Back to Journals » International Medical Case Reports Journal » Volume 16
Efficacy of Remifentanil Intravenous Patient-Controlled Analgesia in Singleton Parturients During the Second Stage of Labor: A Single-Arm, Prospective Study
Authors Li J, Cai J, Li J, Li Z
Received 25 July 2023
Accepted for publication 26 September 2023
Published 11 October 2023 Volume 2023:16 Pages 673—678
DOI https://doi.org/10.2147/IMCRJ.S432093
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jiexiong Li,1,* Jiachun Cai,2,* Jianjin Li,1 Zhitao Li1
1Department of Anesthesiology, Peking University Shenzhen Hospital, Shenzhen, People’s Republic of China; 2Department of Fundus Disease, Shenzhen Eye Hospital, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhitao Li, Email [email protected]
Background: Worrying about increasing the use of instrument-assisted delivery and prolonging the process, midwives in our institution tended to persuade the parturients who had entered the second stage of labor, to give up neuraxial analgesia. Remifentanil seemed to be suitable for this situation due to its advantages of fast onset and metabolism. We designed a single arm and prospective study to explore the analgesic effect and safety of remifentanil intravenous patient-controlled analgesia (PCA) in singleton parturients during the second stage of labor.
Methods: Ten pregnancies were included, whose cervical dilation had exceeded 8 cm when they arrived into the delivery room. The patient was given 40 μg remifentanil intravenously as soon as she felt the contraction with a lockout time of 2 minutes. The maternal heart rate (MHR), mean arterial pressure (MAP), SpO2, fetal heart rate (FHR), OAA/S score, and VAS analgesia score were recorded before and at 5, 10, 20, 30, and 60 minutes after administration. An umbilical artery blood gas analysis and Apgar scores at 1 and 5 minutes were also recorded after the delivery of the newborn. The incidence of adverse events and overall satisfaction with analgesia were recorded.
Results: The VAS score decreased from 9.2 to 4.6 after remifentanil administration. All umbilical artery PH values were beyond 7.20. All 1-minute newborns’ Apgar scores were 8 points or greater, and 9 points or greater in the 5th minute. Adverse events included 3 cases of dizziness, 3 cases of nausea, 1 case of vomiting, and 1 case of parturients had a transient FHR drop at the 10th minute, accompanied by hypoxemia and hypersomnia. The overall satisfaction rate was 70%.
Conclusion: Remifentanil PCA could effectively relieve the severe uterine contraction pain suffered by the parturients in the second stage of labor with safety.
Keywords: labor, analgesia, remifentanil
Introduction
Satisfactory analgesia is of paramount importance in labor. Neuraxial blockade is still the most effective and safe method (gold standard) for labor analgesia. However, part of the parturients could not receive labor analgesia services due to the neuraxial analgesia being contraindicated, or the labor process progressing too fast. Besides, midwives might worry neuraxial analgesia would increase the use of instrument-assisted delivery and prolong the second stage of labor.1,2 An effective systemic analgesia that ideally has rapid onset and offset, matches the time course of uterine contractions, and does not compromise the fetus is required in the case.
Remifentanil is an ultra-short-acting mu-1 opioid receptor agonist with a rapid onset of action and a biexponential decay curve. It is rapidly metabolized and redistributed in the fetus. This pharmacokinetic profile gives remifentanil an advantage over other opioids used for labor analgesia, as an alternative method for parturients who do not want neuraxial analgesia or when its use is contraindicated.3 In some medical institutions in Europe, it was a free choice of remifentanil PCA or neuraxial analgesia; even neuraxial analgesia is not contraindicated.4 Most of the research on remifentanil focused on the first stage of labor, and even some researchers stopped remifentanil PCA in the second stage, concerning the respiratory depression of opioids in the newborn with a background basic continuous infusion.5,6 Little research has studied the analgesic efficacy of remifentanil in the second stage of labor solely. So the main objective of our study is to explore the analgesic effect and safety of a single bolus remifentanil intravenous patient-controlled analgesia in singleton parturients during the second stage of labor.
Methods
The protocol was approved by Medical Ethics Committee and registered at the Chinese Clinical Trials Registry (ChiCTR1900024037). Ten singleton uncomplicated pregnancies, aged 20–40 years, with an ASA rating of 1–2 levels were included, whose uterine orifice dilation had exceeded 8 cm when they were sent to the delivery room. Written informed consent was obtained from all participants. Exclusion criteria: parturients with no contraindication strongly demanding neuraxial analgesia, multiple-birth pregnancies, morbid obesity (BMI >35 kg/m2), allergy to remifentanil, a history of opioids addiction, and suspected of fetus congenital deformity.
Upon arrival in the delivery room, a dedicated intravenous cannula was inserted at the left upper limb peripheral vein, dedicated to remifentanil infusion. Another intravenous cannula was inserted on the other hand and Ringer’s lactate infusion was started. Electrocardiogram, SpO2, MAP, FHR, and uterine contraction pressure were monitored routinely. Supplemental oxygen via a venturi mask at 3 L/min was administered throughout the delivery. Hydrochloric acid remifentanil 1 mg was diluted by 0.9% sodium chloride 100 mL (solution 10 μg/mL). Infusion of remifentanil was administered by a wireless analgesia system, developed by Jiangsu Renxian Medical Technology Co., Ltd. The woman pressed the analgesic button by herself when she felt the contraction. A bolus dose of 40 μg remifentanil was administered intravenously as soon as she pressed the analgesic button followed by a lockout time of 2 minutes, without background infusion. The device was stopped about 10 minutes prior to cord clamping.
As the puerpera entered the labor stage, the uterine contraction interval changed to 1–15 minutes and lasted 30–40 seconds during the incubation period of the first stage of labor, and increased to 3–4 minutes and lasted 40–60 seconds during the active stage, reduced to 60 seconds in duration with 1–2 min rest between contractions in the second stage. Parturients could feel the contraction when the intrauterine pressure reached 10 mmHg and feel pain when it reached 15–20 mmHg. We taught the parturients to press the button when she felt the beginning of contractions. As the infusion rate was 300 mL/h, 48 seconds was needed to inject 40 µg remifentanil (4mL) and the first contraction ended at the same time. By doing so, remifentanil took effect before the next contraction, and the analgesic peak effect just overlapped the peak of the contraction (Figure 1).
Observation Indicators
The MHR, MAP, SpO2, FHR, OAA/S sedation score, and VAS analgesia score were recorded before and at 5th, 10th, 20th, 30th, and 60th minutes after the administration. The VAS score ranged from 0 to 10, a 0 score meant no pain, and a 10 score meant the worst pain imaginable. Gravidity, gestational weeks, duration of analgesia, the dosage of remifentanil, the umbilical artery blood gas analysis, and Apgar scores at 1 and 5 minutes after delivery of the newborn were recorded. The incidence of adverse events such as nausea and vomiting, dizziness, hypersomnia, hypoxemia (SpO2<94%), skin pruritus, and abnormal FHR were all recorded. Maternal satisfaction with analgesia was recorded, followed by another anesthesiologist the day after delivery (classified by very satisfied, satisfied, average, dissatisfied, and very dissatisfied).
Safety Evaluation
Adverse events were used to evaluate the safety of remifentanil intravenous patient-controlled analgesia (PCA), involving bradycardia (heart rate <50 bpm), hypotension (MAP below 60 mm Hg), and hypoxemia (Spo2 dropped below 94%).
If bradycardia happened, the patient would be treated with atropine 0.5 mg i.v. Ephedrine 5 mg and Ringer’s lactate 5 mL/kg were administered i.v. when hypotension occurred. The effect of these treatments was assessed every 1 minute and if the heart rate or the blood pressure did not return to normal 5 minutes after the treatment, the aforementioned treatment would be repeated until they reach normal. If Spo2 dropped below 94% (hypoxemia), the anesthesiologists lightly tapped on the shoulder, verbally urging the patient to breathe. We would use a mask for pressurized oxygen delivery to maintain ventilation when the parturients did not respond to the stimulation. If FHR is of abnormality, suspend the use of remifentanil, adjust the position of parturients, and find the reason actively.
Statistics Analysis
Data were shown as mean ± standard deviation, median (range), or the number of patients. Statistical analyses were performed using SPSS 22.0 (SPSS Inc, Chicago, IL). Charts were made using the GraphPad prism software. P < 0.05 was considered to be statistically significant.
Results
A total of 10 parturients were enrolled. The parturients’ age, height, weight, gravidity, gestational weeks, duration of analgesia, dosage of remifentanil, umbilical artery PH value, and Apgar scores at 1 and 5 minutes after delivery of the newborn are shown in Table 1. There were 2 primiparas and 8 multiparas enrolled, and one of them already had sixth natural births. The average dosage of remifentanil was 570 μg, up to 880 μg. The duration of the analgesia was 53 minutes, ranging from 25 min to 86 min. The VAS scores decreased from 9.2 to 4.6 at 5th min after the first administration of remifentanil, with a decrease rate of 50% (Figure 2). Then, the scores gradually increased, almost reached to 5 points at the 60th minute.
 |
Table 1 Patients’ Demographic Data |
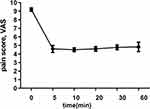 |
Figure 2 The VAS scores decreased from 9.2 to 4.6 after 5 min administrations of remifentanil, with a decrease rate of 50%. |
All umbilical artery PH values were beyond 7.20, ranging from 7.21 to 7.37, and the newborn’s 1-minute Apgar scores were both beyond 8 points and the 5-minute Apgar scores beyond 9 points.
Maternal heart rate decreased significantly in 5 minutes after analgesia with no bradycardia (HR below 50 beats). Adverse events included 3 cases of dizziness, 3 cases of nausea, 1 case of vomiting, and 1 case of parturients had a transient FHR drop at the 10th minute, accompanied by hypoxemia (SpO2 dropped to 91%) and hypersomnia (OAA/S score 3 points). The maternal satisfaction with analgesia was very satisfactory in 2 cases, satisfactory in 5 cases, and average in 3 cases. The overall satisfaction rate was 70%. The 3 cases of parturients who scored as average were all accompanied by nausea and vomiting.
Discussion
Remifentanil could be given as an intermittent patient-controlled bolus at a lockout interval in labor analgesia, and with or without a background infusion. It could be an alternative treatment when there is a contraindication of neuraxial analgesia or no opportunity to implement intraspinal analgesia.5–7 In our small sample size observation study, remifentanil PCA was effective and safe in the second stage of labor. It had no increased severe adverse effects in maternal and neonatal.
Referring to previous studies and considering that the uterine cavity pressure of the parturients in the second stage of labor was significantly higher than that in the first stage, we set the single PCA dose at 40μg and the locking time to 2 minutes.3,7 The effective analgesic dose of remifentanil was reported to have a wide interindividual variation even with different stages of labor, but the timing, the rate of bolus delivery, and the lockout interval may be more important.3 Due to the long interval of 1–15 min between contractions in the first stage, the onset and peak time of a single dose of remifentanil was difficult to be consistent with the next contraction. If the background dose was used or increased, the parturients who lacked pain stimulation were prone to respiratory depression during the interval of contraction. So the effectiveness of remifentanil on labor pain was controversial.8,9 The interval and duration of contraction were both shortened to 1–2 minutes in the second stage. We educated the parturients to master the timing of self-control administration and adjusted the drug concentration of remifentanil (10μg/mL) and the dosing rate of pulse pump (300mL/h) to ensure that remifentanil took effect before the next uterine contraction, and the peak time of drug effect and contraction was relatively consistent. Our results have shown that the average pain VAS score of parturients had decreased from 9.2 to 4.6, and the degree of pain relief was up to 50%. But the pain scores gradually increased, which might be related to drug tolerance and labor progress.8
Another controversial issue with remifentanil PCA was the safety of parturients and neonates, for its respiratory inhibition. There were about 27% of the maternal still needed oxygen supplementation or wake-up stimulation because of hypoxemia, and even several studies have reported that maternal cardiopulmonary arrest caused by respiratory depression.10–12 However, risk factors for respiratory depression included excessive bolus doses of remifentanil, background doses applied in the intermittent contractions, history of opioid abuse, and medication errors.4,8 Studies have shown that the application of background doses did not improve analgesic effects but significantly increased opioid adverse effects.13 In our study, only 1 case was hypoxemia (10%), significantly lower than that reported in other studies. It might be associated with our small sample size, or be without a background dose. A dedicated intravenous cannula was inserted exclusively for remifentanil PCA, to avoid artificial mistakes as much as possible. In addition, the labor pain was intense in the second stage, and the midwife will repeatedly guide the mother to exert force, reducing the risk of respiratory depression.
Remifentanil crossed the placenta rapidly with a mean umbilical vein to maternal artery concentration ratio of 0.88. However, the mean umbilical artery to umbilical vein concentration ratio was 0.29, demonstrating that this drug was rapidly metabolized and redistributed in the fetus.14 Referring to the Swiss RemiPCA safe network (https://www.remipca.org/php/en/index.php), our remifentanil PCA was stopped 10 min prior to cord clamping.4 The PH of umbilical cord gases and Apgar scores at 1 minute and 5 minutes were within normal limits, with no neonatal resuscitation. However, our study sample was too small, and another study reported that about 4% of newborns needed mask-assisted oxygen ventilation.4 It was still necessary to be vigilant and ready for neonatal resuscitation, monitored by neonatal doctors entirely if necessary.
The nausea and vomiting were 40% in our study, significantly higher than the latest data (17%.1) reported by RemiPCA safe network, which might be related to midwives in our hospital tending to advise women to drink broth, which might also be due to inconsistent evaluation criteria of nausea and vomiting among different researchers. In addition, our overall satisfaction rate was 70%. The 3 cases of mothers who scored average were all accompanied by nausea and vomiting which were described as the most unpleasant memory during delivery rather than contraction pain.
Limitations
The main limitation of our study was the small sample size. The medical ethics committee approved only 10 parturients for inclusion in the study because of safety concerns. Another limitation was that there were more subjective indicators and less objective indicators, especially respiration, oxygen saturation, uterine contraction, and other indicators directly related to maternal safety, and only pH value of blood gas in umbilical artery was recorded.
Conclusions
In conclusion, 40 μg bolus of remifentanil PCA with a lockout time of 2 minutes could effectively relieve the severe labor pain of our 10 parturients in the second stage of labor. But it was still necessary to be vigilant about its impact on the breathing of the parturients and the newborn, prevent and treat nausea and vomiting, and improve the overall satisfaction of the parturients. We recommend that anesthesiologists should be stationed in the delivery room to provide one-to-one bedside monitoring services for parturients.
Ethics Statement
The protocol was approved by Medical Ethics Committee and registered at the Chinese Clinical Trials Registry (ChiCTR1900024037). Written informed consent was obtained from all participants. The study was conducted according to the Declaration of Helsinki. Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Acknowledgments
We gratefully acknowledge the kind cooperation of Dr Tao Luo, Dongling Chen, and Rui Zhou, Department of Anaesthesiology, Peking University Shenzhen Hospital, in the preparation and modification of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the General Program for Clinical Research at Peking University Shenzhen Hospital (No. LCYJ202004).
Disclosure
None of the authors declare any conflict of interest for this work.
References
1. Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UK. Effect of low-dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet. 2001;358(9275):19–23.
2. Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour (Review). Cochrane Database Syst Rev. 2011;12(12):D331.
3. Hinova A, Fernando R. Systemic remifentanil for labor analgesia. Anesth Analg. 2009;109(6):1925–1929.
4. Melber AA, Jelting Y, Huber M, et al. Remifentanil patient-controlled analgesia in labour: six-year audit of outcome data of the RemiPCA SAFE Network (2010–2015). Int J Obstet Anesth. 2019;39:12–21.
5. Volmanen P, Sarvela J, Akural EI, et al. Intravenous remifentanil vs. epidural levobupivacaine with fentanyl for pain relief in early labour: a randomised, controlled, double-blinded study. Acta Anaesthesiol Scand. 2010;52(2):249–255.
6. Volmanen PV, Akural EI, Raudaskoski T, et al. Timing of intravenous patient-controlled remifentanil bolus during early labour. Acta Anaesthesiol Scand. 2011;55(4):486–494.
7. Daga V. Intravenous remifentanil patient-controlled analgesia versus intramuscular pethidine for pain relief in labour (RESPITE): an open-label, multicentre, randomised controlled trial. Lancet. 2018;392(10148):662–672.
8. Velde M. remifentanil patient-controlled intravenous analgesia for labor pain relief: is it really an option to consider? Anesth Analg. 2017;124(4):1029–1031.
9. Melber AA. Remifentanil patient-controlled analgesia (PCA) in labour - in the eye of the storm. Anaesthesia. 2019;74(3):277–279.
10. Bonner JC, McClymont W. Respiratory arrest in an obstetric patient using remifentanil patient-controlled analgesia. Anaesthesia. 2012;67(5):538–540.
11. Kinney MA, Rose CH, Traynor KD, et al. Emergency bedside cesarean delivery: lessons learned in teamwork and patient safety. BMC Res Notes. 2012;5:412.
12. Marr R, Hyams J, Bythell V. Cardiac arrest in an obstetric patient using remifentanil patient‐controlled analgesia. Anaesthesia. 2013;68(3):283–287.
13. Balki M, Kasodekar S, Mbbs SD, et al. Remifentanil patient-controlled analgesia for labour: optimizing drug delivery regimens. Obstet Anesth Dig. 2007;28(8):35–36.
14. Kan RE, Hughes SC, Rosen MA, et al. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88(6):1467.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

