Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Eight-week of low-intensive lifestyle modification does improve insulin resistance in adults with metabolic syndrome
Received 17 January 2019
Accepted for publication 5 March 2019
Published 2 May 2019 Volume 2019:12 Pages 613—621
DOI https://doi.org/10.2147/DMSO.S201526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Yi-Hsin Lin,1 Hsuan Huang2
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Taiwan Adventist Hospital, Taipei, Taiwan; 2Department of Surgery, Mackay Memorial Hospital, Taipei, Taiwan
Purpose: Improvements in insulin resistance have been observed by following lifestyle modification (LM) for adults with metabolic syndrome (MetS). However, these improvements are associated with relatively intensive and long-term duration LM, which is unlikely to be a part of routine practice for most people. This study examined the impact of a short-term (eight-week) low-intensive LM program on anthropomorphic parameters and insulin resistance in a community-based population.
Patients and methods: A total of 174 adults (67 with MetS) were enrolled in this retrospective observational study. The effects of the eight-week LM program on anthropomorphic parameters and glucose homeostasis were investigated.
Results: After the LM program, most anthropomorphic parameters in both groups were significantly improved (P<0.05). Glucose homeostasis significantly was improved (P<0.001) in the MetS group. A change in the homeostasis model assessment of insulin resistance (HOMA-IR) was positively associated with the baseline HOMA-IR level (R=0.75, P<0.001).
Conclusion: A low-intensive eight-week LM program is an effective and efficient way to improve the anthropomorphic parameters and to reduce insulin resistance, especially for adults with MetS.
Keywords: lifestyle modification, insulin resistance, glucose homeostasis, metabolic syndrome
Introduction
Throughout the world, the prevalence of metabolic syndrome (MetS) is dramatically rising.1 Dr Reaven first defined the term “syndrome X” in 1988 with simultaneously grouped risk factors such as abdominal obesity, insulin resistance, elevated blood pressure (BP), and obesity-related dyslipidemia.2–4 The core defect of syndrome X, later termed MetS, is insulin resistance, which is highly correlated with type 2 diabetes and atherosclerotic cardiovascular disease.5 The benefits of exercise and/or caloric restriction to improve insulin resistance and glucose homeostasis are well established for adults who are overweight and obese.6–8
To address the worldwide trend of MetS, multiple studies have reported intensive lifestyle modification (LM) programs in obese patients and proved that intensive intervention positively promoted weight loss and reduction of MetS.9–12 For example, after 6 to 12 months of a diet with/without exercise intervention, the prevalence of MetS decreased between 31% and 52.4% in intervention group.9,10 However, it must be noted that the previous studies provided relatively intensive LM for participants, which was unlikely to be a part of routine practice for most people in many countries, not only in the degree of intensity but also the duration. Moreover, some studies reported that long-duration interventions tend to have high attrition rates (around 40%), and dropout rates as high as 50% for weight-control programs could be expected in community-based interventions.13,14
This study aimed to examine whether a short-term (eight-week), low-intensive LM program for a community-based population could achieve weight reduction, regression of MetS, and improvement in insulin resistance.
Patients and methods
Study participants
This was a retrospective observational study which was approved by the Institutional Review Board of Taiwan Adventist Hospital. Participants of this study included 198 volunteers who used to be with sedentary lifestyle at the NEWSTART Exercise Center of Taiwan Adventist Hospital from May 2015 to August 2017. Each of them attended an eight-week LM program, which including exercise and diet regimen. Individuals who had been treated with antihypertensive, antidiabetic, or antihyperlipidemic agents were excluded. Individuals were not enrolled if they were younger than 18 years of age, less than 150 cm in body height, and had acute illness or complications of chronic diseases (such as known heart diseases or anemia with hemoglobin <8.0 g/dL). All the exclusion criteria could affect intensity and efficiency of the exercises. Pregnant or breastfeeding women were ineligible as they could affect the changes in body weight. In addition, neither of them was a smoker, and who were not allowed to use any weight loss medications/supplements during eight-week LM programs. All volunteers had provided written consent for participating in the program. This study was conducted in accordance with the Declaration of Helsinki.
Diet and exercise program
All participants were asked to record a baseline diet and exercise log before intervention. Participants chose their own diet throughout the program, although education on the principles of a healthy diet was provided by registered dieticians every week, and participants were encouraged to decrease intake of calories.
Each participant would do exercise three times per week, every two days, at least 150 mins/week. The exercise program included jogging or rhythmic aerobic exercises set to beat music (such as SPINNING®). During each session, participants were advised to achieve approximately 70% of their maximal heartbeat rate for a minimum of 30 mins. Maximum heartbeat rate was estimated from the formula 220–age (years) with a standard deviation of 10–12 beats/min.15
Data collection
Before and after the program, trained staff would measure the participants’ height, weight, and waist circumference (WC) in light clothing without shoes and after emptying the bladder. Body mass index (BMI) was calculated as weight (in kilograms) divided by squared height (in square meters). The body fat percentage (BFP) and body muscle mass (BMM) were measured by the same bioelectrical body composition analyzer (TANITA® BC-418; TANITA Corp., Tokyo, Japan).
For pre- and post-program laboratory tests, overnight/8-hr blood samples were obtained from the antecubital vein of the arm for measurement of fasting plasma glucose (FPG), serum insulin (FSI), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid (UA), creatinine (Cr), serum glutamate pyruvate transaminase (S-GPT), and hemoglobin (Hb). Renal function was recorded as estimated glomerular filtration rate (eGFR) using the MDRD (modification of diet in renal disease) study equation.16 Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: (FSI × FPG)/405, where insulin is expressed in mIU/L and glucose in mg/dL.17
Metabolic syndrome
MetS was defined according to the National Cholesterol Education Program adult treatment panel III updated guidelines as the presence of three or more of five risk factors: (1) WC >90 cm (men) and >80 cm (women) (ethnic criteria for Asians); (2) Fasting TG  150 mg/dL; (3) HDL-C <40 mg/dL (men) and <50 mg/dL (women); (4) systolic blood pressure (SBP) ≥130 and/or diastolic blood pressure ≥85 mmHg; and (5) FPG >100 mg/dL.18,19
150 mg/dL; (3) HDL-C <40 mg/dL (men) and <50 mg/dL (women); (4) systolic blood pressure (SBP) ≥130 and/or diastolic blood pressure ≥85 mmHg; and (5) FPG >100 mg/dL.18,19
Statistical analysis
Numeric values were presented as mean±standard deviation (SD), with categorical values as n (%). The differences in clinical and laboratory characteristics between MetS and non-MetS groups were analyzed using independent t-tests or Mann-Whitney U tests for parameters withnormal or skewed distributions and analysis of variance for numeric data and Chi-square test for some categorical data. The degrees of association among independent variables for baseline HOMA-IR and the improvement of HOMA-IR, including age, gender, BMI, WC, SBP, eGFR, BFP, BMM, TC, TG, LDL-C, HDL-C, UA, and S-GPT were assessed by multiple regression analyses. A P-value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software (version 22.0, IBM Corp., Armonk, NY, USA).
Results
Baseline demographics and clinical characteristics of participants
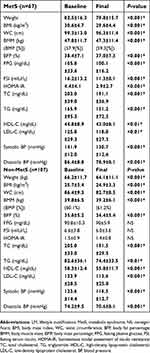
A total of 198 participants in the program were initially screened. Excluding 3 individuals who were younger than 18 years, 3 with anemia (hemoglobin <8.0 g/dL), and 18 with medication for hypertension, diabetes mellitus, or hyperlipidemia, 174 eligible participants completed the programs. Sixty-seven (38.5%) participants were diagnosed with metabolic syndrome (Figure 1). Most of the study population was female 83.9% (146/174) with mean age and BMI of 40.5±11.0 years and 27.6±4.6 kg/m2, respectively. Baseline characteristics of the MetS and Non-MetS groups are shown in Table 1.
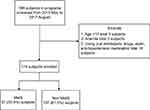 | Figure 1 Study flow. Abbreviation: MetS, metabolic syndrome. |
Insulin resistance was associated with high TG and BMI
Before the program, despite the criteria of MetS (WC, SBP, DBP, TG, FPG, and HDL-C), the height, weight, BMI, S-GPT, UA, BFP, BMM, FSI, and HOMA-IR of the MetS group were significantly greater than in the non-MetS group (all P<0.05). Stepwise multiple regression analysis was performed to elucidate independent determinants for baseline HOMA-IR. It was shown that TG and BMI were positive contributors to insulin resistance, and BMM was a negative contributor. Associations with age, gender, WC, TC, HDL-C, BFP, and BP were statistically excluded (Table 2).
 | Table 2 Multiple regression analysis for determinants of the degree of baseline HOMA-IR: only TG and BMI were positive contributors and BMM was a negative contributor |
Eight-week diet and exercise program results
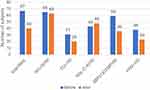
As shown in Table 3, significant improvements in most anthropomorphic parameters were observed in both groups after a short-term modification. However, significant decreases in FPG, FSI, and HOMA-IR were noted only in the MetS group (all P<0.01). The changes from baseline in anthropomorphic parameters for the MetS and non-MetS groups are shown in Figure 2. The MetS group had more significant changes in BMI, BW, FPG, FSI, HOMA-IR, SBP, DBP, and TG than the non-MetS group. As shown in Figure 3, through an eight-week diet and exercise program, the number of MetS factors (WC, TG, SBP/DBP, and FPG) in the MetS group decreased (except for HDL-C), which led to a 40.3% reduction in MetS prevalence.
Improved HOMA-IR after the program in the MetS group
In the MetS group, we performed stepwise multiple regression analysis to elucidate independent determinants for the degree of decrease in HOMA-IR after an eight-week program. It was shown that baseline HOMA-IR level, decreased FPG, and decreased FSI were positive contributors to change in HOMA-IR after the program. This implied that the decrease in HOMA-IR was greater in those with MetS with high baseline HOMA-IR level and those with the greatest reduction of FPG or FSI. However, associations with age, gender, WC, BMI, BFP, BMM, TC, TG, HDL-C, BP, and changes of anthropomorphic parameters (such as WC, BMI, BFP, and BMM) were statistically excluded (Table 4). Single regression analysis showed that the change in HOMA-IR after the program was positively associated with the baseline HOMA-IR level (R=0.75, P<0.001) (Figure 4).
Discussion
In the current study, based on the baseline characteristics of 174 eligible Taiwanese participants, we found that HOMA-IR was associated with high levels of TG and high BMI, which was consistent with the study by McLaughlin et al20,21 and other reports.22 BMM was a negative contributor for insulin resistance, which was like previous reports,23,24 and could be related to high muscle mass resulting in increasing insulin-induced glucose uptake.25,26 Moreover, muscle quality was also thought to play an important role in insulin sensitivity.27–29 There is enough evidence in the present literature to support the association between body fat and insulin resistance.30,31 BFP in our study did not show a statistically significant role in affecting HOMA-IR (coefficient=−0.185, 95% CI=−0.378 to 0.008, P=0.0597), which could be due to small sample size (n=174).
We demonstrated that the eight-week LM program with low intensity could effectively achieve significant improvement in most anthropomorphic parameters in both MetS and non-MetS groups at the community level. Both MetS and non-MetS groups lost a significant amount of BW (−2.7±2.3 kg vs −2.1±1.7 kg, P<0.05), WC (−3.0±4.4 cm vs –3.6±4.1 cm, NS), and BFP (−1.4±1.2% vs −1.4±1.4%, NS) at eight weeks. A major finding of our study was the significant decreases of FPG, FSI, and HOMA-IR in the MetS group. Through the eight-week LM program, reductions in FPG, FSI, and HOMA-IR were not associated with the changes of WC, BFP, and BMM. Unlike LM programs lasting three to six months, a short-term LM program with low intensity was enough to improve glucose homeostasis in the MetS group and minimize the risk of type 2 diabetes and atherosclerotic cardiovascular disease. We suggested that insulin resistance was reversed by the early benefit of exercise and diet control. Changes in LM enhanced insulin signaling to the liver and muscle to reestablish the normal disposal of glucose, even before gross changes of body weight, body fat, and muscle mass.32–34
Another interesting observation of the study was the reduction of HDL-C following the short-term LM program. There are the existing evidences that HDL-C levels could be increased by aerobic exercise of substantial intensity, frequency, and duration.35–37 In contrast, low-fat diet with weight loss tends to reduce HDL-C levels but to modulate the anti-inflammatory properties of HDL, which means HDL function maybe more important than the HDL-C levels associated with cardiovascular disease.38–42 In our study, significant weight loss and reduction of total cholesterol level following short-term LM program with low intensity may be attributed to healthy dietary with decrease of intake of calories, rather than exercise. Low-intensity exercise did not ameliorate diet-induced reduction in HDL-C, suggesting an explanation for HDL-C reduction in our study.
Despite HDL-C reduction, there were significant reductions in the number of participants who met the MetS criteria for WC, TG, BP, and FPG levels in the MetS group in our study. A 40.3% reduction in prevalence of MetS in the MetS group was like results in previous studies, ranging from 31% to 52.4%, after 6 to 12 months of dieting with/without exercise intervention. For example, Esposito et al reported a 48% reduction in the prevalence of MetS through 2 years of a Mediterranean-style dietary intervention when compared with the control diet.43 A 35% reduction in the prevalence of MetS after 6 months of LM with a DASH diet was reported by Azadbakht et al.44 Bihan et al10 and Eui Geum et al45 reported a 52.4% and 45.2% reduction in the prevalence of MetS after 6 months of diet and exercise intervention, respectively. These studies provided relatively intensive LM for participants, which were unlikely to be a part of routine practice for most people in many countries, not only in the degree of intensity but also the duration. Our study affirmed that a short-term LM program with low intensity was easily approached and effective for a community-based population in achieving a reduction of weight and body fat in the whole group, especially improvement of insulin resistance in the MetS group and regression of MetS. Our study also suggested that baseline HOMA-IR level was the identifiable predictor of response to the short-term LM program with low intensity for people with MetS in a community environment.
Limitations
There were some limitations in our study. First of all, this was a retrospective study with a small sample size. The number of male samples was significantly lower than the females due to clustering sampling, and the females may have more health awareness and attitudes to join the program. The 174 volunteer participants were probably more conscious of their own health conditions than the general population, which may not be directly generalized to a community setting. Second, although we focus on the encouraging results of a short-term, low-intensive program, however, the uncertain long-term outcomes are worth for more further investigation.
Conclusion
In conclusion, we demonstrate a short-term (eight-week), low-intensive but easy to complete LM program for a community-based population, which is an effective and efficient way to improve the anthropomorphic parameters and to reduce insulin resistance, especially for adults with MetS.
Acknowledgments
We thank Department of Education and Research, NEWSTART Exercise Center of Taiwan Adventist Hospital for supporting during data collection (
Disclosure
The authors report no conflicts on interest in this work.
References
1. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–636. doi:10.1161/ATVBAHA.107.151092
2. Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607.
3. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi:10.1016/S0140-6736(05)66378-7
4. Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710):181–183. doi:10.1016/S0140-6736(09)61794-3
5. Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi:10.1210/er.2008-0024
6. Bruce CR, Kriketos AD, Cooney GJ, Hawley JA. Disassociation of muscle triglyceride content and insulin sensitiity after exercise training in patients with type 2 diabetes. Diabetologia. 2004;47(1):23–30. doi:10.1007/s00125-003-1265-7
7. Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab. 2004;89(6):2704–2710. doi:10.1210/jc.2003-031827
8. O’Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol (1985). 2006;100(5):1584–1589. doi:10.1152/japplphysiol.01336.2005
9. Bo S, Ciccone G, Baldi C, et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J Gen Intern Med. 2007;22(12):1695–1703. doi:10.1007/s11606-007-0399-6
10. Bihan H, Takbou K, Cohen R, et al. Impact of short-duration lifestyle intervention in collaboration with general practitioners in patients with the metabolic syndrome. Diabetes Metab. 2009;35(3):185–191. doi:10.1016/j.diabet.2008.11.002
11. Oh EG, Bang SY, Hyun SS, et al. Effects of a 6-month lifestyle modification intervention on the cardiometabolic risk factors and health-related qualities of life in women with metabolic syndrome. Metabolism. 2010;59(7):1035–1043. doi:10.1016/j.metabol.2009.10.027
12. Leblanc ES, O’Connor E, Whitlock EP, et al. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434–447. doi:10.7326/0003-4819-155-7-201110040-00006
13. van Gool CH, Penninx BWJH, Kempen GIJM, et al. Determinants of high and low attendance to diet and exercise interventions among overweight and obese older adults: results from the arthritis, diet, and activity promotion trial. Contemp Clin Trials. 2006;27(3):227–237. doi:10.1016/j.cct.2005.11.002
14. Graffagnino CL, Falko JM, La Londe M, et al. Effect of a community-based weight management program on weight loss and cardiovascular disease risk factors. Obesity (Silver Spring). 2006;14(2):280–288. doi:10.1038/oby.2006.36
15. Chaitman BR. Exercise stress testing. In: Braunwald E, editor. Heart Disease: A Textbook of Cardiovascular Medicine.
16. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254.
17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419.
18.
19. Liu J, Grundy SM, Wang W, et al. Ethnic-specific criteria for the metabolic syndrome: evidence from China. Diabetes Care. 2006;29(6):1414–1416. doi:10.2337/dc06-0481
20. McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven GM. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–809.
21. McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96(3):399–404. doi:10.1016/j.amjcard.2005.03.085
22. McAuley KA, Williams SM, Mann JI, et al. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24(3):460–464.
23. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–2903. doi:10.1210/jc.2011-0435
24. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J. 2014;61(1):61–70.
25. Katz LD, Glickman MG, Rapoport S, Ferrannini E, DeFronzo RA. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983;32(7):675–679.
26. Capaldo B, Gastaldelli A, Antoniello S, et al. Splanchnic and leg substrate exchange after ingestion of a natural mixed meal in humans. Diabetes. 1999;48(5):958–966.
27. Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2006;4(1):19–27.
28. Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA;
29. Kim K, Park SM. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: a cross-sectional study. Sci Rep. 2018;8(1):2703. doi:10.1038/s41598-018-21168-5
30. Kim S, Cho B, Lee H, et al. Distribution of abdominal visceral and subcutaneous adipose tissue and metabolic syndrome in a Korean population. Diabetes Care. 2011;34(2):504–506. doi:10.2337/dc10-1364
31. Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5(6):2019–2027. doi:10.3390/nu5062019
32. Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335(18):1357–1362. doi:10.1056/NEJM199610313351804
33. Francois ME, Baldi JC, Manning PJ, et al. ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 2014;57(7):1437–1445. doi:10.1007/s00125-014-3244-6
34. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. doi:10.1172/JCI77812
35. Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991;325(7):461–466. doi:10.1056/NEJM199108153250703
36. Stefanick ML, Mackey S, Sheehan M, et al. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med. 1998;339(1):12–20. doi:10.1056/NEJM199807023390103
37. Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–1492. doi:10.1056/NEJMoa020194
38. Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26(2):194–202.
39. Brinton EA, Eisenberg S, Breslow JL. A low-fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. J Clin Invest. 1990;85(1):144–151. doi:10.1172/JCI114405
40. Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol (1985). 2006;101(6):1727–1732. doi:10.1152/japplphysiol.00345.2006
41. Aicher BO, Haser EK, Freeman LA, et al. Diet-induced weight loss in overweight or obese women and changes in high-density lipoprotein levels and function. Obesity (Silver Spring). 2012;20(10):2057–2062. doi:10.1038/oby.2012.56
42. Kent L, Morton D, Rankin P, et al. The effect of a low-fat, plant-based lifestyle intervention (CHIP) on serum HDL levels and the implications for metabolic syndrome status - a cohort study. Nutr Metab (Lond). 2013;10(1):58. doi:10.1186/1743-7075-10-58
43. Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi:10.1001/jama.292.12.1440
44. Azadbakht L, Mirmiran P, Esmaillzadeh A, et al. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28(12):2823–2831.
45. Eui Geum O, So Youn B, Sa Saeng H, et al. Effects of a 6-month lifestyle modification intervention on the cardiometabolic risk factors and health-related qualities of life in women with metabolic syndrome. Metabolism. 2010;59(7):1035–1043. doi:10.1016/j.metabol.2009.10.027
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.