Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Efficacy, safety, and Pharmacoeconomics of Three Common Strategies for Pediatric Alopecia Areata Patients: A Retrospective Cohort Study
Authors Zhou J, Yang Y, Xu M, Lyu Z, Wu X
Received 14 June 2023
Accepted for publication 14 September 2023
Published 18 October 2023 Volume 2023:16 Pages 2947—2956
DOI https://doi.org/10.2147/CCID.S425534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jiong Zhou,* Yang Yang,* Mengjun Xu, Zhongfa Lyu, Xianjie Wu
Department of Dermatology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianjie Wu, Department of Dermatology, Second Affiliated Hospital, Zhejiang University School of Medicine, 88 Jiefang Road, Hangzhou, 310009, People’s Republic of China, Email [email protected]
Objective: To evaluate and explore the efficacy, safety, and pharmacoeconomics of three common strategies for pediatric alopecia areata.
Methods: Chinese pediatric alopecia areata patients meeting the criteria were included and divided into three groups based on the received treatments. The efficacy, adverse events and pharmacoeconomics of these treatments were retrospectively analyzed.
Results: Twenty-four pediatric AA patients were recruited in this study. 100% (12/12) of patients from the traditional group achieved SALT100. In the tofacitinib group, 40.0% (2/5) of patients achieved SALT50. 20.0% (1/5) of patients achieved SALT75 and 40.0% (2/5) of patients achieved SALT100. In the MN group, 42.86% (3/7) of patients were non-responders. 14.28 (1/7) of patients achieved SALT75 and 42.86% (3/7) of patients achieved SALT100. The adverse effects (AEs) were mild in all three groups, and none of the patients discontinued the treatments due to the AEs. Comparing the other two groups, the MN treatment would be more time-intensive and more expensive.
Conclusion: For newly diagnosed or naive pediatric patients, the traditional treatment was the first-line approach. For long-duration, severe and refractory patients, tofacitinib and microneedling can be alternative options.
Keywords: pediatric, alopecia areata, tofacitinib, microneedling, efficacy
Introduction
Alopecia areata (AA) is an autoimmune disease characterized by non-scarring hair loss. Eleven to 50% of the cases occur in children, and the prevalence rate of pediatric AA is slightly higher than that in adults.1,2 Multiple areas of hair loss on the scalp or and/or other body parts cause significant changes in the appearance of patients, which can result in emotional and psychological burden in childhood. In the study of Bilgic et al, it was found that the degree of psychological influence is positively correlated with the location of hair loss and may eventually contribute to psychological disorders.3 It is reported the most common hair loss location of AA patches is on the occiput of the scalp in both female and male patients.4
Since most pediatric patients with limited AA recover spontaneously, and the response to current therapies is unpredictable, evidence for the efficacy of treatments is mostly weak. Many parents discontinue treatment because there is no curative effect, relapse is common, or they are concerned about side effects. Some patients, however, will develop recurrent symptoms, AA can affect all scalp skin (alopecia totalis), and even involve other hairy body parts such as eyebrows, eyelashes, armpit hair and pubic hair, as well as suffer from significant psychosocial stress.5,6 Rarely all or almost all hair on the body can be lost (alopecia universalis).The commonly accepted treatments for AA include intralesional corticosteroids, topical corticosteroids, minoxidil, topical prostaglandin analogues, systemic corticosteroids, immunosuppressants, laser therapy, JAK inhibitors, biologics, phosphodiesterase 4 inhibitors, PRP therapy, a Low-Dose IL-2 administration, statins, antihistamines and microneedling, while high potency topical corticosteroids are the first-line treatment for pediatric patients.1,2,6,7 The plethora of possible therapies suggests that none are highly are universally effective.
The therapeutic options for AA should be determined by the patients’ age. Intralesional corticosteroids and topical immune therapy are avoided in patients under the age of ten. Children over the age of ten are treated in the same way as adults.8
Pediatric AA is commonly associated with a negative effect on health-related quality of life for both patients and their caregivers, which may impact throughout the lifespan.9
Oral tofacitinib may now be considered as a therapeutic option for preadolescent children with severe AA resulting in psychosocial impairment, with appropriate risk assessment for infection and malignancy.9,10
However, in view of various side effects, cost, the limitation on patients’ age, and the lack of clinical trials, there is no definitive treatment for children. Therefore, the safety and efficacy of pediatric AA treatment require more trials to explore and confirm optimal therapies.
As a minimally invasive treatment, Microneedling (MN) has the effect of enhancing transdermal delivery and has been used in scars, psoriasis and other skin disease treatment.11 By changing the microenvironment and local immune cells, MN is also effective for AA.12 Therefore, we explored the efficacy of MN in pediatric AA.
Materials and Methods
Study Design and Population
The inclusion criteria were met by Chinese pediatric patients (2–14 years old): (1) diagnosed as AA by two independent dermatologists at the dermatology clinic of the Second Affiliated Hospital, Zhejiang University School of Medicine, from Apr 2021 to Nov 2022; (2) with AA subtypes classified as patchy AA, alopecia totalis (AT), alopecia universalis (AU), ophiasis, and sisaipho; (3) age under 14 years-old.
Based on the type of treatment they had received, the included pediatric patients were divided into three groups: 1) the traditional group: topical 0.1% mometasone furoate (Eloson, BAYER PHARMA.), QD and 5% solution of minoxidil (MANDI, 3SBIOI INC.), QN; 2) the tofacitinib group: treated with 5 mg tofacitinib (AIJIEWEI, SIMCERE INC.) by mouth once daily, the patients consented to laboratory tests before treatment and every 3 months after initiating the treatment, including a complete blood count, biochemical blood test, screening for hepatitis B virus infection and tuberculosis); and 3) the microneedling (MN) group: the needles used in the therapy were 31G (Medi Hair), which resulted in 0.35 mm depth wounds in order to allow herbal extracts (Medi Hair) to be absorbed into the skin (Lavandula angustifolia Oil, Swertia japonica extract, Panax ginseng root extract, Larix europaea wood extract, Camellia sinensis extract), treated every two weeks.
Baseline Assessment and Follow-Up
We collected demographic data and Severity of Alopecia Tool (SALT) scores. SALT is an important calculation guideline for quantifying the severity of AA.
Data Analyses and Statistical Methods
Quantitative variables were expressed as the mean ± standard deviation (±SD). Statistical analyses were performed using GraphPad Prism v.9.0.0. The one-way ANOVA and the Kruskal–Wallis test were performed as appropriate to compare the continuous variables among the three groups. P-value < 0.05 was considered statistically significant.
Results
Patient Characteristics
Demographic data and disease characteristics of the 24 recruited patients with AA are listed in Table 1. The patients included 10 females and 14 males, with a median age of 8.9 years (range, 3–14 years). Among them, 15 patients were diagnosed with patchy alopecia, 2 with alopecia totalis, 2 with alopecia universalis, 2 with ophiasis, 1 with sisaipho, and 2 with reticulate alopecia areata. All patients received at least 24 weeks of current treatment.
 |
Table 1 Demographic Data and Characteristics of Pediatric AA Patients in Different Groups |
In the traditional group, all the patients had no prior AA medication history. 20% (1/5) of patients in the tofacitinib group and 57.14% (4/7) of patients in the MN group had previously received treatment for AA.
Among 24 pediatric AA patients, 9 patients had comorbidity (3/12 in the traditional group, 6/7 in the MN group), 2 had atopic dermatitis, 2 had urticaria (1 patient received treatment with omalizumab), 2 reported allergic rhinitis (1 patient received desensitization therapy), 1 had temporary total triiodothyronine elevation, 1 had lichen planus, and 1 had atopic eczema. No patients had a family history of hair loss; however, 2 patients reported a family history of allergic rhinitis (1 patient did not have a history of allergic rhinitis). It is noteworthy that most patients (6/7) with comorbidities were in the MN group.
Efficacy
Treatment responses in each group are shown in Figure 1.
 |
Figure 1 Proportion of efficacy outcomes. |
Notably, 100% (12/12) of patients from the traditional group achieved SALT100.
In the tofacitinib group, 40.0% (2/5) of patients achieved SALT50. 20.0% (1/5) of patients achieved SALT75 and 40.0% (2/5) of patients achieved SALT100.
In the MN group, 42.86% (3/7) of patients were non-responders. 14.28 (1/7) of patients achieved SALT75 and 42.86% (3/7) of patients achieved SALT100.
Results showed that the effect of MN therapy varied within wide limits from the endpoint SALT score (shown in Table 2). There were 1 (8.33%), 1 (20.00%), and 2 (28.57%) patients who experienced recurrence in the traditional group, the tofacitinib group, and the MN group, respectively (shown in Table 2).
 |
Table 2 Endpoint SALT Score and Recurrence in Each Group |
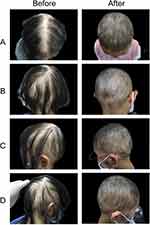
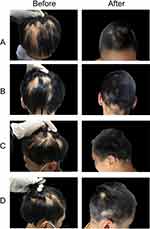
Photos of representative responders in the three groups are shown in Figures 2–4.
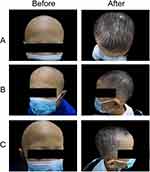 |
Figure 4 Photographs of a representative patient in the MN group: panel A-C showed different regions of scalp before and after treatment ((A) top, (B) left side, (C) right side). |
The percentage of patients who had a long duration of their current hair loss episode (≥1 year) was 33.3% (4/12), 80% (4/5) and 71.4% (5/7) in the traditional group, the tofacitinib group, and the MN group, respectively. All the traditional group patients achieve SLAT100 at the endpoint. The efficacy (SALT change) in the tofacitinib group between patients with a shorter duration (≥1 years) and those with a longer duration (≥1 years) had no difference (P =0.367). Similarly, the efficacy (SALT change) in the MN group between a shorter duration (≥1 years) and a longer duration (≥1 years) had no difference (P =0.775).
Safety
The adverse effects (AEs) were mild in all three groups, and none of the patients discontinued the treatments due to the AEs.
In the tofacitinib group, one patient experienced excessive hair growth.
Microneedling is a minimally invasive procedure, and during therapy, 42.86% (3/7) patients complained of mild transient pain (Numerical Rating Scale, NRS: 1–2).
No side effects of traditional group therapy were described in children with alopecia areata.
Cost in Treatment
In terms of the monthly costs as follows: the traditional group: 59.67—225 Chinese Yuan (CNY); the tofacitinib group: 55 CNY; the MN group: 696 CNY (Table 3), the MN group was significantly higher than the other 2 groups (P<0.0001). Except for MN treatment, the other two treatments can be covered by medical insurance. The frequency of follow-up for the traditional group, the tofacitinib group and the MN group was every 8 weeks, every 4 weeks and every 2 weeks, respectively. As a result, the treatment for the patients of the MN group would be more time-intensive and more expensive.
 |
Table 3 Cost Parameters |
Discussion
Approximately 20% of AA cases occur in children. It is proposed that psychological stress contributes to the development of alopecia areata.13 It has been reported that at least 23% of patients experienced an emotional event or crisis prior to the onset of alopecia areata.14 Aside from stress, the development of AA is also associated with personality disorders, paranoia, stress, depression and anxiety disorders. However, there was no correlation found between the severity of AA and psychiatric disorders.15 Children are under a greater degree of stress now than in the past16 Furthermore, the unpredictable course of AA produces psychological distress;17 as a result, AA patients more often experience depression and anxiety compared to the healthy population.18
Some dermatologists suggest leaving alopecia areata untreated for many patients.19 Hair regrowth can occur spontaneously in approximately 50% of patients without treatment within 1 year,15,18 only approximately 7–10% of AA becoming chronic.15 However, spontaneous hair regrowth of severe AA is rare. Patients of severe forms that started at young age always have poor prognosis.20
However, most Chinese parents find this concept difficult to accept. and the duration of hair loss may be longer than patients expected. There is no definitive guideline for pediatric AA treatment; the management plan should be designed in collaboration with the patients and their parents with full consideration of their goals, benefits and risks of the treatment.1
50% of AA patients have concomitant diseases; mostly atopic disorders, including atopic dermatitis, eczema and allergic rhinitis; and, autoimmune diseases such as thyroiditis and vitiligo; and collagen-vascular diseases such as lupus erythematosus.1,15,21 However, AA can also develop after dupilumab initiation for treating AD.22 Interestingly, was recently examined as a possible therapy for AA.23
In our study, 2 patients had a history of medication (omalizumab and desensitization therapy) before the onset of AA.
Due to possible adverse effects, many parents refuse to consider treatment with intralesional and systemic corticosteroids, or systemic immunosuppressive therapy; they chose topical treatment as their first choice. Previous research found that topical corticosteroids were the preferred first-line treatment.5
We chose 5% topical minoxidil, which has been shown to be more effective than a 2% solution, in combination with topical corticosteroids to improve efficacy15 and decrease irritation for topical therapy. Topical minoxidil may result in unwanted hair growth, distant hypertrichosis, irritation, pruritus and dermatitis.15 In our study, only 1 patient had unwanted hair growth and no other adverse effects that should be correlated with topical corticosteroids.
Despite the fact that there were numerous therapeutic options, including topical treatment, corticosteroid injections, topical immunotherapy, systemic corticosteroid, JAK inhibitor and phototherapy, none of these therapeutic modalities have proven to be consistently efficacious.18,24 The prognosis of pediatric AA is influenced by a number of variables, including age at onset, comorbidities, and the duration of AA. Due to the high relapse rate of AA in children, juvenile onset may cause a decreased rate of recovery,25,26 a reduced response to treatment as the disease progresses, and a lower long-term recovery rate than the initial rate.25 Atopy is a common complication in pediatric AA patients. According to a study of allergy, the yearly cycle of AA in some allergy patients may be specifically caused by seasonal changes in allergen exposure and/or virus load, which reacted a correlation between allergies and the development and recurrence of AA.27
So chronic AA patients had to accept use of other treatments following disease relapse. The duration of disease and medication history influenced the treatment choices of patients and their families. Here, we describe that there were no severely affected patients (SALT < 50) in the traditional group, and the percentages in the tofacitinib group and the MN group were 40% and 57.14%, respectively. The percentage of patients with a long duration of the current hair loss episode (≥1 years) in the tofacitinib group and the MN group was higher than the traditional group (80% versus 33.3%, 71.4%).
In the MN group, 57.14% of subjects had a history of use of medications for AA, while in the tofacitinib group the percentage was 20%, and no patients from the traditional group had a medication history. Patients with a long duration of treatment or a history of treatment failure were more willing to accept tofacitinib or MN treatment.
The curative effect of MN varied from person to person. In our study, 42.86% of patients were non-responders. 14.28% of patients achieved SALT75, and 42.86% of patients achieved SALT100. The cost was highest in this treatment group, which may disappoint the patients and their caregivers. Thus, this issue should be discussed with the patients and their families when formulating a treatment plan.
Limitation
An intrinsic limitation of this study is that it is a retrospective study, and the sample size is small. Because the study focused on pediatric AA patients, many patients were unable to follow-up appropriately, so we simply selected patients for regular check-ups. Also, we did not include a control group, since most patients presented to our hospitals have initially gone to community hospitals, and usually followed up without treatment, so the patients and caregivers refused natural remedy. There were differences in the baseline characteristics of the groups, which had the potential to impact the results in a biased manner. Studies with larger sample sizes will still be needed in the future.
Conclusion
In summary, for newly diagnosed or naive pediatric patients, the traditional treatment was the first-line approach. Although AA may recover despite no treatment with in approximately 1 year, considering the mental stress impacting the patients and their caregivers, traditional therapy can be applied. For long-duration, severe and refractory patients, tofacitinib and microneedling can be alternative options.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
Our study complies with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine (2023-0039).
Consent to Participate
Written informed consent was obtained from the parents. The authors affirm that human research participants provided informed consent for publication of the images in Figures 2-4.
Acknowledgments
Thanks are due to Dr. Richard D Granstein for polish this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is supported by the National Natural Science Foundation of China (82073425, 82003332 and 82373503).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Afford R, Leung AKC, Lam JM. Pediatric alopecia areata. Curr Pediatr Rev. 2021;17(1):45–54. doi:10.2174/1573396316666200430084825
2. Peloquin L, Castelo-Soccio L. Alopecia areata: an update on treatment options for children. Paediatr Drugs. 2017;19(5):411–422. doi:10.1007/s40272-017-0239-z
3. Bilgic O, Bilgiç A, Bahalı K, et al. Psychiatric symptomatology and health-related quality of life in children and adolescents with alopecia areata. J Eur Acad Dermatol Venereol. 2014;28(11):1463–1468. doi:10.1111/jdv.12315
4. Juhasz M, Mesinkovska NA. Are Preferred Scalp Locations for Alopecia Areata Patches a Clue to Neuronal Etiology? Skin Appendage Disord. 2019;5(5):283–287. doi:10.1159/000497392
5. Barton VR, Toussi A, Awasthi S, Kiuru M. Treatment of pediatric alopecia areata: a systematic review. J Am Acad Dermatol. 2022;86(6):1318–1334.
6. Zhou C, Li X, Wang C, et al. Alopecia Areata: an Update on Etiopathogenesis, Diagnosis, and Management. Clin Rev Allergy Immunol. 2021;61(3):403–423. doi:10.1007/s12016-021-08883-0
7. Fertig RM, Gamret AC, Cervantes J, et al. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32(4):564–569. doi:10.1111/jdv.14722
8. Waskiel-Burnat A, Kołodziejak M, Sikora M, et al. Therapeutic management in paediatric alopecia areata: a systematic review. J Eur Acad Dermatol Venereol. 2021;35(6):1299–1308. doi:10.1111/jdv.17187
9. Craiglow BG, King BA. Tofacitinib for the treatment of alopecia areata in preadolescent children. J Am Acad Dermatol. 2019;80(2):568–570. doi:10.1016/j.jaad.2018.08.041
10. Kamath S. Pediatric Game Changers *: oral tofacitinib for the treatment of alopecia areata in children. J Am Acad Dermatol. 2021;84(6):1799. doi:10.1016/j.jaad.2021.02.014
11. Yang D, Chen M, Sun Y, et al. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021;121:119–133. doi:10.1016/j.actbio.2020.12.004
12. English RS Jr, Ruiz S, DoAmaral P. Microneedling and Its Use in Hair Loss Disorders: a Systematic Review. Dermatol Ther (Heidelb). 2022;12(1):41–60. doi:10.1007/s13555-021-00653-2
13. Minokawa Y, Sawada Y, Nakamura M. Lifestyle Factors Involved in the Pathogenesis of Alopecia Areata. Int J Mol Sci. 2022;23(3):1038. doi:10.3390/ijms23031038
14. Brajac I, Tkalčić M, Dragojević DM, et al. Roles of stress, stress perception and trait-anxiety in the onset and course of alopecia areata. J Dermatol. 2003;30(12):871–878. doi:10.1111/j.1346-8138.2003.tb00341.x
15. Hon KL, Luk DCK, Leung AKC, et al. Childhood Alopecia Areata: an Overview of Treatment and Recent Patents. Recent Pat Inflamm Allergy Drug Discov. 2020;14(2):117–132. doi:10.2174/1872213X14999200728145822
16. Nearchou F, Flinn C, Niland R, et al. Exploring the Impact of COVID-19 on Mental Health Outcomes in Children and Adolescents: a Systematic Review. Int J Environ Res Public Health. 2020;17(22):8479. doi:10.3390/ijerph17228479
17. Hunt N, McHale S. The psychological impact of alopecia. Br Med J. 2005;331(7522):951–953. doi:10.1136/bmj.331.7522.951
18. Sterkens A, Lambert J, Bervoets A. Alopecia areata: a review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med. 2021;21(2):215–230. doi:10.1007/s10238-020-00673-w
19. Messenger AG, McKillop J, Farrant P, et al. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166(5):916–926. doi:10.1111/j.1365-2133.2012.10955.x
20. Fernandez-Gonzalez P, Saceda‐Corralo D, Pindado‐Ortega C, et al. Spontaneous hair regrowth in eight patients with severe alopecia areata. Australas J Dermatol. 2018;59(4):e318–e319. doi:10.1111/ajd.12840
21. Lee S, Lee H, Lee CH, et al. Comorbidities in alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):466–477 e16. doi:10.1016/j.jaad.2018.07.013
22. Stander S, Trense Y, Thaçi D, et al. Alopecia areata development in atopic dermatitis patients treated with dupilumab. J Eur Acad Dermatol Venereol. 2020;34(10):e612–e613. doi:10.1111/jdv.16493
23. Guttman-Yassky E, Renert‐Yuval Y, Bares J, et al. Phase 2a randomized clinical trial of dupilumab (anti-IL-4Ralpha) for alopecia areata patients. Allergy. 2022;77(3):897–906. doi:10.1111/all.15071
24. Juarez-Rendon KJ, Rivera Sánchez G, Reyes-López MÁ, et al. Alopecia Areata. Current situation and perspectives. Arch Argent Pediatr. 2017;115(6):e404–e411. doi:10.5546/aap.2017.eng.e404
25. Burroway B, Griggs J, Tosti A. Alopecia totalis and universalis long-term outcomes: a review. J Eur Acad Dermatol Venereol. 2020;34(4):709–715. doi:10.1111/jdv.15994
26. Kridin K, Renert-Yuval Y, Guttman-Yassky E, et al. Alopecia Areata Is Associated with Atopic Diathesis: results from a Population-Based Study of 51,561 Patients. J Allergy Clin Immunol Pract. 2020;8(4):1323–1328 e1. doi:10.1016/j.jaip.2020.01.052
27. Zhang X, McElwee KJ. Allergy promotes alopecia areata in a subset of patients. Exp Dermatol. 2020;29(3):239–242. doi:10.1111/exd.14027
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.