Back to Journals » Drug Design, Development and Therapy » Volume 13
eIF2α promotes vascular remodeling via autophagy in monocrotaline-induced pulmonary arterial hypertension rats
Authors Guo L, Li Y, Tian Y, Gong S, Chen X, Peng T, Wang A, Jiang Z
Received 29 April 2019
Accepted for publication 15 July 2019
Published 13 August 2019 Volume 2019:13 Pages 2799—2809
DOI https://doi.org/10.2147/DDDT.S213817
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Linya Guo,1,* Yanbing Li,2,3,* Ying Tian,4,5,* Shaoxin Gong,6 Xi Chen,1 Tianhong Peng,1 Aiping Wang,1,4,5,7 Zhisheng Jiang7
1Clinical Anatomy & Reproductive Medicine Application Institute, School of Medicine, University of South China, Hengyang 421001, People’s Republic of China; 2National Key Discipline of Human Anatomy, Southern Medical University, Guangzhou 510000, Guangdong, People’s Republic of China; 3Guangdong Engineering Research Center for Translation of Medical 3D Printing Application, Guangzhou, 510000, Guangdong, People’s Republic of China; 4Institute of Clinical Research, Affiliated Nanhua Hospital, University of South China, Hengyang 421002, Hunan, People’s Republic of China; 5Postdoctoral Research Institute on Basic Medicine, University of South China, Hengyang, 421001, Hunan, People’s Republic of China; 6Department of Pathology, First Affiliated Hospital, University of South China, Hengyang 421001, Hunan, People’s Republic of China; 7Key Laboratory for Arteriosclerology of Hunan Province, Institute of Cardiovascular Disease, University of South China, Hengyang 421001, Hunan, People’s Republic of China
*These authors contributed equally to this work
Purpose: Eukaryotic initiation factor 2α (eIF2α) plays important roles in the proliferation and survival of pulmonary artery smooth muscle cells (PASMCs) in animal hypoxia-induced pulmonary hypertension models. However, the underlying mechanism remains unknown at large. Autophagy has been reported to play a key role in the vascular remodeling in pulmonary arterial hypertension (PAH). The purposes of this study are to determine the functions of eIF2α and autophagy in the vascular remodeling of the monocrotaline-induced PAH rats and to clarify the correlation between eIF2α and autophagy.
Methods: We established a rat model of monocrotaline-induced PAH, and we established a cell model of platelet derived growth factor (PDGF)-induced PASMCs proliferation. The vascular morphology and the expression of eIF2α, LC3B, and p62 were assessed in the pulmonary arterial tissue of Sprague-Dawleyrats and PDGF-induced PASMCs.
Results: Autophagy was significantly active in monocrotaline model group (MCT)-induced PAH rats, which obviously promotes vascular remodeling in MCT-induced PAH rats. Furthermore, the proliferation of PASMCs was induced by PDGF in vitro. The expression of LC3B, eIF2α was increased in the PDGF-induced PASMCs proliferation, and the expression of p62 was reduced in the PDGF-induced PASMCs proliferation. Moreover, eIF2α siRNA downregulated the expression of eIF2α and LC3B, and upregulated the expression of p62 in PDGF-induced PASMCs proliferation. eIF2α siRNA inhibited the PDGF-induced PASMCs proliferation. Finally, chloroquine can upregulate the protein expression of LC3B and p62, it also can inhibit proliferation in PDGF-induced PASMCs.
Conclusion: Based on these observations, we conclude that eIF2α promotes the proliferation of PASMCs and vascular remodeling in monocrotaline-induced PAH rats through accelerating autophagy pathway.
Keywords: eIF2α, autophagy, PASMCs, PAH, monocrotaline
Introduction
Pulmonary arterial hypertension (PAH) is an extremely malignant disease of the cardiovascular system. Its main clinical features are increased pulmonary vascular resistance and pulmonary artery pressure, which ultimately leads to right heart failure and even death.1–3 The pathophysiological mechanism of PAH is very complicated, and pulmonary vascular remodeling is the main pathological change in PAH. The influencing factors of pulmonary vascular remodeling involve a variety of cells, importantly, the abnormal proliferation of pulmonary artery smooth muscle cells (PASMCs) is the most important cause of pulmonary vascular remodeling in PAH.4,5 However, the underlying mechanism remains unknown. Therefore, to further study the pathophysiological mechanism of PAH and find new drugs to effectively treat PAH have become a top priority.
Recent studies have shown that translation initiation factors play a key role in regulating certain functional proteins to promote cell proliferation.6 However, eukaryotic initiation factor 2α (eIF2α) plays a key role in regulating cell proliferation and hypertrophy as the regulatory subunit of the translation initiation factor family, and participated in the regulation of smooth muscle cell proliferation and migration.7–10 Our previous study also confirmed that the expression of p-eIF2α and eIF2α was upregulated in both pulmonary artery tissue of HPH rats and hypoxic-treated PASMCs proliferation.11 Meanwhile, after transfected with eIF2α siRNA in hypoxia-promoted PASMCs proliferation, it can significantly inhibit the proliferation of PASMCs.11 These results reveal for the first time the role of eIF2α as a new pro-proliferating protein in pulmonary vascular remodeling in HPH. However, the underlying mechanism remains unknown.
Autophagy is an efficient subcellular degradation pathway of lysosomal-dependent in eukaryotic cells, which plays an important role in scavenging waste, cell growth, maintaining protein metabolism balance, and stabilization of intracellular environment.12–14 Abnormal autophagy breaks the balance of the body and leads to disease development in some pathological environments.15 Recent studies have shown that autophagy plays a vital role in various human diseases.16–19 More and more studies have reported that autophagy plays an important role in cardiovascular diseases, and recent studies have found that activation of autophagy promotes the development of monocrotaline-induced PAH; moreover, inhibition of autophagy can inhibit the development of monocrotaline-induced PAH.20,21 Furthermore, studies have shown that eIF2α can activate endoplasmic reticulum autophagy after oxidative damage in the endoplasmic reticulum.22 Based on the above studies, we speculated that eIF2α could regulate pulmonary vascular remodeling in monocrotaline-induced PAH via autophagy pathway. In this study, we will reveal a key role of eIF2α in monocrotaline-induced PAH.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (weighing 150–200 g) were obtained from the Laboratory Animal Center, School of Medicine, School of Medicine, University of South China (Hengyang, People’s Republic of China). All surviving animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the medicine animal welfare committee of Medicine School, University of South China (Hengyang, People’s Republic of China).
Animals experiments
Animals experiments each SD rat is 150–200 g/head. After 1 week of adaptive feeding, each rat was weighed and labeled; rats were randomly divided into three groups: 1) control group (Control); 2) vehicle group (Vehicle), each rat was intraperitoneally injected with the same dose of vehicle; 3) monocrotaline model group (MCT), each rat was intraperitoneally injected with monocrotaline (60 mg/kg); pulmonary hypertension model was established for 4 weeks. Rats in each group were weighed and treated separately. Rats were intraperitoneally injected with chloral hydrate (30 mg/kg). Right ventricular systolic pressure (RVSP) and mean pulmonary arterial pressure (mPAP) were monitored for each rat. Subsequently, the right ventricle and left ventricle (RV, LV) and interventricular septum (S) of the rats were dissected separately. The length of the tibia was measured and weighed to calculate the ratio of RV to (LV+S) and tibia length to RV, which is a key indicator for assessing RV hypertrophy. Pulmonary tissue was isolated for analysis of mRNA and protein expression.
HE staining
The isolated rat lung tissue was stained with HE to assess the degree of pulmonary vascular remodeling. After paraffin sectioning, the sections are placed in distilled water and then placed in an aqueous solution of hematoxylin for staining for about 10 mins; then, the slices were placed into ammonia and acid water for several seconds; after the slice was rinsed in running water for 1 hr, it was placed in distilled water for several seconds; then, the sections were dehydrated in alcohol at concentrations of 90% and 70% for 10 mins each; subsequently, the sections were stained with eosin staining solution for about 2–3 mins; after sectioning, it is dehydrated with 100% alcohol and then placed in xylene; the sections were sealed and placed in an incubator for drying; finally, the sections were photographed with a microscope to observe the morphology of the blood vessels. Pulmonary arterial wall thickness (WT) was calculated by the following formula: WT (%)=areaext−areaint/areaext×100, where areaext and areaint are the area bounded by external and internal elastic lamina, respectively.
Western blot analysis
Protein concentration was measured after protein extraction from pulmonary artery tissue and primary cultured PASMCs. Extracted proteins were detected by Western blot analysis Primary antibody was purchased from Cell Signaling Technology (CST), USA. Optical density analysis was performed using Image J 1.43 (National Institutes of Health). Primary antibodies against eIF2α (1:1000 dilution, CST, Danvers, MA, USA, #5324s) were purchased from CST. Primary antibodies against p-eIF2α (1:1000 dilution, CST, #3398s) were purchased from CST, Primary antibodies against LC3B (1:1000 dilution, CST, #12741s) were obtained from CST, Primary antibodies against p62 (1:1000 dilution, CST, #88588) were purchased from CST. Primary antibody against β-actin (BM0627, 1:250) was purchased from Boster. Goat anti-rabbit IgG secondary antibody was purchased from Beyotime (A0408, 1:2000 dilution).
Real-time PCR analysis
RNA concentration was measured by spectrophotometer. Total RNA was extracted by TRIzol. Reverse transcription reaction using PrimeScriptTM RT reagent (TaKaRa, Shiga, Japan). Fluorescence quantification using SYBR® PremixEx TaqTM II kit (TaKaRa, Shiga, Japan). Real-time PCR amplification using the ABI 7500 Real-Time PCR System (Applied Biosciences, Foster City, CA, USA). The data were quantified using the ΔΔCT method, and the β-actin gene was used as a reference.
Cell culture and experiments
SD rats were anesthetized with 10% chloral hydrate (0.3 mg/kg) by intraperitoneal injection. The chest and abdomen hair of rat were removed and disinfected, and then the chest of rat was dissected immediately on the ultra-clean operating table to take the pulmonary artery tissue. The endovascular cell and adventitia of the pulmonary artery were removed. The pulmonary artery tissue was cut into 1×1 mm3 with ophthalmic scissor and inoculated in containing 20% FBS- DMEM high glycosylation. The third generation of cells was used for subsequent experiments after the PASMCs growing to 70–80%. After the cells were grown to 70−80% confluence, the medium was replaced with 10% FBS, serum was starved for 24 hrs, and the cells were synchronized to G0 phase. Platelet-derived growth factor-BB (PDGF-BB, R&D Systems, USA) (10 ng/mL) stimulated PASMCs at different times (6, 12, 24, 48, 72 hrs), cells were collected at the peak of cell viability; to explore autophagy in the PDGF-induced PASMCs proliferation, we designed the following experiment: PASMCs (passage 2–3) were plated in 24-well plates grown to subconfluence, then quiesced in serum-free medium for 24 hrs before chloroquine (CLQ, Sigma, USA) (10 μM) treatment in 10% FCS for 24 hrs. The cultured cells were preincubated with autophagy inhibitor chloroquine for 2 hrs, and PDGF-BB (10 ng/mL) was stimulated for 48 hrs.
Proliferation assay
Cell Counting Kit-8 (CCK-8, Yiyuan Biotechnologies, Guangzhou, People’s Republic of China) specific steps are according to the manufacturer’s instructions. Cell suspension (100 μL) was cultured in a 96-well plate, and the plate was pre-incubated at 37°C and 5% CO2 condition for 24 hrs. CCK-8 (10 μL) solution was added to the plate for 1–4 hrs. Finally, the OD value was read at the absorbance of 450 nm.
Small interference RNA transfection
The eIF2α siRNA were purchased from Ribobio (Guangzhou, People’s Republic of China). The eIF2α siRNA segment was transfected by transient transfection. The cells were planted in 24-well plates, the transfection was treated when the confluence of PASMCs grown at about 30%. Subsequently, according to the manufacturer’s instructions, the irrelevant fragment transfection complex and the eIF2α siRNA transfection complex were, respectively, transfected into PDGF-induced PASMCs according to manufacturer’s instructions. After transfection, PASMCs were cultured with a concentration of 5% CO2 and a temperature of 37°C for 4–6 hrs. Then exchange for fresh medium (20% fetal bovine serum) , continue to culture cells for 48 hrs in PDGF treatment condition. The specific experiments were divided into four groups: control group (Control), PDGF group (PDGF), PDGF+negative group (+Ng), PDGF+siRNA group (+50 nM).
Statistical analysis
All data were statistically analyzed and the data were expressed as mean±standard error (x±s.e.m). The q-PCR results were processed by ABI 7500 real-time PCR instrument; the Western blot data were analyzed by Image J software. All the data were statistically analyzed by SPSS 20.0 statistical software. The bar graph was made by Graphpad Prism 6.0. Statistical analysis was performed by unpaired Student’s t-test for two groups or analysis of variance followed by Newman-Student-Keuls test for multiple groups Bilateral P<0.05 was considered statistically significant.
Results
Vascular remodeling in MCT-induced PAH rats
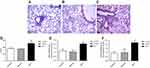
In order to clarify whether the establishment of the MCT-induced PAH rat model was successful, we performed hemodynamic analysis on SD rats in each group. The mPAP, RVSP, RV/(LV+S), and right ventricular/tibia length ratios were significantly increased in the MCT model group compared with the control group (Figure 1A-D). There was no change in the vehicle group compared with that of the control group. HE staining results showed that the pulmonary arteries of the lung tissue in the MCT-induced PAH rats showed significant thickening (Figure 2A-C) and the percentage of the medial thickness of the pulmonary arterioles (WT%) was significantly increased (Figure 2D), showing obvious characteristics of pulmonary arteriole remodeling. The above results indicated that the MCT-induced PAH model in SD rats was successfully constructed.
The mRNA expression of Ki-67 and PCNA in MCT-induced PAH rats
The mRNA expression of proliferation markers Ki-67 and PCNA in pulmonary arteries tissue was detected by real-time PCR. The results showed that the mRNA expression levels of Ki-67 and PCNA were significantly increased in the pulmonary arteries of MCT-induced PAH rats (Figure 2E, F) which was significantly different from that of control group (P<0.05). The results suggest that PASMCs significantly proliferate in MCT-induced PAH rats.
The expression of p-eIF2α, eIF2α, LC3B, and p62 in MCT-induced PAH rats
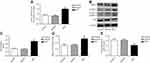
The mRNA expression of eIF2α in pulmonary arteries was detected by real-time PCR, the results showed that the mRNA level of eIF2α in the pulmonary arteries tissue of MCT-induced PAH rats was significantly upregulated (Figure 3A). To examine whether eIF2α and autophagy were activated in MCT-induced PAH rats, the protein levels of eIF2α, p-eIF2α, LC3B, and p62 were examined by Western blotting. In MCT-induced PAH rats, the levels of p-eIF2α, eIF2α, and LC3B protein were increased in the pulmonary arteries compared with that of control group (Figure 3B), which was significantly different from the control group (P<0.05); however, the protein expression of p62 was reduced in the pulmonary arteries tissue of the MCT-induced PAH rats (Figure 3B). Statistical analysis of protein gray value scans showed statistical significance (Figure 3C-E); these results suggest that eIF2α and autophagy both are activated in MCT-induced PAH rats.
PDGF-induced PASMCs proliferation
PASMCs were treated with PDGF for stimulating proliferation, cells proliferation was tested by CCK-8. As depicted in Figure 4A, PDGF-induced PASMCs proliferation successfully, which was significantly different from the control group (P<0.05). The mRNA expression of Ki-67 and PCNA in PDGF-induced PASMCs was detected by real-time PCR, the results showed that the mRNA expression levels of Ki-67 and PCNA were significantly increased in the PDGF-induced PASMCs proliferation (Figure 4B, 4C); the result indicates that PDGF successfully induces the proliferation of PASMCs.
Effect of eIF2α siRNA on PDGF-induced PASMCs proliferation
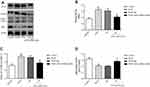
To confirm the role of eIF2α in PDGF-induced PASMCs proliferation, we transfected eIF2α siRNA (50 µM) into PDGF-induced PASMCs to observe whether eIF2α siRNA can inhibit the proliferation of PASMCs. The protein expression of eIF2α after transfection with eIF2α siRNA was detected by Western blotting. As depicted in Figure 5A, the protein expression of eIF2α and p-eIF2α were upregulated in the PDGF group, but eIF2α siRNA significantly downregulated the protein expression of eIF2α and p-eIF2α compared with that of PDGF-induced PASMCs group (Figure 5B, P<0.05). The effect of eIF2α siRNA on the proliferation of PASMCs was analyzed by CCK-8 to observe whether eIF2α siRNA can inhibit the proliferation of PDGF-induced PASMCs. As shown in Figure 6A, PDGF obviously induced the PASMCs proliferation, whereas eIF2α siRNA markedly inhibited PDGF-induced PASMC proliferation in vitro. Real-time PCR results show that the mRNA expression of Ki67 and PCNA was inhibited after transfection with eIF2α siRNA in PDGF-induced PASMCs (Figure 6B, C), which was significantly different from the PDGF group (P<0.05). The above results indicate that eIF2α siRNA inhibits the expression of eIF2α and the PDGF-induced PASMCs proliferation.
Effect of chloroquine on PDGF-induced PASMCs proliferation
To confirm that eIF2α siRNA can inhibit autophagy activation, the protein expression of LC3B and p62 after transfection with eIF2α siRNA was detected by Western blotting. As depicted in Figure 5A, the protein expression of LC3B was upregulated and the protein expression of p62 was downregulated, but eIF2α siRNA significantly downregulated the protein expression of LC3B and upregulated the protein expression of p62 compared with that of PDGF-group (Figure 5C, 5D, P<0.05). To confirm the role of autophagy in PDGF-induced PASMCs proliferation, PASMCs were pretreated with chloroquine, a specific autophagy inhibitor, and then PDGF-induced proliferation. First, the protein expression of LC3B and p62 was detected by Western blotting after treatment with chloroquine in the PDGF-induced PASMCs proliferation. As depicted in Figure 7A, chloroquine significantly upregulated the protein expression of LC3B and p62 in CLQ-group compared with that of PDGF-group (chloroquine is an autophagic lysosome inhibitor) (Figure 7B, C, P<0.05). We examined the effects of chloroquine on the PDGF-induced PASMCs proliferation by using CCK-8. As shown in Figure 7D, chloroquine extremely inhibited the proliferation of PDGF-induced PASMCs. Real-time PCR results showed that the mRNA expression of Ki67 and PCNA was significantly inhibited after treatment with chloroquine (Figure 7E, F). These results indicate that chloroquine can inhibit PDGF-induced PASMCs proliferation. In conclusion, autophagy plays an important role in the pulmonary vascular remodeling of MCT-induced PAH, importantly, eIF2α plays a key role via autophagy pathway in MCT-induced PAH.
Discussion
The primary findings of the study were as follows: 1) pulmonary arterial remodeling was observed in rats treated with monocrotaline, which also exhibited elevated the protein expression of eIF2α, p-eIF2α, LC3B and reduced the protein expression of p62; 2) eIF2α plays an important role via autophagy pathway in the pulmonary vascular remodeling in MCT-induced PAH; and 3) hloroquine attenuated PDGF-induced PASMC proliferation and expression of autophagy. Autophagy plays an important role in the pulmonary vascular remodeling in MCT-induced PAH.
Increased pulmonary vascular resistance and pulmonary vascular remodeling are two basic pathological features of PAH.23–25 In this study, we have used monocrotaline to induce pulmonary vascular remodeling of PAH rats. Studies have proved that PASMCs play a crucial role in the pulmonary vasculature of PAH.26–28 In our study, primary PASMCs are treated with PDGF to induce the proliferation. The above results showed the animal models and cell models were successfully established.
eIF2α is a regulatory subunit of eukaryotic translation initiation factor 2, which is a key protein that catalyzes the initiation of protein synthesis.29,30 eIF2α, as an important member of the translation initiation factor family, plays a key role in the regulation of cell proliferation, in addition, it is involved in the regulation of smooth muscle cell proliferation and migration.7–10 Our previous studies also confirmed that the expression of p-eIF2α and eIF2α was upregulated in both HPH rats in pulmonary arterial tissue and hypoxic-treated PASMCs proliferation, and all of these effects were inhibited by eIF2α siRNA.11 But the underlying mechanism remains unknown. In our study, we established monocrotaline-induced PAH rats and PDGF-induced PASMCs proliferation. Our study has found the following results: 1) both p-eIF2α and eIF2α expression were upregulated in both PDGF-induced PASMCs proliferation and MCT-induced PAH rats; 2) the mRNA expression of Ki-67 and PCNA in pulmonary arterial tissue was significantly increased in monocrotaline-induced PAH rats and PDGF-induced PASMCs proliferation; 3) eIF2α expression was significantly upregulated, and the above effects were inhibited by eIF2α siRNA in PDGF-induced PASMCs proliferation; and 4) eIF2α siRNA significantly inhibited the proliferation of the PDGF-induced PASMCs. These results reveal the important role of eIF2α as a new pro-proliferating protein in pulmonary vascular remodeling in MCT-induced PAH. These results indicate that eIF2α can be used as a new target for the prevention and treatment of PAH.
The expression of eIF2α is elevated in monocrotaline-induced PAH rats and PDGF-induced PASMCs proliferation, but what kind of downstream mechanism does eIF2α play role by regulating? This is one of the main issues to be explored in this experiment. Autophagosomes bind to lysosomes to form autophagolysosome during autophagy, which can degrade damaged macromolecules and organelles in the cytoplasm. It can provide raw materials for the normal survival and metabolism of cells by degrading products.31,32 Many researchshow that autophagy is closely related to the occurrence and development of lung diseases.21,33 Recently, some studies have shown that autophagy is involved in the development of PAH induced by monocrotaline.21,33 Studies have shown that eIF2α can active endoplasmic reticulum autophagy after oxidative damage in the endoplasmic reticulum. Therefore, we speculate that the proliferative effect of eIF2α in MCT-induced PAH may be related to the activation of autophagy. We also found the following results: 1) the expression of LC3B was significantly up-regulated in both PDGF-induced PASMCs proliferation and MCT-induced PAH rats, and the expression of p62 was down-regulated, consistent with the activation of autophagy; and (2) eIF2α siRNA significantly inhibited the expression of LC3B and increased the expression of p62 in PDGF-induced PASMCs proliferation. To further confirm the effect of autophagy on the proliferation of PASMCs, next we use chloroquine to inhibits autophagy pathways in PDGF-induced PASMCs. The expression of LC3B protein was up-regulated in PDGF-induced PASMCs. Chloroquine treatment further increased LC3B expression in these cells consistent (Chloroquine is an autophagic lysosome inhibitor). We examined the effects of chloroquine on p62. The results show that the expression of p62 was markedly reduced in PDGF-induced PASMCs, but chloroquine treatment up-regulated p62 expression in PDGF-induced PASMCs, and treatment with chloroquine profoundly inhibited the proliferation of PDGF-induced PASMCs. These results show that autophagy is involved in the development of PAH and can be inhibited by eIF2α siRNA.
Taken together, in this experimental study, we found that the eIF2α expression was up-regulated both in the rat PAH model induced by monocrotaline and the PASMCs induced by PDGF. The high expression of eIF2α leads to the activation of autophagy, which in turn promotes the proliferation of PASMCs, and leads to the pulmonary vascular remodeling in PAH. We further found that the pathophysiological process that the important role of eIF2α promoting PASMCs proliferation and activating of autophagy can be blocked by eIF2α siRNA. At the same time, chloroquine can inhibit the proliferation of PASMCs by inhibiting autophagy. These results indicate that eIF2α is an important proliferation protein in the pulmonary vascular remodeling in PAH. As a kind of protein closely related to protein translation, people’s understanding of eIF2α tends to be deeper. The relationship between eIF2α and tumor provides new ideas for clinical tumor treatment. At present, there are some exploratory studies using eIF2α as a therapeutic target.34,35 This suggests indicate that eIF2α may be a very promising target for clinical treatment. Although there are still some problems to be solved so far, some existing research results have pointed out the direction for future research and provided new ideas for the treatment of PAH. We have reason to believe that eIF2α protein can be used as a new target for the prevention and treatment of PAH in the further research, and blocking the function of eIF2α may reduce and delay the development of PAH and improve the therapeutic effect of PAH. These research will provide a key regulatory molecule and a new target for the exploration of PAH.
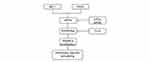
In summary, our research found that eIF2α promotes the proliferation of PASMCs and vascular remodeling in monocrotaline-induced PAH rats through accelerating autophagy pathway (Figure 8).
Acknowledgment
This project was financed by the National Natural Science Foundation of China (grant number 81600040 to Wang A; 31871169 to Tian Y), the Province Natural Science Foundation of Hu Nan (grant number 2017JJ3279 to Wang A), the Postdoctoral Science Fund of China (grant number 2017M622589 to Wang A), Key Project of Education Department of Hu Nan (grant number 18A243 to Wang A).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Miura Y, Fukumoto Y, Sugimura K, et al. Identification of new prognostic factors of pulmonary hypertension. Circ J. 2010;74(9):1965–1971.
2. Lan NSH, Massam BD, Kulkarni SS, Lang CC. Pulmonary arterial hypertension: pathophysiology and Treatment. Diseases. 2018;6(2):
3. Guiot J, Parzibut G, Weber T, et al. Pulmonary arterial hypertension. Rev Med Liege. 2019;74(3):139–145.
4. Santos-Ribeiro D, Mendes-Ferreira P, Maia-Rocha C, Adão R, Leite-Moreira AF, Brás-Silva C. Pulmonary arterial hypertension: basic knowledge for clinicians. Arch Cardiovasc Dis. 2016;109(10):550–561. doi:10.1016/j.acvd.2016.03.004
5. Yu L, Tu Y, Jia X, et al. Resveratrol protects against pulmonary arterial hypertension in rats via activation of silent information regulator 1. Cell Physiol Biochem. 2017;42(1):55–67. doi:10.1159/000477115
6. Zheng Q, Ye J, Cao J. Translational regulator eIF2alpha in tumor. Tumour Biol. 2014;35(7):6255–6264. doi:10.1007/s13277-014-1789-0
7. Jiang L, Zang D, Yi S, et al. A microRNA-mediated decrease in eukaryotic initiation factor 2alpha promotes cell survival during PS-341 treatment. Sci Rep. 2016;6:21565. doi:10.1038/srep21565
8. Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc. 2008;5(1):89–96. doi:10.1513/pats.200705-063VS
9. Liu X, Bennett RL, Cheng X, Byrne M, Reinhard MK, May WS
10. Bennett RL, Pan Y, Christian J, Hui T, May WS
11. Wang AP, Li XH, Yang YMA, et al. Critical role of the mTOR/eIF2alpha pathway in hypoxia-induced pulmonary hypertension. PLoS One. 2015;10(6):e0130806. doi:10.1371/journal.pone.0130806
12. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi:10.1038/nature06639
13. Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12(9):814–822. doi:10.1038/ncb0910-814
14. Yang Z, Zeng B, Pan Y, Huang P, Wang C. Autophagy participates in isoliquiritigenin-induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed Pharmacother. 2018;97:248–254. doi:10.1016/j.biopha.2017.10.070
15. Yorimitsu T, Klionsky DJ. Eating the endoplasmic reticulum: quality control by autophagy. Trends Cell Biol. 2007;17(6):279–285. doi:10.1016/j.tcb.2007.04.005
16. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Intern Med. 2013;132(1):27–42.
17. Grootaert MOJ, Roth L, Schrijvers DM, De Meyer GRY, Martinet W. Defective autophagy in atherosclerosis: to die or to senesce? Oxid Med Cell Longev. 2018;2018:7687083. doi:10.1155/2018/7687083
18. Roy S, Debnath J. Autophagy and Tumorigenesis. Semin Immunopathol. 2010;32(4):383–396. doi:10.1007/s00281-010-0213-0
19. Yuan H, Jiang C, Zhao J, et al. Euxanthone attenuates Aβ1-42-induced oxidative stress and apoptosis by triggering autophagy. J Mol Neurosci. 2018;66(4):512–523. doi:10.1007/s12031-018-1175-2
20. Zhou Y, Wang Y, Wang X, et al. The protective effects of kappa-opioid receptor stimulation in hypoxic pulmonary hypertension involve inhibition of autophagy through the AMPK-MTOR pathway. Cell Physiol Biochem. 2017;44(5):1965–1979. doi:10.1159/000485886
21. Long L, Yang X, Southwood M, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013;112(8):1159–1170. doi:10.1161/CIRCRESAHA.111.300483
22. Tsai TC, Lai KH, Su JH, Wu YJ, Sheu JH. 7-acetylsinumaximol B induces apoptosis and autophagy in human gastric carcinoma cells through mitochondria dysfunction and activation of the PERK/eIF2alpha/ATF4/CHOP signaling pathway. Mar Drugs. 2018;16(4):
23. Kim D, George MP. Pulmonary hypertension. Med Clin North Am. 2019;103(3):413–423. doi:10.1016/j.mcna.2018.12.002
24. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–1436. doi:10.1056/NEJMra040291
25. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American college of cardiology foundation task force on expert consensus documents and the American heart association developed in collaboration with the American college of chest physicians; American thoracic society, inc.; and the pulmonary hypertension association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi:10.1016/j.jacc.2009.01.004
26. Li X, He Y, Xu Y, et al. KLF5 mediates vascular remodeling via HIF-1alpha in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;310(4):L299–L310. doi:10.1152/ajplung.00189.2015
27. Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res. 2004;68(2):75–103. doi:10.1016/j.mvr.2004.06.001
28. Morrell NW, Adnot S, Archer SL. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi:10.1016/j.jacc.2009.04.018
29. Morrell NW, Adnot S, Archer SL. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv Nutr. 2012;3(1 Suppl):307–321. doi:10.3945/an.112.002113
30. Page AB, Owen CR, Kumar R, et al. Persistent eIF2alpha(P) is colocalized with cytoplasmic cytochrome c in vulnerable hippocampal neurons after 4 hours of reperfusion following 10-minute complete brain ischemia. Acta Neuropathol. 2003;106(1):8–16. doi:10.1007/s00401-003-0693-2
31. Lin NY, Beyer C, Giessl A, et al. Autophagy regulates TNFalpha-mediated joint destruction in experimental arthritis. Ann Rheum Dis. 2013;72(5):761–768. doi:10.1136/annrheumdis-2012-201671
32. Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi:10.1016/j.molcel.2010.09.023
33. Liu X, Cao H, Li J, et al. Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination. Cell Death Differ. 2017;24(4):683–693. doi:10.1038/cdd.2017.1
34. Shi B, Shao B, Yang C, Guo Y, Fu X, Gan N. Upregulation of JHDM1D-AS1 protects PDLSCs from H2O2-induced apoptosis by decreasing DNAJC10 via phosphorylation of eIF2α. Biochimie. 2019;9084(19):30191–30199.
35. Sengupta S, Sevigny CM, Bhattacharya P, Jordan VC, Clarke R. Estrogen-induced apoptosis in breast cancers is phenocopied by blocking dephosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) protein. Mol Cancer Res. 2019;17(4):918–928. doi:10.1158/1541-7786.MCR-18-0481
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.