Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Efficacy of zofenopril in combination with thiazide diuretics in patients with acute myocardial infarction: a pooled individual data analysis of four randomized, double-blind, controlled, prospective studies
Authors Borghi C, Omboni S , Reggiardo G, Bacchelli S, Degli Esposti D, Ambrosioni E
Received 15 February 2018
Accepted for publication 30 April 2018
Published 9 July 2018 Volume 2018:14 Pages 1185—1190
DOI https://doi.org/10.2147/TCRM.S165629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Claudio Borghi,1 Stefano Omboni,2 Giorgio Reggiardo,3 Stefano Bacchelli,1 Daniela Degli Esposti,1 Ettore Ambrosioni1
On behalf of the SMILE Working Project
1Unit of Internal Medicine, Policlinico S. Orsola, University of Bologna, Bologna, Italy; 2Clinical Research Unit, Italian Institute of Telemedicine, Varese, Italy; 3Mediservice S.r.l., Agrate Brianza, Italy
Background: In the Survival of Myocardial Infarction Long-Term Evaluation (SMILE) studies, early administration of zofenopril after acute myocardial infarction (AMI) was prognostically beneficial as compared to placebo and other angiotensin-converting enzyme inhibitors (ACEIs), such as lisinopril and ramipril. Here, we investigated whether zofenopril efficacy could be affected by a concomitant use of thiazide diuretics (TDs).
Methods: This was a post hoc analysis of pooled individual patient data from the SMILE studies. Patients treated with other diuretics than TDs were excluded. The primary study endpoint was the 1-year combined occurrence of death or hospitalization for CV causes, with or without TD.
Results: Among 2,995 patients, 263 (8.8%) were treated with a combination including a TD (TD+), whereas 2,732 (91.2%) were not treated with any diuretic (TD-). Proportions of subjects who were treated with TD were equally distributed (p=0.774) within the placebo, zofenopril, and other ACEIs groups. The 1-year risk of major cardiovascular events was similar in TD+ (18.3%) and TD- (16.8%) patients (hazard ratio [HR] 1.04; 95% CI 0.74–1.45; p=0.838). After stratifying per concomitant treatment and TD, the 1-year risk of CV events was significantly lower with zofenopril than with placebo (HR 0.70; 95% CI 0.55–0.88; p=0.002) and other ACEIs (HR 0.58; 95% CI 0.46–0.74; p=0.0001). Treatment with ACEIs and TD as concomitant therapy was associated with a larger blood pressure (BP) reduction (p=0.0001 for systolic BP and p=0.045 for diastolic BP).
Conclusion: In post AMI patients, zofenopril maintained its positive impact on prognosis compared to placebo or other ACEIs, regardless concomitant TD administration. In this setting, TD shows advantages in managing the most difficult hypertensive patients.
Keywords: acute myocardial infarction, drug therapy, angiotensin-converting enzyme inhibitors, thiazide diuretics, cardiovascular risk
Introduction
Hypertension is a key risk factor for cardiovascular (CV) diseases and it should be strictly controlled in patients after acute myocardial infarction (AMI) who are at very high CV risk.1 For all patients with hypertension, European Society of Cardiology guidelines recommend lifestyle measures, including weight control, increased physical activity, alcohol moderation, sodium restriction, and increased consumption of fruits and vegetables.1 In addition, for patients with any grade of hypertension at very high risk, including those with AMI, a drug treatment should be started early to lower blood pressure (BP) to <140/90 mmHg, or to <130/80 mmHg in case of diabetes or chronic kidney disease.2 All antihypertensive drugs are similarly effective to control BP, but a combination of several molecules is often required to achieve the BP target.1 The antihypertensive effects may be enhanced when two complementary drugs are administrated: a combination may counteract the regulatory mechanisms triggered whenever pharmacological intervention on BP lowering is initiated.3 Indeed, it has been demonstrated that the combination of a renin–angiotensin system blocker and low dose of diuretic can counteract the renin rise; the efficacy of combination in terms of BP control is increased compared to monotherapy, without diminishing tolerability.3 In pivotal clinical trials, the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) initiated early after AMI has been widely established to prevent ventricular remodeling, decrease the risk of heart failure, and improve overall survival.4 The American College of Cardiology/American Heart Association strongly recommends that an ACEI or ARB should be started and continued indefinitely in all patients recovering from an acute coronary syndrome with left ventricular ejection fraction (LVEF) ≤40% and for those with hypertension, diabetes mellitus, or chronic kidney disease (class 1, level A).5 The same guidelines recommend that ACEI may be combined with beta-blockers, aspirin, and diuretics if required and if the patient is hemodynamically stable (class 1, level C). Among ACEIs, zofenopril has been extensively tested in post-AMI setting in the Survival of Myocardial Infarction Long-Term Evaluation (SMILE) program where it has showed prognostic advantages in reducing the incidence of major CV events at 1 year, compared to ramipril, lisinopril, or placebo.6–9 Zofenopril may be combined with hydrochlorothiazide, a short-acting thiazide diuretic (TD) that reduces the reabsorption of electrolytes from the renal tubules, to lower BP in patients with uncontrolled hypertension,10,11 diabetes,12 and metabolic syndrome.13 In these settings, fixed-dose combination of zofenopril and hydrochlorothiazide has showed a favorable profile in terms of efficacy and safety. Data on the effects of zofenopril and TD combination in post-AMI management are lacking. This post hoc analysis on individual patient data from the four SMILE studies evaluates whether a concomitant treatment with TD may affect the efficacy of zofenopril in preventing the 1-year CV risk, compared with placebo or other ACEIs.
Methods
Study design and population
This post hoc analysis was carried out on the pooled Individual Patient Data of the SMILE Database. Briefly, the SMILE program comprised four double-blind, randomized, parallel-group clinical trials that analyzed the efficacy and safety of zofenopril compared to placebo (SMILE-1 and 3),6,8 lisinopril (SMILE-2),7 or ramipril (SMILE-4)9 in post-AMI phase. Main inclusion criteria were as follows: 1) AMI diagnosis within <24 hours, not eligible for thrombolytic therapy (SMILE-1);6 2) AMI diagnosis and a prior thrombolytic treatment within 12 hours from onset of AMI clinical symptoms (SMILE-2);7 3) prior AMI within 6±1 weeks with LVEF >40%, treated with thrombolytic therapy and ACE-inhibitors (SMILE-3);8 and 4) AMI within <24 hours, with or without thrombolysis, with primary percutaneous transluminal angioplasty or coronary artery bypass graft, and clinical and/or echocardiographic evidence of left ventricular dysfunction (SMILE-4).9 Refer to the original study for details on study design and inclusion and exclusion criteria.
All studies were conducted in accordance with the Guidelines for Good Clinical Practice and the Declaration of Helsinki and were approved by the Institutional Review Board of the University of Bologna (study coordinator) as well as by the local ethics committees when required (a list of centers may be found in the original study publications).6–9 Written informed consent was obtained from each patient before enrolment.
Eligible patients were randomized in two groups to receive zofenopril or comparator, in addition to standard recommended therapy for AMI. Zofenopril dosing was 7.5 mg twice daily on day 1 and 2, 15 mg twice daily on day 3 and 4, and 30 mg twice daily from day 5 onward. Lisinopril and ramipril were uptitrated up to 10 mg once daily and to 5 mg twice daily, respectively.
Statistical analysis
The primary study endpoint of this retrospective analysis was the 1-year combined occurrence of death or hospitalization for CV causes in patients who were treated with a combination including thiazides and in those who did not receive any diuretics. All randomized patients treated with at least one dose of study medication and documenting at least once the measure of primary efficacy assessment, even in case of protocol violation or premature withdrawal from the study were included in the analysis. Patients who were treated with any diuretics different from thiazides were excluded from the analysis. The efficacy end point was calculated after weighing for the number of subjects contributing from each study.
Due to different duration across studies, the relative risk of the composite endpoint was estimated using a time-dependent Cox proportional-hazard regression model. Hazard ratios (HRs) and 95% CI were calculated, and survival curves were modeled. The absolute occurrence of events was compared by a logistic regression analysis and adjusted for sex, age (≥65 years), and metabolic syndrome. A patient had the metabolic syndrome when at least 3 out of the following 5 risk factors were present: 1) elevated waist circumference (≥102 cm males and ≥88 females); 2) elevated triglycerides (≥150 mg/dL) or under specific lipid-lowering pharmacological treatment; 3) reduced HDL cholesterol (<40 mg/dL in males and <50 mg/dL in females) or under specific lipid-lowering pharmacological treatment; 4) elevated office BP (systolic ≥130 mmHg and/or diastolic ≥85 mmHg) or under antihypertensive drug treatment; and 5) elevated fasting glucose (≥100 mg/dL) or treated with antidiabetic drugs.14 Data on waist circumference were not available; therefore, central obesity was defined as BMI ≥25 kg/m2.
BP changes from baseline to the end of study were compared in a general linear model analysis that included treatment and metabolic syndrome as factors, and age and baseline BP as covariates. Categorical variables were compared using chi-squared test, while continuous variables were compared using Student’s t-test. All p-values were 2 sided, and statistical significance was set at p<0.05. Data are shown as mean±SD or as mean and 95% CI or as absolute (n) and relative (%) frequencies.
Results
Patient characteristics
Among 2,995 patients included in the analysis, hereafter defined as “overall population”, 833 (27.8%) were treated with placebo, 1,479 (49.4%) with zofenopril, and 683 (22.8%) with other ACEIs. Concomitantly, 263 (8.8%) patients received a combination including thiazides (TD+) and 2,732 (91.2%) were not treated with any diuretics (TD−). The mean follow-up was similar in the two groups (TD+ 7.1 vs TD− 7.4 months, p=0.268).
Baseline characteristics are summarized in Table 1. TD+ patients were older (65.7±9.5 vs 60.9±10.7 years; p<0.001), more frequently women (36.1 vs 23.0%; p<0.001), with higher systolic BP (139.9±24.1 vs 136.2±21.1 mmHg; p=0.006) and diastolic BP (84.5±12.7 vs 82.6±12.3 mmHg; p=0.015) and heart rate (84.1±18.7 vs 78.9±15.5 bpm; p<0.001). Except for BP, these differences were maintained as significant after stratifying the population per treatment.
Cardiovascular outcomes
In the overall population, the proportion of patients who died or were hospitalized at least once for CV causes during the study period was similar between TD+ (18.3%) and TD− patients (16.8%); concomitant treatment with thiazides did not affect the 1-year risk for combined occurrence of CV events (HR 1.04; CI 95% 0.74, 1.45; p=0.838, logistic regression analysis). Consistently, when the overall population was stratified per treatment, the prevalence of major CV events with or without TD was even similar.
As shown in Figure 1, no difference was observed in the prevalence of major CV events between TD+ and TD− patients treated with zofenopril (13.8% vs 13.9%; p=0.522) or the other two ACEIs (14.3% vs 21.4%; p=0.228), whereas in placebo-treated patients a larger prevalence of events was observed in patients treated with a diuretic (TD+: 28.6% vs TD−: 18.3%), although the difference did not achieve the statistical significance (p=0.100). In the logistic regression analysis accounting for concomitant TD treatment, the 1-year risk of CV events was significantly lower with zofenopril than with placebo (HR 0.70; 95% CI 0.55–0.88; p=0.002) and other ACEIs (HR 0.58; 95% CI 0.46–0.74; p=0.0001).
Among patients who received any ACEIs, the combination of zofenopril plus TD significantly decreased the 1-year risk of major CV events by 42% compared to other ACEIs combined with TD (Figure 2).
Effect on blood pressure
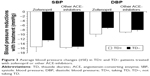
Average office systolic and diastolic BP changes from baseline to the end of study are shown in Figure 3. Regardless of ACEIs used, BP reduction was slightly but significantly greater with concomitant TD (p=0.0001 for systolic BP and p=0.045 for diastolic BP).
Discussion
This post hoc analysis of the SMILE studies evaluated whether concomitant administration of TDs may interfere with zofenopril efficacy in post-AMI setting. The results showed that thiazides did not affect the 1-year risk of CV events in patients treated with either zofenopril or other ACEIs; the zofenopril superiority in preventing CV risk compared to other ACEIs was also confirmed, regardless the concomitant use of diuretics. However, the combination with thiazides was more effective in lowering BP, thus suggesting that the protective properties of zofenopril were beyond its antihypertensive effect. Ancillary characteristics of ACEIs may explain these results. Angiotensin-converting enzyme regulates the balance between the vasoconstrictive and salt-retentive renin–angiotensin system and the vasodilatory and natriuretic kallikrein–kinin one.15 Therefore, in addition to their initial use as antihypertensive drugs, ACEIs have became a fundamental treatment for congestive heart failure, left ventricular dysfunction after AMI, and diabetic and nondiabetic nephropathies.16 Sulfhydryl-containing ACEIs, such as zofenopril, can act as antioxidants by scavenging superoxide anion as well as non-superoxide radicals.17,18 In animal models of NO deficiency-induced hypertension, the combination zofenopril plus hydrochlorothiazide has been more efficient to reduce glomerular and tubular alterations than enalapril plus diuretic; the protective effect has been associated to an increased eNOS expression and an oxidative stress decrease.19 The relationship among zofenopril, NOS activation, and oxidative stress has been further confirmed in essential hypertensive patients, thus suggesting a possible role in delaying vascular dysfunction and atherosclerosis.20 In addition, long-term treatment with zofenopril has slowed the progression of intima-media thickness of carotid arteries in newly diagnosed mildly hypertensive patients, compared to enalapril.21 The antioxidant activity of zofenopril has been associated to both the presence of a sulfhydryl group and its high lipophilicity, which guarantees more pronounced intracellular effects.16,22 Furthermore, high lipophilicity favors tissue accumulation of zofenopril when it is concomitantly administered with hydrochlorothiazide, thus further prolonging its antihypertensive action.23 In this retrospective analysis, patients who received a TD had higher BP and heart rate. Therefore, even if zofenopril has been resulted as more efficient without TD, major improvement in hypertension may be beneficial in patients difficult to manage. The combination of hydrochlorothiazide and zofenopril has demonstrated high efficacy in several settings to control BP.11,12,24 In patients with metabolic syndrome, the fixed combination of zofenopril plus hydrochlorothiazide has been more efficient than zofenopril alone in reducing BP, thus providing a valuable therapeutic option for these patients who are at a greater risk for CV events.13 Similar outcomes have been reported in patients with essential hypertension and high risk of CV diseases, where a combined treatment with zofenopril and hydrochlorothiazide has been associated with greater BP decrease than monotherapy with zofenopril. The between-treatment difference has been proportionally greater in patients at higher risk, thus indicating that they may have more benefits from the combined treatment.24 In elderly patients (older than 65 years) with isolated systolic hypertension untreated or uncontrolled by previous monotherapy, daytime systolic BP has been normalized with zofenopril and hydrochlorothiazide within 18 months, without reporting significant drug-related adverse events.25
The study has some limitations. First, it has been designed as a post hoc analysis. The population size of thiazide group is limited compared to overall population and other antihypertensive treatments, except diuretics, were administered. This may represent a confounding factor that may lead to misinterpretation of results. Furthermore, baseline characteristics were not completely homogeneous, and the higher BP values at entry in the thiazide group may have interfered with the clinical outcomes at 1 year. There are two important points that deserve to be discussed as potential study confounders. A marginally (non-significantly) larger prevalence of CV events was observed in the placebo group treated with TD compared to those patients of the same group not treated with TD. The discrepancy may be due to the fact that patients in the placebo group were treated with standard CV drugs with the exclusion of ACEI. There might have been an unbalance in the type and intensity of treatment between the two TD+/TD− groups treated with placebo. Another point regards the fact that we only looked at TDs, which might not be the ideal diuretic to be used in post-AMI. However, the combination of zofenopril and a TD is a common recommended treatment for patients with hypertension or CV disease and we were interested to check for the efficacy of such a combination.
Conclusion
In post-AMI patients, zofenopril confirmed its efficacy in preventing CV events at 1 year, regardless the concomitant treatment with thiazides. TDs could be useful to manage uncontrolled hypertensive patients and, in any cases, did not interfere with the beneficial effect of zofenopril after AMI. Further prospective studies should be advisable to identify patients who could have major benefits from the combination of zofenopril and TD.
Acknowledgments
This work was financially supported by Menarini International Operations Luxembourg S.A. through an unconditional and unrestricted grant. The funding source did not influence or commented on planned methods, protocol, data analysis, and the draft report.
Disclosure
Prof Claudio Borghi receives consultancy fee from Boehringer Ingelheim, Menarini International, Sanofi, Amgen, Takeda, Novartis, Ely Lilly, and Servier. The other authors report no conflicts of interest in this work.
References
Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;252:207–274. | ||
Aronow WS. Office management after myocardial infarction. Am J Med. 2010;123(7):593–595. | ||
Waeber B. Combination therapy with ACE inhibitors/angiotensin II receptor antagonists and diuretics in hypertension. Expert Rev Cardiovasc Ther. 2003;1(1):43–50. | ||
Bainey KR, Armstrong PW, Fonarow GC, et al. Use of renin-angiotensin system blockers in acute coronary syndromes: findings from Get With the Guidelines-Coronary Artery Disease Program. Circ Cardiovasc Qual Outcomes. 2014;7(2):227–235. | ||
Amsterdam EA, Wenger NK, Brindis RG, et al; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):2354–2394. | ||
Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. N Engl J Med. 1995;332(2):80–85. | ||
Borghi C, Ambrosioni E; Survival of Myocardial Infarction Long-Term Evaluation-2 Working Party. Double-blind comparison between zofenopril and lisinopril in patients with acute myocardial infarction: results of the Survival of Myocardial Infarction Long-Term Evaluation-2 (SMILE-2) Study. Am Heart J. 2003;145(1):80–87. | ||
Borghi C, Ambrosioni E; Survival of Myocardial Long-term Evaluation Study Group. Effects of zofenopril on myocardial ischemia in post-myocardial infarction patients with preserved left ventricular function: the Survival of Myocardial Infarction Long-term Evaluation (SMILE)-ISCHEMIA Study. Am Heart J. 2007;153(3):445.e7–e14. | ||
Borghi C, Ambrosioni E, Novo S, Vinereanu D, Ambrosio G; SMILE-4 Working Party. Comparison between zofenopril and ramipril in combination with acetylsalicylic acid in patients with left ventricular systolic dysfunction after acute myocardial infarction: results of a randomized, double-blind, parallel-group, multicenter, European study (SMILE-4). Clin Cardiol. 2012;35(7):416–423. | ||
Omboni S, Malacco E, Parati G. Zofenopril plus hydrochlorothiazide fixed combination in the treatment of hypertension and associated clinical conditions. Cardiovasc Ther. 2009;27(4):275–288. | ||
Borghi C, Omboni S. Zofenopril plus hydrochlorothiazide combination in the treatment of hypertension: an update. Expert Rev Cardiovasc Ther. 2014;12(9):1055–1065. | ||
Agabiti-Rosei E, Manolis A, Zava D, Omboni S; ZODIAC Study Group. Zofenopril plus hydrochlorothiazide and irbesartan plus hydrochlorothiazide in previously treated and uncontrolled diabetic and non-diabetic essential hypertensive patients. Adv Ther. 2014;31(2):217–233. | ||
Malacco E, Omboni S. Antihypertensive efficacy of zofenopril plus hydrochlorothiazide fixed combination for treatment in metabolic syndrome. Adv Ther. 2007;24(5):1006–1015. | ||
Alberti KG, Eckel RH, Grundy SM, et al; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(6):1640–1645. | ||
Riordan JF. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4(8):225. | ||
Desideri G, Grassi D, Croce G, et al. Different effects of angiotensin converting enzyme inhibitors on endothelin-1 and nitric oxide balance in human vascular endothelial cells: evidence of an oxidant-sensitive pathway. Mediators Inflamm. 2008;2008:305087. | ||
Liu X, Engelman RM, Rousou JA, Cordis GA, Das DK. Attenuation of myocardial reperfusion injury by sulfhydryl-containing angiotensin converting enzyme inhibitors. Cardiovasc Drugs Ther. 1992;6(4):437–443. | ||
Mak IT, Freedman AM, Dickens BF, Weglicki WB. Protective effects of sulfhydryl-containing angiotensin converting enzyme inhibitors against free radical injury in endothelial cells. Biochem Pharmacol. 1990;40(9):2169–2175. | ||
García-Estañ J, Ortiz MC, O’Valle F, et al. Effects of angiotensin-converting-enzyme inhibitors in combination with diuretics on blood pressure and renal injury in nitric oxide-deficiency-induced hypertension in rats. Clin Sci (Lond). 2006;110(2):227–233. | ||
Napoli C, Sica V, de Nigris F, et al. Sulfhydryl angiotensin-converting enzyme inhibition induces sustained reduction of systemic oxidative stress and improves the nitric oxide pathway in patients with essential hypertension. Am Heart J. 2004;148(1):e5. | ||
Napoli C, Bruzzese G, Ignarro LJ, et al. Long-term treatment with sulfhydryl angiotensin-converting enzyme inhibition reduces carotid intima-media thickening and improves the nitric oxide/oxidative stress pathways in newly diagnosed patients with mild to moderate primary hypertension. Am Heart J. 2008;156(6):1154.e1–e8. | ||
Evangelista S, Manzini S. Antioxidant and cardioprotective properties of the sulphydryl angiotensin-converting enzyme inhibitor zofenopril. J Int Med Res. 2005;33(1):42–54. | ||
Westendorp B, Schoemaker RG, van Gilst WH, van Veldhuisen DJ, Buikema H. Hydrochlorothiazide increases plasma or tissue angiotensin-converting enzyme-inhibitor drug levels in rats with myocardial infarction: differential effects on lisinopril and zofenopril. Eur J Pharmacol. 2005;527(1–3):141–149. | ||
Malacco E, Omboni S; Study Group. Antihypertensive effect of zofenopril plus hydrochlorothiazide versus zofenopril monotherapy in patients with essential hypertension according to their cardiovascular risk level: a post hoc analysis. Curr Ther Res Clin Exp. 2008;69(3):232–242. | ||
Modesti PA, Omboni S, Taddei S, et al. Zofenopril or irbesartan plus hydrochlorothiazide in elderly patients with isolated systolic hypertension untreated or uncontrolled by previous treatment: a double-blind, randomized study. J Hypertens. 2016;34(3):576–587. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.