Back to Journals » Journal of Pain Research » Volume 11
Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double-blind, placebo-controlled trial
Authors Mao Y, Zuo YM, Mei B, Chen LJ, Liu XS , Zhang Z, Gu EW
Received 31 January 2018
Accepted for publication 15 May 2018
Published 11 September 2018 Volume 2018:11 Pages 1811—1819
DOI https://doi.org/10.2147/JPR.S164225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Yu Mao,1,2,* Youmei Zuo,2,* Bin Mei,2 Lijian Chen,2 Xuesheng Liu,2 Zhi Zhang,1 Erwei Gu2
1Key Laboratory of Brain Function and Disease of Chinese Academy of Science, Department of Biophysics and Neurobiology, University of Science and Technology of China, Hefei City, Anhui 230027, People’s Republic of China; 2Department of Anaesthesiology, First Affiliated Hospital of Anhui Medical University, Hefei City, Anhui 230031, People’s Republic of China
*These authors equally contributed to this work
Purpose: The purpose of this study was to assess the efficacy of perineural dexamethasone with ropivacaine in multimodal analgesia for thoracic paravertebral block (TPVB) in patients undergoing elective thoracotomy.
Patients and methods: Ninety-six patients undergoing thoracotomy were enrolled in this trial and randomized to adjuvant therapy for TPVB: group S (saline), group R (0.5% ropivacaine), or group RD (5 mg dexamethasone and 0.5% ropivacaine). Postoperative analgesia, recovery duration, and chronic pain were recorded.
Results: Groups R and RD spent less time in the postanaesthesia care unit, had earlier out-of-bed activity, and had shorter postoperative hospital stays compared with group S. The RD group regained consciousness faster and had lower acute pain scores and used less patient-controlled analgesia during the first 72 h after surgery compared with group S. Postthoracotomy pain was decreased in group RD (19.0%) compared with group S (47.6%) 3 months postoperatively, p = 0.050.
Conclusion: Perineural dexamethasone with ropivacaine for TPVB improves postoperative analgesia quality, reduces recovery time, and may decrease the incidence of chronic pain after thoracotomy with an opioid-based anesthetic regimen.
Keywords: chronic pain, dexamethasone, nerve block, thoracotomy
Introduction
Thoracotomy procedures are painful,1 and inadequate pain control during the perioperative period after the procedure leads to postoperative complications such as pneumonia, atelectasis, or respiratory failure.2 Furthermore, the incidence of chronic postthoracotomy pain (CPTP), persisting at least 2 months after thoracotomy, is ~30%–50%, which significantly decreases patients’ quality of life.1 It has been reported that acute pain control after thoracotomy using preemptive3,4 and multimodal5,6 approaches reduces the incidence of chronic pain.1,7 Thus, effectively controlling pain during any phase of the perioperative procedure may prevent or reduce the risk of developing chronic pain after surgery.8,9
Thoracic epidural analgesia (TEA) is a standard analgesic technique after thoracotomy,10,11 but it is limited by coagulopathy and other side effects.12 Thus, thoracic paravertebral block (TPVB), an alternative to TEA, may offer comparable analgesia postthoracotomy13,14 and a fewer side effects including decreased risk of serious neurologic complications, fewer hemodynamic problems, and better preserved postoperative respiratory function.14,15 Several studies have indicated that preoperative paravertebral blockade may decrease neuropathically mediated chronic pain after breast surgery, which has similar underlying mechanisms of CPTP.16,17 However, the role of TPVB in preventing CPTP is not clear.
Because single-dose local anesthetics offer pain relief of limited duration, adjuvants have been applied to prolong analgesia for peripheral nerve block.18–20 Perineural dexamethasone can prolong the duration of local anesthetic blockade,21,22 so we hypothesize that dexamethasone plus long-acting local ropivacaine for TPVB not only offers effective acute pain control with fewer side effects during the first 72 h after surgery but also reduces the incidence of CPTP.
Patients and methods
Protocol
Ninety-six patients undergoing elective transthoracic esophagectomy for esophageal carcinoma or open surgery for lung cancer were enrolled in this prospective, double-blind, randomized, placebo-controlled clinical study. The study was approved by the ethics committee of First Affiliated Hospital of Anhui Medical University (kuai2016-06-08) and registered at clinicaltrials.gov (NCT 02871193). Patients were enrolled between August 2016 and January 2017. Subjects aged 18–80 years with an American Society of Anesthesiologists (ASA) physical status of I–II were recruited. The exclusion criteria were allergy to local anesthetics or narcotics, preoperative chronic medication with opioids, coagulopathy, heart disease, central and peripheral neuropathies, severe chronic obstructive pulmonary diseases, severe pulmonary emphysema, liver or renal failure, peptic ulcer, a history of previous thoracotomy, or local puncture site infection.
After written informed consent was obtained from each patient, all patients were randomized to one of three groups using computer-generated random numbers and a 1:1:1 allocation ratio. Allocation concealment was fulfilled by an assistant not involved in the study, and randomization was achieved in sequentially numbered, sealed, opaque envelopes, which were opened after patient’s arrival to the operation room. Blinding of research personnel was maintained throughout the study, including postoperative follow-ups.
TPVB technique
Patients were placed in a standard lateral position to apply TPVB after induction of anesthesia. An anesthetic assistant neither involved in the study nor participating in the perioperative period or the postoperative follow-up prepared study drugs in a 20-mL syringe. Groups received isotonic saline (S), 0.5% ropivacaine (R), or 0.5% ropivacaine with 5 mg dexamethasone (RD) in the paravertebral space. TPVB was performed using an ultrasound-guided parasagittal out-plane approach. The skin was prepared with chlorhexidine in isopropyl alcohol and then covered with a sterile sheet. A 22G, 120-mm needle (stimuplex D; B. Braun Melsungen AG, Melsungen, Germany) was guided using a real-time ultrasound machine (SonoSite M-Turbo, Bothell, WA, USA) with a C60x transducer (2–5 MHz) draped with a sterile cover (3M Tegaderm, St. Paul, MN, USA). Local anesthetic or saline was administered at the paravertebral spaces between T3–T4, T4–T5, and T5–T6 vertebrae with a bolus of 5–7 mL in each interspace region. Ultrasonography confirmed that the pleura shifted downward due to the local anesthesia.
Anesthesia and perioperative treatment
When patients were transferred into the operating room, peripheral intravenous (iv), right internal jugular vein, and radial artery catheters were placed. Electrocardiogram (leads II and V5), invasive blood pressure, central venous pressure, heart rate, pulse oximetry, and the bispectral index (BIS) (Vista; Aspect Medical Systems Inc., Norwood, MA, USA) were monitored throughout surgery. Propofol (Diprivan; AstraZeneca plc, London, UK) was administered with a target-controlled infusion according to pharmacokinetics of the Marsh model23 (Graseby 3500; Smiths Medical, Watford, UK) during anesthetic induction. After an initial target concentration of 1.0 µg/mL was achieved, the concentration progressively increased by 0.3 µg/mL until the BIS value reached 40–60. Then, 0.03 mg/kg midazolam and 0.5 µg/kg sufentanil were injected (iv). Rocuronium bromide (0.9 mg/kg) was used to facilitate double-lumen endobronchial intubation. After tracheal intubation, lungs were ventilated with 100% oxygen, and a volume-cycled ventilator was applied with the following settings: tidal volume, 8 mL/kg ideal body weight; inspiratory-to-expiratory ratio, 1:2; and a respiratory frequency of 8 breaths/min. Propofol and remifentanil were continuously infused to maintain anesthesia, and sufentanil and cisatracurium were administered as needed. BIS values were maintained from 40 to 60 throughout surgery by changing the effect-site concentration of propofol. The ventilation mode was switched to one-lung ventilation before the surgical procedure, and the frequency and tidal volume were adjusted to maintain pulse oximetry and end-tidal carbon dioxide. Propofol and remifentanil were discontinued at the end of the last skin suture. Neostigmine (20 μg/kg) and atropine (5–10 μg/kg) were administered according to tidal volume and frequency to reverse residual muscle relaxation at the end of surgery. Patients were admitted to the postanaesthesia care unit (PACU) until spontaneous breathing was recovered. Patients were extubated in the PACU according to standard extubation criteria and subjects were moved to the ward when a Steward recovery score exceeded 4.
Flurbiprofen (50 mg, iv) was injected before skin incision, and sufentanil (0.1–0.2 µg/kg) and flurbiprofen (50 mg, iv) were given, followed by patient-controlled analgesia (PCA) pump use before the end of the surgery. PCA capacity was 250 mL and contained 7.5 µg/kg sufentanil and 250 mg flurbiprofen. The infusion rate was maintained at 2 mL/h, and the patient-controlled bolus was 2 mL with a lockout interval of 15 min. Patients were trained to press for an additional bolus if a 10 cm visual analog scale (VAS) for postoperative pain exceeded 3.
Throughout anesthesia, mean arterial pressure (MAP) was maintained between -20% and +20% of the baseline value. Hypotension was defined as a 20% decrease below the baseline MAP or an MAP <60 mmHg lasting more than 30 s. Phenylephrine (40 µg, iv) was given when fluid therapy was not appropriate. Atropine (0.3 mg, iv) was given for bradycardia, which was defined as an HR <60 bpm. Ephedrine (3–6 mg, iv) was given to treat bradycardia and hypotension.
Study outcomes
The primary end point was PCA use during the first 6 h postoperatively. Second outcomes included: 1) duration of surgery and one-lung ventilation, sufentanil use, fluid volume (colloid and crystalloid solutions), phenylephrine consumption during anesthesia; 2) changes in hemodynamics including heart rate and blood pressure at various time points: baseline (T0), 5 min after induction (T1), 5 min after paravertebral block (T2), 10 min after skin incision (T3), 10 min after one-lung ventilation (T4), 1 h after one-lung ventilation (T5), 10 min after the end of one-lung ventilation (T6), at the end of surgery (T7), at transfer to the PACU (T8), upon awakening (T9), upon extubation (T10), and with transfer from the PACU (T11); 3) PACU recovery data referring to awake time, extubation time, and length of stay; 4) a 10 cm VAS for pain (0–10; 0, no pain; 10, worst imaginable pain); 5) PCA machine use and side effects including postoperative nausea and vomiting (PONV) intensity (0, no nausea and vomiting; 1, mild; 2, moderate; and 3, severe) and the incidence of pruritus at 6, 12, 24, 48 and 72 h after surgery; 6) short-time recovery including major complications, postoperative days for first out-of-bed activity, hospital stay, and hospitalization cost; and 7) CPTP: 3 months after surgery during a telephone interview.
Statistical analysis
Sample size calculations were performed using an online power sample size calculator based on our previous pilot study showing a decreased mean effective pressing number of PCA for patients under general anesthesia combined with TPVB using ropivacaine and ropivacaine with dexamethasone (2.0 ± 2.2 and 1.6 ± 1.9, respectively) compared with patients undergoing general anesthesia combined with TPVB using saline (5.1 ± 4.0) at 6 h after surgery. To detect differences in PCA use 6 h postoperatively with an SD of σ = 3, the sample size was calculated as 21 per group at a power of 80% and a two-tailed α-error of 5%. We enrolled 96 patients in total (N = 32/group) to countervail potential dropouts.
Data analysis was primarily performed using SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA). Patient hemodynamics were analyzed using repeated-measure analysis of variance (ANOVA). Quantitative variables, reported as mean ± SD, were calculated using one-way ANOVA, and a least significant difference (LSD) procedure was used for post hoc comparisons. PONV intensity was assessed with using the Kruskal–Wallis test. Mann–Whitney U tests were applied for intergroup comparisons when a significant difference was detected among groups. Categorical variables were compared using chi-squared or Fisher’s exact test (p < 0.05 was considered as a statistical significant difference among groups). p-values were corrected to 0.017 using Bonferroni adjustment for repeated outcome measurements. Analysis of covariance (ANCOVA) was performed to identify the effect of confounding factors.
Results
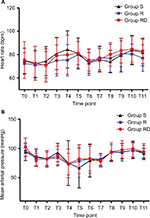
The study flow is depicted in Figure 1. Table 1 lists patient data. There was no significant difference in intraoperative characteristics among groups except that consumption of sufentanil was significantly lower in group R compared with that in group S (Table 2). Figure 2 shows no differences in heart rate and blood pressure among all three groups. Table 2 depicts patients in group RD with faster recovery and shorter duration in PACU than those in group S. Table 3 shows that postoperative VAS scores at all time points and total PCA machine use in group RD decreased significantly compared with that in group S. The intensity of PONV did not differ significantly among groups (Table 4). No patient experienced pruritus, pleural effusion, subjective symptoms of local anesthetic toxicity, infection, or hematoma at the insertion site. Table 3 demonstrates that groups R and RD had earlier ambulation and reduced postoperative stay compared with group S. Two group S subjects had severe pneumonia and received a tracheostomy. The incidence of postthoracotomy pain syndrome 3 months after surgery was significantly different among the three groups, and chronic pain was reduced in the RD group. Group S seems to be older than group R or group RD, which was almost significant (p = 0.062), so ANCOVA was performed in order to understand the effect of the age as a confounding factor. The results showed that age was a confounding factor for time of awaking only (Table 5). To identify whether this confounding factor was caused by allocation, we analyzed all basic data of patients in three groups before loss to follow-up. The result presented that the comparison of ages in three groups was not significantly different (Table 6).
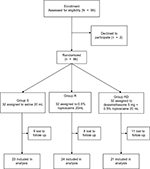  | Figure 1 Flowchart of the study. Notes: S, saline; R, 0.5% ropivacaine; RD, 5 mg dexamethasone and 0.5% ropivacaine. |
  | Table 2 Intraoperative data and characteristics of recovery in PACU Notes: Data represent mean (SD). p1, group R vs group S; p2, group RD vs group S; p3, group RD vs group R. *Statistically significant differences (p < 0.05). S, saline; R, 0.5% ropivacaine; RD, 5 mg dexamethasone and 0.5% ropivacaine. Abbreviation: PACU, postanaesthesia care unit. |
  | Table 3 Postoperative analgesia and recovery duration Notes: Data represent mean (SD). p1, group R vs group S; p2, group RD vs group S; p3, group RD vs group R. *Statistically significant differences (p < 0.05). S, saline; R, 0.5% ropivacaine; RD, 5 mg dexamethasone and 0.5% ropivacaine. CNY= Chinese Yuan. Abbreviation: VAS, visual analog scale. |
  | Table 4 Intensity of PONV 72 h after surgery and chronic postoperative pain incidence Notes: p1, group R vs group S; p2, group RD vs group S; p3, group RD vs group R. *Statistically significant differences (p < 0.05). S, saline; R, 0.5% ropivacaine; RD, 5 mg dexamethasone and 0.5% ropivacaine. Abbreviation: PONV, postoperative nausea and vomiting. |
  | Table 5 ANCOVA of age factor with time of awaking as a dependent variable Abbreviation: ANCOVA, analysis of covariance. |
Discussion
Patients after open thoracotomy experienced severe pain, and inadequate pain control impedes coughing, deep breathing, and remobilization, culminating in atelectasis, bronchospasm, and pneumonia.24 Since the multifactorial pathophysiology including nociceptive and neuropathic mechanisms involved in the onset and progress of postoperative pain, a multimodal approach was mandated to provide analgesia.25,26
A complimentary analgesic activity within multimodal analgesia should not only provide sufficient pain control with a few side effects after surgery but also decrease the incidence and scores of chronic pain. TPVB can be used for regional anesthesia to control acute pain after thoracotomy by blocking thoracic sympathetic and somatic nerves, with fewer adverse events,15 but it does not provide complete postoperative analgesia for thoracotomy.27–29 Flurbiprofen axetil, a nonselective nonsteroidal anti-inflammatory drug, reduced postoperative opioid consumption and offered postoperative analgesia30,31 by reducing local inflammation and preventing peripheral and central sensitization.30,32 Low-dose opiates plus flurbiprofen was reported to reduce postoperative sufentanil consumption33 and facilitate analgesic effects.30,34 It has been reported that as a strategy of preventive analgesia, preoperative administration of flurbiprofen can significantly reduce postoperative pain scores.31,35 Since the anesthesiologists responsible for the surgery were blind to the allocation and the response of patients in different interventional groups to the incision and the use of sufentanil may be unpredictable, so the preoperative administration of sufentanil was according to the need rather than compulsory. In addition, sufentanil with flurbiprofen as an alternative multimodal analgesia for postoperative pain control was given as a loading dose for PCA at the end of surgery. Studies have shown that postoperative analgesia with opioid plus flurbiprofen resulted in lower pain scores,36,37 thus, this strategy of multimodal postoperative analgesia was applied in this study. Even though the routine use of PCA with a background infusion is not recommended, it remains suitable in patients after thoracotomy who require high opioid consumption or complain of waking due to severe pain at night.38 We reported that TPVB plus iv infusion of sufentanil and flurbiprofen for postoperative analgesia for patients undergoing elective thoracotomy decreased PACU stay, reduced postoperative pain scores, required less PCA, reduced recovery duration and reduced costs.
Clinical trials indicated benefits of various adjuncts with local anesthetics for single injection, but blockade prolongation was unsatisfactory.39–45 We observed that subjects of group RD had better postoperative analgesia and less pain intensity at all time points with decreased PCA use during the first postoperative 72 h compared with those of group S. In addition, perineural dexamethasone was superior to a single injection of ropivacaine for decreasing acute pain and reducing PCA machine use at 24 h after thoracotomy. Our data agreed with previous results that perineural dexamethasone can prolong analgesic duration21,46–48 by decreasing nociceptive C fiber activity via direct modulation of glucocorticoid receptors.21
Early acute postoperative pain was reported to be a positive and independent predictive factor for subsequent chronic pain,49,50 so our primary outcome and sample size estimation were based on PCA machine use with effective pain control 6 h after surgery. We noted that CPTP in group RD decreased, and this was similar to PCA machine use during the first postoperative 72 h compared with group S. So, dexamethasone as an adjunct is not only directed at prolonging analgesia but may also decrease the incidence of chronic pain. Although the sample size was not sufficiently large to offer appropriate power for a conclusion, analgesic demand during 72 h may predict decreased incidence of CPTP compared with maximum postoperative pain intensity. Thus, the cumulative effect of acute postoperative pain may influence the development of CPTP after thoracotomy.51,52
In our trial, intraoperative sufentanil consumption was significantly decreased for group R subjects who received a single-dose local anesthetic compared with group S, and this was consistent with previous findings.53 In addition, compared with group S, the length of stay and costs decreased in group R, not in group RD, which may be due to diagnostic heterogeneity and operative sites among groups. Lost to follow-up was ~29% due to inaccurate or insufficiently detailed contact information for each patient, which may be the reason for the bias of age. Follow-up data were derived from telephone interviews, and patients were asked if they had pain at the surgical site at rest or during activities of daily living. The pain type, severity, and effects on activities of daily life54 should be assessed in a more comprehensive study with well-trained staff to develop a relationship with respondents in the future.
Conclusion
Dexamethasone, used as an adjuvant of TPVB with ropivacaine, provides effective acute pain control after surgery, requires less anesthetic consumption, and reduces complications. It also reduces recovery duration and may reduce chronic pain for patients undergoing elective thoracotomy. More adequately powered trials to assess TPVB are needed to fully assess the benefits for chronic pain relief.
Acknowledgment
We thank Liangqiang Tao and Jiajia Fang for clinical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
Khelemsky Y, Noto CJ. Preventing post-thoracotomy pain syndrome. Mt Sinai J Med. 2012;79(1):133–139. | ||
Sabanathan S, Eng J, Mearns AJ. Alterations in respiratory mechanics following thoracotomy. J R Coll Surg Edinb. 1990;35(3):144–150. | ||
Mishra A, Afzal M, Mookerjee S, Bandyopadhyay K, Paul A. Pre-emptive analgesia: recent trends and evidences. Indian J Pain. 2013;27(3):114–120. | ||
Vadivelu N, Mitra S, Schermer E, Kodumudi V, Kaye AD, Urman RD. Preventive analgesia for postoperative pain control: a broader concept. Local Reg Anesth. 2014;7:17–22. | ||
Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22(5):588–593. | ||
Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America. 2005;23(1):185–202. | ||
Gessling EA, Miller M. Efficacy of thoracic paravertebral block versus systemic analgesia for postoperative thoracotomy pain: a systematic review protocol. JBI Database System Rev Implement Rep. 2017;15(1):30–38. | ||
Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013;111(5):711–720. | ||
Atchabahian A, Andreae M. Long-term functional outcomes after regional anesthesia: a summary of the published evidence and a recent Cochrane review. Refresh Courses Anesthesiol. 2015;43(1):15–26. | ||
Senturk M, Ozcan PE, Talu GK, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002;94(1):11–15, table of contents. | ||
Obata H, Saito S, Fujita N, Fuse Y, Ishizaki K, Goto F. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anaesth. 1999;46(12):1127–1132. | ||
Hansdottir V, Philip J, Olsen MF, Eduard C, Houltz E, Ricksten SE. Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery: a randomized controlled trial on length of hospital stay and patient-perceived quality of recovery. Anesthesiology. 2006;104(1):142–151. | ||
Ding X, Jin S, Niu X, Ren H, Fu S, Li Q. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One. 2014;9(5):e96233. | ||
Komatsu T, Sowa T, Takahashi K, Fujinaga T. Paravertebral block as a promising analgesic modality for managing post-thoracotomy pain. Ann Thorac Cardiovasc Surg. 2014;20(2):113–116. | ||
Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:Cd009121. | ||
Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006;103(3):703–708. | ||
Ibarra MM, GC S-Carralero, Vicente GU, Cuartero del Pozo A, Lopez Rincon R, Fajardo del Castillo MJ. [Chronic postoperative pain after general anesthesia with or without a single-dose preincisional paravertebral nerve block in radical breast cancer surgery]. Rev Esp Anestesiol Reanim. 2011;58(5):290–294. | ||
Wu HH, Wang HT, Jin JJ, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9(3):e93114. | ||
Tiwari AK, Tomar GS, Agrawal J. Intrathecal bupivacaine in comparison with a combination of nalbuphine and bupivacaine for subarachnoid block: a randomized prospective double-blind clinical study. Am J Ther. 2013;20(6):592–595. | ||
Tomar GS, Godwin RB, Gaur N, et al. A double-blind study on analgesic effects of fentanyl combined with bupivacaine for extradural labor analgesia. Anesth Essays Res. 2011;5(2):147–152. | ||
Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. 2015;70(1):71–83. | ||
Uda Y, Cowie B, Kluger R. Comparison of preoperative and intraoperative assessment of aortic stenosis severity by echocardiography. Br J Anaesth. 2017;118(5):699–704. | ||
Thomson AJ, Nimmo AF, Engbers FH, Glen JB. A novel technique to determine an ‘apparent ke0’ value for use with the Marsh pharmacokinetic model for propofol. Anaesthesia. 2014;69(5):420–428. | ||
Sengupta S. Post-operative pulmonary complications after thoracotomy. Indian J Anaesth. 2015;59(9):618–626. | ||
Mesbah A, Yeung J, Gao F. Pain after thoracotomy. BJA Educ. 2016;16(1):1–7. | ||
Hughes R, Gao F. Pain control for thoracotomy. Contin Educ Anaesth Crit Care Pain. 2005;5(2):56–60. | ||
Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95(3):771–780. | ||
Batra RK, Krishnan K, Agarwal A. Paravertebral block. J Anaesthesiol Clin Pharmacol. 2011;27(1):5–11. | ||
Schumann R, Shikora S, Weiss JM, Wurm H, Strassels S, Carr DB. A comparison of multimodal perioperative analgesia to epidural pain management after gastric bypass surgery. Anesth Analg. 2003;96(2):469–474, table of contents. | ||
Takada M, Fukusaki M, Terao Y, et al. Preadministration of flurbiprofen suppresses prostaglandin production and postoperative pain in orthopedic patients undergoing tourniquet inflation. J Clin Anesth. 2007;19(2):97–100. | ||
Yamashita K, Fukusaki M, Ando Y, et al. Preoperative administration of intravenous flurbiprofen axetil reduces postoperative pain for spinal fusion surgery. J Anesth. 2006;20(2):92–95. | ||
DE G. Priciples of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Baltimore: Lippincott Williams & Wilkins; 2008. | ||
Lin X, Zhang R, Xing J, Gao X, Chang P, Li W. Flurbiprofen axetil reduces postoperative sufentanil consumption and enhances postoperative analgesic effects in patients with colorectal cancer surgery. Int J Clin Exp Med. 2014;7(12):4887–4896. | ||
Mihara R, Soen M, Kusaka H, et al. [Effect of continuous intravenous infusion of flurbiprofen axetil and tramadol hydrochloride for postoperative pain management of laparoscopic colectomy]. Masui Jpn J Anesthesiol. 2011;60(12):1364–1369. | ||
Wang K, Luo J, Zheng L, Luo T. Preoperative flurbiprofen axetil administration for acute postoperative pain: a meta-analysis of randomized controlled trials. J Anesth. 2017;31(6):852–860. | ||
Lin WQ, Cao LH, Zhong ZJ, Wen LL, Bai XH. [Postoperative analgesia with fentanyl combined with flurbiprofen axetil following gynecologic surgery for turnor]. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(2):313–315. | ||
Geng W, Hong W, Wang J, et al. Flurbiprofen axetil enhances analgesic effects of sufentanil and attenuates postoperative emergence agitation and systemic proinflammation in patients undergoing tangential excision surgery. Mediators Inflamm. 2015;2015:6 (601083). | ||
Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87(1):36–46. | ||
Axelsson K, Gupta A. Local anaesthetic adjuvants: neuraxial versus peripheral nerve block. Curr Opin Anaesthesiol. 2009;22(5):649–654. | ||
Wang CG, Ding YL, Han AP, et al. Adding dexmedetomidine to ropivacaine for lumbar plexus and sciatic nerve block for amputation of lower limb in high-risk patient: a case report. Int J Clin Exp Med. 2015;8(8):14184–14187. | ||
Wang LZ, Liu X, Zhang YF, Hu XX, Zhang XM. Addition of fentanyl to the ultrasound-guided transversus abdominis plane block does not improve analgesia following cesarean delivery. Exp Ther Med. 2016;11(4):1441–1446. | ||
Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One. 2015;10(9):e0137312. | ||
Dutta V, Kumar B, Jayant A, Mishra AK. Effect of continuous paravertebral dexmedetomidine administration on intraoperative anesthetic drug requirement and post-thoracotomy pain syndrome after thoracotomy: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2016;31(1):159–165. | ||
Goravanchi F, Kee SS, Kowalski AM, Berger JS, French KE. A case series of thoracic paravertebral blocks using a combination of ropivacaine, clonidine, epinephrine, and dexamethasone. J Clin Anesth. 2012;24(8):664–667. | ||
Kalava A, Clendenen S, McKinney JM, Bojaxhi E, Greengrass RA. Bilateral thoracic paravertebral nerve blocks for placement of percutaneous radiologic gastrostomy in patients with amyotrophic lateral sclerosis: a case series. Rom J Anaesth Intensive Care. 2016;23(2):149–153. | ||
Ilfeld BM. Continuous peripheral nerve blocks: an update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg. 2017;124(1):308–335. | ||
Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2015;32(11):751–758. | ||
Liu F, Zhang H, Zuo Y. Bilateral thoracic paravertebral block for immediate postoperative pain relief in the PACU: a prospective, observational study. BMC Anesthesiol. 2017;17(1):89. | ||
Shimizu H, Kamiya Y, Nishimaki H, Denda S, Baba H. Thoracic paravertebral block reduced the incidence of chronic postoperative pain for more than 1 year after breast cancer surgery. JA Clin Rep. 2015;1(1):19. | ||
Schnabel A, Pogatzki-Zahn E. [Predictors of chronic pain following surgery. What do we know?]. Schmerz. 2010;24(5):517–531; quiz 532–513. | ||
Bisgaard T, Rosenberg J, Kehlet H. From acute to chronic pain after laparoscopic cholecystectomy: a prospective follow-up analysis. Scand J Gastroenterol. 2005;40(11):1358–1364. | ||
Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010;112(6):1494–1502. | ||
Hill SE, Keller RA, Stafford-Smith M, et al. Efficacy of single-dose, multilevel paravertebral nerve blockade for analgesia after thoracoscopic procedures. Anesthesiology. 2006;104(5):1047–1053. | ||
Fink R. Pain assessment: the cornerstone to optimal pain management. Proc (Bayl Univ Med Cent). 2000;13(3):236–239. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.