Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Efficacy of Neutrophil-to-Lymphocyte Ratio for Cancer-Specific Survival in Elderly Patients with Localized Colon Cancer: A Single Center Propensity Score-Matched Analysis
Authors Tominaga T, Nonaka T, Oyama S, Takamura Y, Hashimoto S, Shiraishi T, Sawai T, Nagayasu T
Received 9 August 2022
Accepted for publication 22 November 2022
Published 5 January 2023 Volume 2023:16 Pages 1—9
DOI https://doi.org/10.2147/CEG.S385207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Santosh Shenoy
Tetsuro Tominaga, Takashi Nonaka, Shosaburo Oyama, Yuma Takamura, Shintaro Hashimoto, Toshio Shiraishi, Terumitsu Sawai, Takeshi Nagayasu
Department of Surgical Oncology, Nagasaki University Graduate School of Biomedical Science, Nagasaki, Japan
Correspondence: Tetsuro Tominaga, Department of Surgical Oncology, Nagasaki University Graduate School of Biological Sciences, 1-7-1 Sakamoto, Nagasaki, 852-8501, Japan, Tel +81-95-819-7304, Fax +81-95-819-7306, Email [email protected]
Purpose: The prognostic value of neutrophil-to-lymphocyte ratio (NLR) has been studied for colorectal cancer. Elderly patients in general tend to have comorbidities and decreased organ function that potentially influence the NLR score. The aim of this study was to investigate the relationship between NLR and cancer-specific survival in elderly patients with colon cancer, using a propensity score-matched analysis.
Patients and Methods: A total of 203 patients aged over 75 years who underwent curative resection for colon cancer and were diagnosed pathologically with stage II/III disease were eligible for entry to the study. Patients were divided into two groups according to NLR score: NLR-High (NLR≥ 4.5) group (NLR-H, n=60) and NLR-Low (NLR< 4.5) group (NLR-L, n=143). After propensity score matching, 57 patients in each group were matched.
Results: Before matching, Charlson comorbidity index was significantly higher in the NLR-H group (4 vs 2, p< 0.001). After matching, all factors were similar between the groups. The median follow-up period was 43 months (range, 1– 160 months). Five-year relapse-free-survival (69.8% vs 87.3%, p=0.030) and cancer-specific survival (83.0% vs 96.0%, p=0.042) were significantly lower in the NLR-H group.
Conclusion: NLR appears to be a cancer-specific prognostic marker in elderly patients with colon cancer.
Keywords: cancer-specific survival, colon cancer, neutrophil-to-lymphocyte ratio
Introduction
The incidence of colon cancer (CC) continues to increase, and is the second leading cause of cancer death worldwide.1 The prognosis in CC has recently improved due to advances in surgical techniques and chemotherapy.2,3 Therefore, it is crucial to obtain appropriate staging and prognostic scores to decide the optimal treatment strategy and thus improve cancer prognosis.4,5
Inflammation-based score (IBS) has recently been reported as a prognostic marker for various cancers.6–8 Neutrophil-to-lymphocyte ratio (NLR) is an IBS calculated as the serum neutrophil count divided by the lymphocyte count.9 A correlation of NLR with survival outcomes has been reported in colorectal cancer.10–12
With aging of the population, the number of elderly patients with cancer has increased.13 In general, elderly patients tend to have more comorbidities, worse performance status, and decreased organ function compared with younger patients.14,15 These conditions can potentially influence systemic inflammation and malnutrition.15 Previous studies have shown correlations between IBS, Charlson comorbidity index, nutrition score, and age-related complications including dementia in elderly patients.15–18
Furthermore, these scores are also reported to correlate with cancer prognosis in elderly patients.8,15,19,20 With regard to colorectal cancer, NLR could be a potential marker for cancer prognosis in these patients.10,21 However, to the best of our knowledge, this hypothesis has not been evaluated in any large-scale prospective studies or randomized controlled trials. In addition, the uni- and multi-variate analyses used in previous studies have led to potential confounding factors and selection bias.10 The incidence of non-cancer death increases as patients age.22,23 Some studies have examined the efficacy of NLR as a predictor of non-cancer death in the elderly.24,25
The purpose of the present study was to investigate the relationship between NLR and cancer-specific survival in elderly patients with colon cancer using a propensity score-matched analysis.
Materials and Methods
We retrospectively investigated patients with colon cancer aged >75 years who underwent curative surgery and were pathologically diagnosed with stage II or stage III disease at Nagasaki University Hospital between April 2008 and December 2018. In this study, colon cancer was defined for tumors located between the cecum and the sigmoid. The exclusion criteria were incomplete laboratory data, neoadjuvant treatment, elective stoma construction, and emergency surgery. A final total of 203 patients were eligible for analysis. This study was performed in line with the principles of the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all patients. This study was reviewed and approved by the Clinical Research Review Board of Nagasaki University Hospital.
NLR is calculated as the serum neutrophil count (/mm3) divided by the serum lymphocyte count (/mm3). A receiver-operating characteristic (ROC) curve was used to assess the optimal cut-off value of NLR. Patients were divided into two groups according to NLR score: NLR-High group (NLR-H, n=60) and NLR-Low group (NLR-L, n=143).
Propensity score matching was applied to minimize selection bias and balance covariates that could affect cancer-specific survival. The following covariates were included in the score matching: age, sex, BMI, comorbidities, Charlson comorbidity index (CCI), and clinical T/N status. Nearest-neighbor matching was performed in a 1:1 ratio, with the caliper set at 0.25. Finally, 57 patients in each group were matched.
To compare the clinical features between two groups, the following data were collected: sex, age at operation (middle-old, 75–84 years; oldest-old, >85 years), body mass index (BMI), CCI, past history of abdominal surgery, tumor location, surgical approach, multivisceral resection, clinical T/N status, and laboratory data (neutrophils, lymphocytes). Perioperative data including operation time, estimated blood loss, pathological T/N status, histological type, tumor size, lymphovascular invasion, postoperative complications, length of hospital stay, and the presence or absence of adjuvant chemotherapy were also collected. Pathological classification and staging were determined according to the American Joint Committee on Cancer criteria. Complications experienced within 30 days of surgery were defined as postoperative complications. Detailed data of comorbidities were collated in each patient, and the CCI score was calculated as previously reported.26
Patients diagnosed with pathological stage III disease received 5-fluorouracil-based adjuvant chemotherapy within 2 months of the initial surgery. The indication for adjuvant chemotherapy and the type of adjuvant chemotherapy regimen depended on the patient’s performance status, patient’s choice, and the out-patient doctor’s decision. Patients were followed up every 3 months during the five years after surgery. Blood tests including tumor markers were performed every three months. Chest and abdominal CT were performed every 6 months.
Statistical analysis was performed using Bell Curve for Excel software, version 2.02 (Social Survey Research Information Co., Ltd., Tokyo, Japan). The data are presented as median values with ranges. Differences in categorical variables were compared using Fisher’s exact test or Chi square test. Differences in continuous variables were analyzed with the Mann–Whitney U-test. Relapse-free survival (RFS) was defined as the time from surgery to the appearance of new recurrent metastases or death. Overall survival (OS) was defined as the time from surgery to death or to the last follow-up visit. Cancer-specific survival (CSS) was defined as the time from surgery to cancer-related death or last follow-up visit. RFS, OS, and CSS were calculated using the Kaplan–Meier method. Differences between groups were tested for significance using the Log rank test. Clinical variables with a p value < 0.20 in univariate analysis were included in the multivariate analysis. All p values < 0.05 were considered significant.
Results
Figure 1 shows the ROC curve of NLR for RFS. The area under the curve was 0.588, and NLR of 3.0 had the highest sensitivity (64.2) and specificity (54.8).
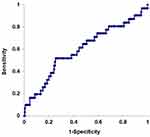 |
Figure 1 ROC curve of neutrophil-to-lymphocyte ratio (NLR) for relapse-free survival. The area under the curve was 0.588. NLR of 4.5 had the highest sensitivity (64.2) and specificity (54.8). |
Figure 2 shows survival curves for the NLR-H and NLR-L groups before matching (NLR-H; n=60, NLR-L; n=143). The median follow-up period was 43 months (1–160 months). Five-year RFS was significantly lower in the NLR-H group (NLR-H; 65.6% vs NLR-L; 85.8%, p=0.001) (Figure 2A). Five-year OS (NLR-H; 75.6% vs NLR-L; 89.3%, p=0.103) and CSS (NLR-H; 84.6% vs NLR-L; 95.3%, p=0.065) were similar between the groups (Figure 2B and C).
Clinical differences between the NLR-H and NLR-L groups are presented in Table 1. Before matching (NLR-H; n=60, NLR-L; n=143), Charlson comorbidity index was significantly higher in the NLR-H group (4 vs 2, p<0.001). Other factors such as sex, age, BMI, past history of abdominal surgery, tumor location, surgical approach, multivisceral resection, and clinical T/N status were similar between the groups. After matching (NLR-H; n=57, NLR-L; n=57), all factors were similar between the groups. Regarding laboratory data, serum neutrophil count was higher and serum lymphocyte count was lower in the NLR-H group, both before and after propensity score matching.
 |
Table 1 Clinical Characteristics in the NLR-H and NLR-L Groups Before and After Matching |
Table 2 shows a comparison of perioperative characteristics between the NLR-H and NLR-L groups. Operation time, blood loss, pathological T/N status, histological type, tumor size, lymphovascular invasion, postoperative complications, length of hospital stay, and adjuvant chemotherapy were similar between the groups, before and after matching.
 |
Table 2 Perioperative Characteristics in the NLR-H and NLR-L Groups Before and After Matching |
Figure 3 shows survival curves for the NLR-H and NLR-L groups after propensity score matching (NLR-H; n=57, NLR-L; n=57). Five-year RFS (NLR-H; 69.8% vs NLR-L; 87.3%, p=0.030) and CSS (NLR-H; 83.0% vs NLR-L; 96.0%, p=0.042) were significantly lower in the NLR-H group (Figure 3A and C). Five-year OS was similar between the groups (NLR-H; 73.6% vs NLR-L; 92.1%, p=0.124) (Figure 3B).
Table 3 lists the sites of recurrence in the matched groups. Recurrence occurred in 16 patients in the NLR-H group (28.1%) and in 9 patients in the NLR-L group (15.8%). The sites of recurrence were the liver (n=8), lung (n=2), local (n=3), paraaortic lymph nodes (n=2), and peritoneal carcinomatosa (n=1) in the NLR-H group; and the liver (n=5), lung (n=2), local (n=1), and peritoneal carcinomatosa (n=1) in the NLR-L group. There was no significant difference in recurrence site between the groups (p=0.723).
 |
Table 3 Sites of Recurrence in the Matched Groups |
Discussion
We examined the correlation between NLR and cancer-related prognosis after colectomy for elderly stage II/III CC patients using propensity score matching. Before matching, ASA-PS was worse and the presence of comorbidities was higher in the NLR-H group than the NLR-L group. After matching, the background was similar between the groups. Five-year RFS and CSS were significantly lower in the NLR-H group.
Systemic inflammation and malnutrition are important patient-related factors that affect cancer prognosis.27,28 Neutrophil count is usually elevated in systemic inflammation, and lymphocyte count is often low when immunity is depressed.29 Low lymphocyte levels reportedly correlate with poor prognosis; in addition, neutrophilia suppresses lymphocyte-mediated cytolysis and is also associated with poor prognosis.30,31 NLR utilizes two factors (neutrophil/lymphocyte count) and high NLR score is reported to correlate with poor prognosis in patients with colorectal cancer.32–34
In addition, elderly patients have a greater tendency than younger patients to die from surgical complications or non-cancer causes.14,22,23 A correlation has also been reported between NLR and surgical complications and non-cancer death from such as cardiovascular disease and pulmonary disease.29,35–38
Few reports have examined the correlation between NLR and prognosis in elderly patients with colorectal cancer.39 Cruz-Ramos and colleagues assessed the impact of NLR on prognosis in patients aged over 65 years with colorectal cancer, and found that NLR-H was correlated with worse outcome in terms of RFS (10 months vs 16 months, p=0.002) and OS (20 months vs 26 months, p=0.002), which was in agreement with previous results.39 However, that study examined only patients with metastatic CRC, who vary in their general condition because of the influence of systemic chemotherapy and the degree of disease progression. Furthermore, their study examined RFS and OS, but not CSS. In the present study, we examined elderly CC patients who underwent curative resection and were diagnosed with stage II/III disease pathologically. In addition, we used propensity score-matching analysis to minimize background selection bias. Our results showed poor RFS and CSS in the NLR-H group after matching. This finding suggests that NLR is a potential prognostic factor even in elderly patients with CC.
A previous study of colorectal cancer patients identified an NLR cut-off value ranging from 2.0 to 5.0 using ROC curve analysis.40 The heterogeneity of the cut-off value might be due to tumor stage, tumor location (colon or rectum), and patient background. An optimal cut-off value has not yet been established. In the present study, we used a cut-off value of 4.5, which is higher than those used in previous reports.41 One possible explanation for the discrepancy is the gradual change in blood cells with aging.42 The number and percentage of lymphocytes decrease along with the reduction in lymphoid tissue that occurs with age.42 Furthermore, the elderly have high rates of comorbidities that increase production of inflammatory cytokines, leading to neutrophilia and to the different NLR patterns between younger patients and elderly patients.14
A previous study reported NLR as a predictor of the recurrence pattern of colorectal cancer.43 Verter and colleagues examined the correlation between NLR and the survival/recurrence pattern in patients with R0 resection after colorectal cancer liver metastasis.43 Median OS (3.8 years vs 5.2 years, p=0.01) and RFS (0.8 years vs 1.2 years, p=0.049) were significantly shorter in the NLR-H group compared with the NLR-L group. In terms of recurrence pattern, recurrence with an extrahepatic pattern (but not intrahepatic pattern) was higher in the NLR-H group (p=0.03). They hypothesized that high NLR was a surrogate marker for aggressive systemic disease, which in turn is correlated with high risk of extrahepatic recurrence. In the present study, there was no significant difference in recurrence pattern between the NLR-H and NLR-L groups (p=0.723). These conflicting results might be due to the small number of patients with recurrence. However, recurrence was significantly higher in the NLR-H group, and NLR-H was clearly correlated with aggressive tumor progression.
Several guidelines recommend adjuvant chemotherapy after curative resection to improve prognosis in pathological stage III patients, even in elderly patients.29,44–46 In our study, no significant difference was found in RFS, OS, or CSS in terms of the presence or absence of adjuvant chemotherapy (Supple. Figure 1A–C). Indeed, due to the age of the patients, there were few pathological stage III patients in the present study and only a small number of stage III patients received adjuvant chemotherapy, which would have influenced the results. However, adjuvant chemotherapy tended to improve CSS in the NLR-H group (Supple. Figure 1D–F). Indeed, elderly patients with NLR-H have higher risk of dementia, which could lead to non-indication for adjuvant chemotherapy.18,47 However, NLR could be a surrogate marker for selecting candidates for adjuvant chemotherapy among elderly patients with pathological stage III CC.
There were several limitations in this study. First, the study was a retrospective, single center study, and we enrolled only a small number of patients. In addition, several selection and methodological biases could exist. The small number of patients might have affected the low sensitivity and specificity of NLR. Second, the choice of whether or not to perform adjuvant chemotherapy and selection of the chemo-regimen was at the discretion of the surgeon. Third, there was no significant correlation between nutritional score including prognostic nutritional index (PNI), CRP to albumin ratio (CAR), modified Glasgow prognostic index (mGPS) and cancer prognosis (Supple. Figure 2A–I). Close correlations of CRP and albumin to production of inflammatory cytokines and malnutrition have been reported.46 Close correlations of nutritional scores such as PNI, CAR, and mGPS with prognosis have also been reported in colorectal cancer patients.8,20,46 However, these scores were not correlated with prognosis in the present study, possibly because our study only included patients who underwent surgery. Before surgery, we could improve their general condition and nutritional status to enable them to better tolerate invasive surgery. Indeed, serum CRP/albumin levels were normal in most patients, and the median status of CRP was 0.12 (range, 0.01–11) and of albumin was 4.0 (2.0–5.0), which might have influenced the results.
Conclusion
In conclusion, neutrophil to lymphocyte ratio shows potential as a prognostic marker in elderly patients with colon cancer. This score might also be suitable as a surrogate marker for selecting candidates for adjuvant chemotherapy.
Disclosure
The authors report no conflicts of interest with regard to this work.
References
1. Arnold M, Sierra MS, Laversanne M, Soejomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi:10.1136/gutjnl-2015-310912
2. Ait Ouakrim D, Pizot C, Boniol M, et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ. 2015;351:h4970. doi:10.1136/bmj.h4970
3. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–378. doi:10.3322/caac.20038
4. Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi:10.1002/cncr.25537
5. Rizzi DA. Medical prognosis--some fundamentals. Theor Med. 1993;14:365–375. doi:10.1007/BF00996342
6. Sugimoto K, Komiyama H, Kojima Y, Goto M, Tomiki Y, Sakamoto K. Glasgow prognostic score as a prognostic factor in patients undergoing curative surgery for colorectal cancer. Dig Surg. 2012;29:503–509. doi:10.1159/000346002
7. Wang F, He W, Jiang C, et al. Prognostic value of inflammation-based scores in patients receiving radical resection for colorectal cancer. BMC Cancer. 2018;18:1102. doi:10.1186/s12885-018-4842-3
8. Roxburgh CS, Crozier JE, Maxwell F, et al. Comparison of tumour-based (Petersen Index) and inflammation-based (Glasgow Prognostic Score) scoring systems in patients undergoing curative resection for colon cancer. Br J Cancer. 2009;100:701–706. doi:10.1038/sj.bjc.6604926
9. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi:10.1002/jso.20329
10. Mazaki J, Katsumata K, Kasahara K, et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20:922. doi:10.1186/s12885-020-07429-5
11. Liu Q, Xi Y, He G, Li X, Zhan F. Dynamics of neutrophil-to-lymphocyte ratio predict outcomes of metastatic colorectal carcinoma patients treated by FOLFOX. J Gastrointest Oncol. 2021;12:2846–2853. doi:10.21037/jgo-21-716
12. Hayama T, Hashiguchi Y, Okada Y, et al. Significance of the 7th postoperative day neutrophil-to-lymphocyte ratio in colorectal cancer. Int J Colorectal Dis. 2020;35:119–124. doi:10.1007/s00384-019-03463-3
13. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
14. Hashimoto S, Tominaga T, Nonaka T, et al. The C-reactive protein to albumin ratio predicts postoperative complications in oldest-old patients with colorectal cancer. Int J Colorectal Dis. 2020;35:423–431. doi:10.1007/s00384-019-03491-z
15. Tominaga T, Nonaka T, Hisanaga M, et al. Prognostic value of the preoperative prognostic nutritional index in oldest-old patients with colorectal cancer. Surg Today. 2020;50:449–459. doi:10.1007/s00595-019-01910-w
16. Tang PL, Lin HS, Hsu CJ. Predicting in-hospital mortality for dementia patients after Hip fracture surgery - A comparison between the Charlson Comorbidity Index (CCI) and the Elixhauser Comorbidity Index. J Orthopaedic Sci. 2021;26:396–402. doi:10.1016/j.jos.2020.04.005
17. Lin JX, Huang YQ, Xie JW, et al. Association of the age-adjusted Charlson Comorbidity Index and systemic inflammation with survival in gastric cancer patients after radical gastrectomy. Eur J Surg Oncol. 2019;45:2465–2472. doi:10.1016/j.ejso.2019.07.010
18. Zhao Y, Yue J, Lei P, et al. Neutrophil-lymphocyte ratio as a predictor of delirium in older internal medicine patients: a prospective cohort study. BMC Geriatr. 2021;21(1):334. doi:10.1186/s12877-021-02284-w
19. Loong HH, Wong CKH, Wei Y, et al. Prevalence and prognostic impact of comorbidities and peripheral blood indices in sarcomas. ESMO Open. 2020;5:e001035. doi:10.1136/esmoopen-2020-001035
20. Gupta A, Gupta E, Hilsden R, et al. Preoperative malnutrition in patients with colorectal cancer. Can J Surg. 2021;64:621–629. doi:10.1503/cjs.016820
21. Peng H, Tan X. The Prognostic Significance of Sarcopenia and the Neutrophil-to-Lymphocyte Ratio in Elderly Patients with Esophageal Squamous Cell Carcinoma. Cancer Manag Res. 2021;13:3209–3218. doi:10.2147/CMAR.S302274
22. Itatani Y, Kawada K, Sakai Y. Treatment of Elderly Patients with Colorectal Cancer. Biomed Res Int. 2018;2018:2176056. doi:10.1155/2018/2176056
23. Lu L, Ma L, Zhang X, Susanne Mullins C, Linnebacher M. Analyzing non-cancer causes of death of colorectal carcinoma patients in the US population for the years 2000-2016. Cancer Med. 2021;10:2740–2751. doi:10.1002/cam4.3673
24. Cataudella E, Giraffa CM, Di marca S, et al. Neutrophil-To-Lymphocyte Ratio: an Emerging Marker Predicting Prognosis in Elderly Adults with Community-Acquired Pneumonia. J Am Geriatr Soc. 2017;65:1796–1801. doi:10.1111/jgs.14894
25. Yang C, Yang H, Feng S, Qin J. Value of peripheral blood neutrophil-to-lymphocyte ratio for clinical diagnosis and prognosis of elderly patients with chronic heart failure and atrial fibrillation. Cardiovasc J Afr. 2021;32:178–181. doi:10.5830/CVJA-2021-004
26. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8
27. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi:10.1038/nature01322
28. Sellitto A, Galizia G, De Fanis U, et al. Behavior of circulating CD4+CD25+Foxp3+ regulatory T cells in colon cancer patients undergoing surgery. J Clin Immunol. 2010;31:1095–1104. doi:10.1007/s10875-011-9585-8
29. Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14.
30. Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–1605. doi:10.1038/bjc.2014.46
31. Sakai T, Tsushima T, Kimura D, Hatanaka R, Yamada Y, Fukuda I. A clinical study of the prognostic factors for postoperative early recurrence in patients who underwent complete resection for pulmonary adenocarcinoma. Ann Thorac Cardiovasc Surg. 2011;17:539–543. doi:10.5761/atcs.oa.11.01660
32. Tsai PL, Su WJ, Leung WH, Lai CT, Liu CK. Neutrophil-lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: a systematic review and meta-analysis. J Cancer Res Ther. 2016;12:582–589. doi:10.4103/0973-1482.144356
33. Chiang SF, Hung HY, Tang R, et al. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27:1347–1357. doi:10.1007/s00384-012-1459-x
34. Hosseini SV, Maleknejad A, Salem SA, Pourahmad S, Zanangirfard Z, Zamani M. The pre- and postoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios: the comparison of laparoscopy and laparotomy in colorectal cancer patients. Asian J Endosc Surg. 2022;15:44–50. doi:10.1111/ases.12962
35. Park JJ, Jang HJ, Oh IY, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi:10.1016/j.amjcard.2012.11.012
36. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607–612. doi:10.1016/j.ejso.2018.02.003
37. Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. 2020;20:208. doi:10.1186/s12885-020-6698-6
38. Liu X, Wang Y, Fu Z. Impact of enhanced recovery after surgery on postoperative neutrophil-lymphocyte ratio in patients with colorectal cancer. J Int Med Res. 2020;48:300060520925941. doi:10.1177/0300060520925941
39. Cruz-Ramos M, Del Puerto-Nevado L, Zheng B, et al. Prognostic significance of neutrophil-to lymphocyte ratio and platelet-to lymphocyte ratio in older patients with metastatic colorectal cancer. J Geriatr Oncol. 2019;10:742–748. doi:10.1016/j.jgo.2018.10.002
40. Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017;115:470–479. doi:10.1002/jso.24523
41. Valiathan R, Ashman M, Asthana D. Effects of Ageing on the Immune System: infants to Elderly. Scand J Immunol. 2016;83:255–266. doi:10.1111/sji.12413
42. Verter E, Berger Y, Perl G, et al. Neutrophil-to-Lymphocyte Ratio Predicts Recurrence Pattern in Patients with Resectable Colorectal Liver Metastases. Ann Surg Oncol. 2021;28:4320–4329. doi:10.1245/s10434-021-10000-6
43. Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi:10.1007/s10147-017-1101-6
44. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi:10.1093/annonc/mdw235
45. Benson AB 3rd, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Net. 2017;15:370–398. doi:10.6004/jnccn.2017.0036
46. Pan Y, Lou Y, Wang L. Prognostic value of C-reactive protein to albumin ratio in metastatic colorectal cancer: a systematic review and meta-analysis. Medicine. 2021;100:e27783. doi:10.1097/MD.0000000000027783
47. Katipoglu B, Naharci MI. Could neutrophil-to-lymphocyte ratio predict mortality in community-dwelling older people with delirium superimposed on dementia? Aging Clin Exp Res. 2022;34:1819–1826. doi:10.1007/s40520-022-02108-w
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


