal hypersensitivity and rectal compliance. The repetitive EA over a period of 2 weeks was, however, effective in treating only rectal hypersensitivity but not rectal compliance. To the best of our knowledge, this was the first study investigating the effects of EA on rectal compliance and hypersensitivity in DSS-treated inflammation in remission in rats.
According to the results of DAI, level of TNF-α, CMDI score and histological score, no inflammation was observed 35 days after the DSS treatment in any of the groups. Our findings suggested that the DSS-induced inflammation was recovered in symptoms, levels of inflammatory cytokines in the blood and pathological assessment of the colon tissue 5 weeks after the 7-day DSS treatment. However, the rectal compliance was still reduced after the complete recovery of inflammation and collagen fibers were significantly increased based on the Masson’s trichrome staining. It was reported that fibrosis was also one of common complications in IBD and fibrosis resulted in wall stiffness and further affected colonic motility and absorbent functions, causing symptoms of abdominal discomfort, rectal urgency, diarrhea, and incontinence.26,27 It was reported that the mucosal layer became thickened and excessive extracellular matrix components deposited in the submucosal layer, leading to an obvious alteration under the mucosal surface with absence of macroscopic and microscopic inflammation.28,29 A previous study showed that EA at ST36 with 0.4 mA, 2 Hz, 15 min every other day for 28 days could inhibit excessive fibrosis by improving blood flow and antioxidant capacities after contusion in rabbits.30 However, in our study, EA at ST36 with parameter P1 for 2 weeks did not improve the fibrosis in the colon. The discrepancy could be attributed to differences in species and EA methodologies.
The number of mast cells was increased in the post-inflammation period in the DSS-treated rats and EA was able to effectively suppress such an increase. The mast cell, containing abundant granules, is a pivotal cell that participated in the visceral hypersensitivity of gastrointestinal tract. Mast cells in the mucosa, located close to sensory nerve endings, degranulate to release mediators, such as histamine, tryptase and serotonin, which could activate and increase the expression of TRPV1 on visceral afferent neurons.31 Previous studies indicated that the mast cells in the gut wall were activated and associated with visceral hypersensitivity in patients with irritable bowel syndrome (IBS).32 Mast cells were reported increased in the colon in patients with ulcerative colitis.33 In patients with ulcerative colitis in remission, IBS-like symptoms were reported to be associated with rectal hypersensitivity, attributing to the mucosal presence and activation of mast cells.34 These findings were consistent with the observed visceral hypersensitivity and increased number of mast cells in the post-inflammation period in the DSS-treated rats in the present study. EA was previously reported to alleviate visceral hypersensitivity by reducing the number of mast cells and inhibiting the activation of mast cells in colon-sensitized rats,35 which was in agreement with the findings in our study.
NGF was elevated in the post-inflammation period in the DSS-treated rats and substantially suppressed after the 2-week EA treatment. NGF is an important neurotrophic factor, secreted and used by various kinds of cells such as mast cells, epithelial cells, fibroblasts, smooth muscle cells, glial cells and lymphocytes, participating in promoting axon growth, proliferation and survival of sensory neurons.36 The activation of NGF/TrkA pathway was previously reported in patients with IBD.37 In the present study, the expressions of NGF and TrkA in the DSS-treated rats in remission were found to be increased. Nori et al reported hyperalgesia and increased NGF in diabetic rats and inhibitory effects of EA on NGF and NGF receptors (TrkA and p75NTR) as well TRPVl.38 Similarly, we also found that EA decreased the expressions of NGF and TrkA in the colon of DSS-treated rats in remission. These findings support a potential therapeutic role of EA for visceral hypersensitivity in different diseases.
EA was capable of suppressing DSS-induced increase in TRPV1 in both colon tissues and S2-S4 DRGs. The TRPV1 is expressed in sensory nerve fibers and mainly distributed in the submucosal nerve plexus and myenteric nerve plexus of the gastrointestinal tract.39 A nearly 3.9-fold increase in TRPV1-immunoreactive fibers was reported in the rectosigmoid biopsies in patients with quiescent IBD but IBS symptoms.39 In the present study, the expression of TRPV1 was increased in the distal colon of the DSS-treated rats in remission. EA at ST36 and ST37 for 15 min was reported to significantly attenuate colorectal hypersensitivity and TRPV1 in a zymosan-induced IBS mice model.40 A randomized controlled clinical study showed that acupuncture at ST36 together with other acupoints alleviated visceral hypersensitivity by suppressing the expression of colonic TRPV1 in patients with IBS.41
The DRG is an aggregation of the cell bodies of primary sensory neurons, which transmits sensory information from the peripheral nerve to the spinal cord. In the present study, we found that the expression of TRPV1 was increased in the DRG of the DSS-treated rats and reduced after the 2-week treatment of EA at ST36. An increased expression of TRPV1 in the DRG was reported in rats with chronic inflammation.42 Furthermore, EA at ST36 was shown to reverse hyperalgesia in mice and inhibit the expression of TRPV1.43 Our findings, together with these previous findings suggest a sensory afferent pathway involved in the ameliorating effect of EA.
The findings of the present study have the following clinical implications: in addition to persistent rectal hypersensitivity, rectal compliance is also impaired in post-inflammation stage in DSS-treated rats. The post-inflammation rectal hypersensitivity and reduced rectal compliance observed in this animal study might partially explain symptoms of pain, fecal urgency and incontinence observed in IBD patients in remission; the noninvasive method of EA at ST36 with the special set of parameters might be used to treat visceral pain in IBD patients in remission.
Conclusions
Reduced rectal compliance and visceral hypersensitivity are present in the post-inflammation state in rats with DSS-induced colitis. EA at ST36 with 100 Hz, 0.1 s-on, 0.4 s-off, 0.5 ms and 0.5 mA reduces visceral hypersensitivity possibly via the mast cells-triggered NGF/TrkA/TRPV1 peripheral afferent pathway. These strategies would complement anti-inflammatory treatments routinely used in IBD patients. A potential benefit would be in correctly treating motility/hypersensitivity pathology in patients who are in remission from an inflammatory perspective but continue to have symptoms. In addition, it is likely that motility/hypersensitivity alterations contribute to symptoms even in patients who have ongoing rectal inflammation and the correct identification of percent contribution of etiologies (hypersensitivity vs inflammation) would allow the personalized and granular treatment of these patients. Clinical studies are warranted to investigate therapeutic potential of EA in treating visceral pain in patients with IBD.
Acknowledgments
We thank Dr. Ling Li for her assistance with the samples and ordering some reagents.
Disclosure
None of the authors had any conflicts of interest, financial or otherwise.
References
1. Casanova MJ, Santander C, Gisbert JP. Rectal hypersensitivity in patients with quiescent ulcerative colitis. J Crohns Colitis. 2015;9(7):592. doi:10.1093/ecco-jcc/jjv070
2. Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis. 2006;12(1):38–46. doi:10.1097/01.MIB.0000195391.49762.89
3. Wang JY, Abbas MA. Current management of fecal incontinence. Perm J. 2013;17(3):65–73. doi:10.7812/TPP/12-064
4. Li H, He T, Xu Q, et al. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol. 2015;21:8304–8313.
5. Chu D, Cheng P, Xiong H, Zhang J, Liu S, Hou X. Electroacupuncture at ST-36 relieves visceral hypersensitivity and decreases 5-HT(3) receptor level in the colon in chronic visceral hypersensitivity rats. Int J Colorectal Dis. 2011;26:569–574. doi:10.1007/s00384-010-1087-2
6. Jin H, Guo J, Liu J, et al. Autonomically mediated anti-inflammatory effects of electrical stimulation at acupoints in a rodent model of colonic inflammation. Neurogastroenterol Motil. 2019;31(8):e13615. doi:10.1111/nmo.13615
7. Eskander MA, Ruparel S, Green DP, et al. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci. 2015;35(22):8593–8603. doi:10.1523/JNEUROSCI.3993-14.2015
8. Li WT, Luo QQ, Wang B, et al. Bile acids induce visceral hypersensitivity via mucosal mast cell-to-nociceptor signaling that involves the farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 axis. FASEB J. 2019;33(2):2435–2450. doi:10.1096/fj.201800935RR
9. Martin JC, Bériou G, Josien R. Dextran sulfate sodium (DSS)-induced acute colitis in the rat. Methods Mol Biol. 2016;1371:197–203. doi:10.1007/978-1-4939-3139-2_12
10. Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132(2):615–627. doi:10.1053/j.gastro.2006.11.014
11. Sun Y, Tan Y, Song G, Chen JD. Effects and mechanisms of gastric electrical stimulation on visceral pain in a rodent model of gastric hyperalgesia secondary to chemically induced mucosal ulceration. Neurogastroenterol Motil. 2014;26(2):176–186. doi:10.1111/nmo.12248
12. Murakami H, Li S, Foreman R, Yin J, Hirai T, Chen JDZ. Ameliorating effects of electroacupuncture on dysmotility, inflammation, and pain mediated via the autonomic mechanism in a rat model of postoperative ileus. J Neurogastroenterol Motil. 2019;25(2):286–299. doi:10.5056/jnm18094
13. Tu L, Gharibani P, Yang Y, et al. A novel approach in spinal cord stimulation for enhancing gastric motility: a preliminary study on canines. J Neurogastroenterol Motil. 2020;26(1):147–159. doi:10.5056/jnm19101
14. Zhang B, Xu F, Hu P, et al. Needleless transcutaneous electrical acustimulation: a pilot study evaluating improvement in post-operative recovery. Am J Gastroenterol. 2018;113(7):1026–1035. doi:10.1038/s41395-018-0156-y
15. Jin H, Guo J, Liu J, et al. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2017;313(3):G192–G202. doi:10.1152/ajpgi.00254.2016
16. Ravnefjord A, Brusberg M, Larsson H, Lindström E, Martínez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br J Pharmacol. 2008;155(3):407–416. doi:10.1038/bjp.2008.259
17. Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi:10.1053/gast.2000.19576
18. Jiang L, Zhang N, Zhang S, Chen JD. Sacral nerve stimulation with optimized parameters improves visceral hypersensitivity in rats mediated via the autonomic pathway. Mol Pain. 2019;15:1744806919880651. doi:10.1177/1744806919880651
19. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249.
20. Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131(4):1122–1130. doi:10.1053/j.gastro.2006.08.016
21. Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology. 2008;135(6):2075–2083. doi:10.1053/j.gastro.2008.08.051
22. Salvioli B, Bharucha AE, Rath-Harvey D, Pemberton JH, Phillips SF. Rectal compliance, capacity, and rectoanal sensation in fecal incontinence. Am J Gastroenterol. 2001;96(7):2158–2168. doi:10.1111/j.1572-0241.2001.03954.x
23. Carrington EV, Scott SM, Bharucha A, et al. Expert consensus document: advances in the evaluation of anorectal function. Nat Rev Gastroenterol Hepatol. 2018;15(5):309–323. doi:10.1038/nrgastro.2018.27
24. Zhou J, Li S, Wang Y, et al. Inhibitory effects and mechanisms of electroacupuncture via chronically implanted electrodes on stress-induced gastric hypersensitivity in rats with neonatal treatment of iodoacetamide. Neuromodulation. 2017;20(8):767–773. doi:10.1111/ner.12602
25. Zhou J, Li S, Wang Y, et al. Effects and mechanisms of auricular electroacupuncture on gastric hypersensitivity in a rodent model of functional dyspepsia. PLoS One. 2017;12(3):e0174568. doi:10.1371/journal.pone.0174568
26. Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20(11):2198–2206. doi:10.1097/MIB.0000000000000080
27. Gordon IO, Agrawal N, Willis E, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47(7):922–939. doi:10.1111/apt.14526
28. Latella G, Rieder F. Time to look underneath the surface: ulcerative colitis-associated fibrosis. J Crohns Colitis. 2015;9(11):941–942. doi:10.1093/ecco-jcc/jjv142
29. Ippolito C, Colucci R, Segnani C, et al. Fibrotic and vascular remodelling of colonic wall in patients with active ulcerative colitis. J Crohns Colitis. 2016;10(10):1194–1204. doi:10.1093/ecco-jcc/jjw076
30. Wang R, Luo D, Xiao C, et al. The time course effects of electroacupuncture on promoting skeletal muscle regeneration and inhibiting excessive fibrosis after contusion in rabbits. Evid Based Complement Alternat Med. 2013;2013:869398.
31. Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65(1):155–168. doi:10.1136/gutjnl-2015-309151
32. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi:10.1053/j.gastro.2003.11.055
33. Nolte H, Spjeldnaes N, Kruse A, Windelborg B. Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut. 1990;31(7):791–794. doi:10.1136/gut.31.7.791
34. van Hoboken EA, Thijssen AY, Verhaaren R, et al. Symptoms in patients with ulcerative colitis in remission are associated with visceral hypersensitivity and mast cell activity. Scand J Gastroenterol. 2011;46(7–8):981–987. doi:10.3109/00365521.2011.579156
35. Yang J, Shang B, Shi H, Zhu S, Lu G, Dai F. The role of toll-like receptor 4 and mast cell in the ameliorating effect of electroacupuncture on visceral hypersensitivity in rats. Neurogastroenterol Motil. 2019;31(6):e13583. doi:10.1111/nmo.13583
36. Micera A, Puxeddu I, Aloe L, Levi-Schaffer F. New insights on the involvement of nerve growth factor in allergic inflammation and fibrosis. Cytokine Growth Factor Rev. 2003;14(5):369–374. doi:10.1016/S1359-6101(03)00047-9
37. Di Mola FF, Friess H, Zhu ZW. Nerve growth factor and Trk high affinity receptor (TrkA) gene expression in inflammatory bowel disease. Gut. 2000;46(5):670–679. doi:10.1136/gut.46.5.670
38. Nori SL, Rocco ML, Florenzano F, Ciotti MT, Aloe L, Manni L. Increased nerve growth factor signaling in sensory neurons of early diabetic rats is corrected by electroacupuncture. Evid Based Complement Alternat Med. 2013;2013:652735. doi:10.1155/2013/652735
39. Akbar A, Yiangou Y, Facer P, et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59(6):767–774. doi:10.1136/gut.2009.194449
40. Wang SJ, Yang HY, Xu GS. Acupuncture alleviates colorectal hypersensitivity and correlates with the regulatory mechanism of TrpV1 and p-ERK. Evid Based Complement Alternat Med. 2012;2012:483123. doi:10.1155/2012/483123
41. Pei L, Chen H, Guo J, et al. Effect of acupuncture and its influence on visceral hypersensitivity in IBS-D patients: study protocol for a randomized controlled trial. Medicine (Baltimore). 2018;97(21):e10877. doi:10.1097/MD.0000000000010877
42. Yu L, Yang F, Luo H, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4:61. doi:10.1186/1744-8069-4-61
43. Liao HY, Hsieh CL, Huang CP, Lin YW. Electroacupuncture attenuates CFA-induced inflammatory pain by suppressing Nav1.8 through S100B, TRPV1, opioid, and adenosine pathways in mice. Sci Rep. 2017;7(1):42531. doi:10.1038/srep42531
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.