Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Efficacy and Tolerability of Cosmetic Serums Enriched with Five Forms of Hyaluronic Acid as Part of Biweekly Diamond Tip Microdermabrasion Treatments for Facial Skin Dryness and Age-Associated Features
Authors Makino ET, Huang PC , Emmerich T, Jiang LI , Mehta RC
Received 5 January 2023
Accepted for publication 15 April 2023
Published 27 April 2023 Volume 2023:16 Pages 1123—1134
DOI https://doi.org/10.2147/CCID.S399846
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Elizabeth T Makino,1 Priscilla C Huang,1 Tanja Emmerich,2 Lily I Jiang,2 Rahul C Mehta1
1SkinMedica - Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA; 2SGS Stephens Inc, Richardson, TX, USA
Correspondence: Elizabeth T Makino, SkinMedica - Allergan Aesthetics, an AbbVie Company, 18581 Teller Ave, Irvine, CA, 92612, USA, Tel +1 714 246-2598, Email [email protected]
Purpose: There is growing interest in combining topical treatments with aesthetic procedures to combat signs of aging skin. This study aimed to assess the efficacy and tolerability of a novel cosmetic serum containing 5 different forms of HA (HA5 DG) when used via a proprietary diamond-tip microdermabrasion procedure (DG) to treat skin dryness, fine lines/wrinkles, rough texture, and dullness.
Patients and Methods: In this open-label, single-center study, participants received HA5 DG as part of a biweekly DG procedure on the face and neck for 12 weeks. Study participants also applied another take-home HA5 serum to the face twice daily at home, along with a basic skincare regimen. The efficacy of the combined treatment was measured by clinical quantification of multiple skin appearance features, analysis of bioinstrumental measurements, and digital photography.
Results: This study enrolled 27 participants, with an average age of 42.7 years and Fitzpatrick skin phototypes I–III (59.3%), IV (18.5%), and V–VI (22.2%), and 23 participants completed the study. The combined treatment had effects in fine lines/wrinkles, skin dryness, smoothness, radiance, firmness, and hydration 15 minutes post-DG. Furthermore, the significant improvements observed in dryness, fine lines/wrinkles, skin smoothness, and radiance were still visible 3 days after and maintained at week 12. Additionally, smoothing of coarse lines/wrinkles, improvement of skin tone evenness, hyperpigmentation, photodamage, and transepidermal water loss were observed at week 12. The treatment had a favorable tolerability profile and was perceived as efficacious and highly satisfactory.
Conclusion: This novel combined treatment delivered immediate and prolonged skin hydration and high participant satisfaction, proving it can be an excellent approach for skin rejuvenation.
Keywords: microdermabrasion, skin aging, hyaluronic acid, combined treatment, facial rejuvenation, skin dryness
Introduction
Skin aging is a multifactorial process that results from a combination of intrinsic and extrinsic mechanisms. Intrinsic or innate skin aging is a natural and inevitable process that occurs along with the deterioration of internal organs. Extrinsic skin aging is caused by external factors, mainly by ultraviolet irradiation (also referred to as photoaging).1 Both mechanisms cause accumulative structural and molecular changes in the skin layers that are encompassed by changes in skin appearance, especially photo-exposed skin areas. Phenotypically, the intrinsically aged skin appears dry, dull, and finely wrinkled with uneven texture. In contrast, photodamaged skin presents coarse wrinkles, mottled hyperpigmentation, significant loss of elasticity, and a rough-textured appearance.2–4
At the molecular level, one of the most dramatic changes that occurs during intrinsic aging and photoaging is the loss of the skin’s ability to retain water. A key molecule involved in skin moisture is hyaluronic acid (HA), the major glycosaminoglycan (GAG) in the epidermal and dermal extracellular matrix.1 As skin ages, there is a marked loss of epidermal HA, while HA is still present at the dermis level but more avidly bound to tissue structures.5 In photoaged skin, there is a reduction of HA levels that is accompanied by an increase of other GAGs components with no water retaining properties,1,6 and an abnormal distribution of GAGs with elastotic material that impairs their proper function.7 Synthesis of lower-sized HA fragments that have less capacity to retain water has also been reported.1,6 Overall, these changes lead to decreased hydration that is associated with wrinkling, skin atrophy, and all the other hallmarks of the appearance associated with aging.
Permeation of HA into the skin varies with its molecular weight as well as its functional properties.6,8 High molecular weight HA molecules have greater hygroscopicity than low molecular weight ones, but are too large to penetrate the stratum corneum.8 Furthermore, topically applied HA degrades rapidly in the skin surface.8 To overcome these difficulties, a new serum containing 5 different forms of HA (labile cross-linked HA, time-release encapsulated HA, and HA with 3 different molecular weights) in combination with a blend of Vitis Flower Stem Cell extract, a milk peptide complex, and marine polysaccharides as key ingredients was developed to restore endogenous HA homeostasis (HA5® Rejuvenating Hydrator or HA5 SkinMedica, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA).6 When applied twice daily to individuals with mild to severe periocular lines, HA5 boosted endogenous epidermal HA synthesis and decreased HA degradation by reducing the levels of hyaluronidases. In addition, the HA5 serum delivered instant effects in smoothing fine lines/wrinkles and increasing skin moisture within 15 minutes post-application that were sustained over 8 weeks.6 In combination with a neuromodulator injection, HA5 provided instant improvements in the appearance of superficial fine lines and wrinkles, and long-term improvements in areas not treated by the neuromodulator.9
Combining skincare cosmetic products with other facial rejuvenation procedures is becoming a popular trend to maximize outcomes and improve patient satisfaction. Microdermabrasion is an advanced exfoliation method designed to remove the superficial layers of the skin and give the skin a luminous and radiant appearance. Among the benefits of microdermabrasion in skin health are a reduction of mottled hyperpigmentation in photoaging, smoothing of wrinkles, a decrease in roughness, and an improvement in skin texture.10–12 In addition, microdermabrasion has been shown to improve the permeation of different molecules into the skin.13,14 Different modalities of microdermabrasion are available on the market, which differ in the nature of the abrasive component used (eg, aluminum oxide, diamonds). A diamond-tip microdermabrasion handpiece allows a more precise exfoliation of hard-to-reach surfaces and leaves no crystal particles on the skin.11 The DiamondGlow® (DG) microdermabrasion device simultaneously exfoliates the skin, removes impurities, and delivers a customizable serum to the skin’s surface (SkinMedica® Pro-Infusion Serums) for a treatment tailored to patient-specific needs.
The intention of this study was to determine whether a new HA5 cosmetic hydrating serum (SkinMedica HA5 DiamondGlow Serum or HA5 DG, Allergan Aesthetics, an AbbVie Company, Irvine, CA) when used as part of the DG procedure, along with a complementary take-home skincare regimen containing HA5, could improve skin hydration, texture, and appearance in participants with facial dryness.
Methods
Study Design and Participants
A 12-week, open-label, single-center study was conducted to evaluate the efficacy and tolerability of the in-clinic HA5 DG serum when infused into participants’ skin as part of a biweekly DG procedure on the face and neck. In addition to the in-clinic treatment, participants received pre-weighed units of the take-home HA5 serum to apply on the face daily, every morning and evening, along with a basic skincare regimen consisting of a facial cleanser, moisturizer, and sunscreen (SkinMedica Facial Cleanser, SkinMedica Ultra Sheer Moisturizer, and SkinMedica Essential Defense Mineral Shield SPF 35). Participants were instructed to wash their face with the provided cleanser before the application of a thin layer of the take-home HA5 to the entire face, and then a thin layer of the moisturizer. The sunscreen was to be applied in the morning to the entire face, and when needed throughout the day.
Participants eligible for study participation were females aged 25 to 65, with Fitzpatrick skin types I to VI, and mild or greater conditions for facial skin dryness, roughness, lack of radiance, and fine lines or wrinkles, as assessed clinically with a score≥3 in the 10-point modified Griffiths scale (where 0=none and 9=severe). The final study protocol, informed consent form and accompanying materials provided to study participants were reviewed and approved by an institutional review board. All participants who met the entry criteria voluntarily signed the IRB-approved informed consent form and photo release consent form prior to study participation.
Enrolled participants were asked to refrain from waxing, threading, and using chemical depilatories, electrolysis, or laser hair removal on the face for at least 1 week before the baseline visit. In addition, they were instructed to avoid application of any topical moisturizing products to the face for at least 3 days prior to the baseline visit, in order to verify presentation of dry skin. In preparation for each visit, participants were required to wash the face and/or remove all makeup at least 30 minutes before and to not apply any other topical products to the face or eye area until visit completion. Furthermore, participants were asked to abstain from any activities that would increase body temperature or induce sweating, such as drinking hot beverages, smoking, exercising, etc, for at least 1 hour before every visit.
Clinical Efficacy Assessments
Clinical assessments were conducted at baseline, immediately after the first DG procedure (15 min post-application), at Day 3, and at weeks 2, 4, 6, 8, 10 and 12. All assessments were made using the 10-point modified Griffiths scale according to these numerical definitions: 0=none (best possible condition); 1–3=mild; 4–6=moderate; 7–9=severe (worst possible condition). Half-points were used to describe the skin condition more accurately. Each participant was clinically graded on the global face for the following parameters: fine lines/fine wrinkles, coarse wrinkles, dryness (visual), skin smoothness (visual), skin smoothness (tactile), skin tone evenness, hyperpigmentation, photodamage, radiance, and firmness (visual) (Table 1).
 |
Table 1 Grading Scale of Clinically Assessed Efficacy Parameters |
Standardized Digital Photography
To document changes in facial skin conditions over time, 3 photographs with the left, center, and right views of each participant’s face were taken using the VISIA-CR photo station (Canfield Imaging Systems, Fairfield, NJ) with a Canon Mark II digital SLR camera (Canon Incorporated, Tokyo, Japan). Digital images were taken at baseline and at each study visit.
Corneometer Measurements
At each study visit, skin hydration was measured in triplicate on participants’ right or left cheek using a Corneometer® CM 825 (Courage + Khazaka electronic GmbH, Cologne, Germany). The exact measurement location was at the intersection of lines extending up from the corner of the mouth and horizontally across the bottom of the nose. The corneometer is a capacitance-based instrument that measures the epidermal moisture content with a penetration depth of 10–20 µm, which is approximately the normal thickness of the stratum corneum.15 The measurement increases with skin hydration.16
Tewameter Measurements
The transepidermal water loss (TEWL) or transfer of water through the stratum corneum was measured using a Tewameter® TM300 (Courage + Khazaka electronic GmbH). One single measurement was taken at the center of each check, at the intersection of lines extending up from the corner of the mouth and horizontally across the bottom of the nose, on the opposite cheek of the one randomized for corneometer measurements. A decrease in TEWL values reflects an improvement in the barrier properties of the skin.17
Participant Questionnaires
Participants completed questionnaires to evaluate the self-perceived efficacy and satisfaction with the treatment on facial and neck skin at 15 min post-DG, day 3, and weeks 2, 4, 6, 8, 10, and 12.
Tolerability Assessment
Tolerability evaluations were conducted at each study visit by clinically assessing for signs and symptoms of edema and erythema, and by participants reporting on the degree of burning, stinging, and itching on the face. When assessing subjective irritation at the baseline visit, participants reported the symptoms they were currently experiencing. At subsequent timepoints, participants reported the degree of any of these symptoms since the previous visit. Irritation signs and symptoms were graded on a scale from 0 to 3 (where 0=none, 1=mild, 2=moderate, and 3=severe). Half points were used as needed to better describe the skin condition.
Statistical Analysis
The Wilcoxon signed-rank test was used to assess for differences on investigator-graded efficacy scores and tolerability evaluations at each timepoint compared to baseline. Bioinstrumental measurements were examined with a paired t-test, whereas participant-reported outcomes were analyzed with a binomial test (sign test). P values <0.05 were regarded as statistically significant. Participants with a baseline score of 0 for any specific parameter were excluded from the analysis. Additionally, data from the visits attended by participants who dropped out prematurely from the study were included in the intent-to-treat population analysis.
Results
Study Participants
A total of 23 females completed the study out of the 27 who enrolled. Two participants were lost to follow-up, one was discontinued by investigator decision because of being unable to attend the scheduled post-baseline visits, and another one requested withdrawal after missing one visit. Participants were on average 42.7 years old and had Fitzpatrick skin types I to III (59.3%), skin type IV (18.5%), and skin types V to VI (22.2%). Caucasians comprised 70.4% of the study participants, followed by African Americans (25.9%), and Asians (3.7%).
Efficacy Assessments
Results from the investigator-graded efficacy analysis showed statistically significant improvements in the appearance of fine lines/wrinkles, dryness (visual), skin smoothness (visual), skin smoothness (tactile), and radiance of the facial area immediately after the first DG treatment (P ≤0.007; Figure 1). Remarkably, the improvement in these parameters was still appreciated 3 days after treatment with no further intervention than the take-home skincare routine (Figure 1). These changes remained consistent through week 12 (P ≤ 0.007; Figure 2). Firmness (visual) grades significantly improved after each DG+ HA5 DG application, at 15 min post-treatment and weeks 2 through 12 (P ≤ 0.008). In addition, significant positive results on skin tone evenness and hyperpigmentation were seen starting week 2 through 12 (P ≤ 0.016), while improvements from baseline in photodamage scores were observed from week 4 through week 12 (P ≤ 0.008). Coarse wrinkles were also significantly reduced from week 8 to the end of the study (P =0.031).
 |
Figure 2 Mean percent change from baseline in clinically assessed efficacy parameters scores at weeks 2 through 12. *Statistically significant vs baseline, all P < 0.001; Wilcoxon signed-rank test. |
Bioinstrumental analyses demonstrated substantial improvements in facial skin hydration and its barrier properties over time (Figure 3). A significant increase in epidermal hydration was seen as early as 15 min after treatment by 88.9% of study participants (P < 0.001) when compared to baseline, and at weeks 2 through 12 (P ≤ 0.049). A statistically significant decrease in transepidermal water loss was observed at weeks 2, 4 and 12. The effects of the combined treatment on water loss peaked at week 12 with a mean decrease of 23.8% (P = 0.001), indicating an improvement in facial skin barrier properties vs baseline.
 |
Figure 3 Mean percent changes from baseline in corneometer and tewameter measurements at different timepoints. *Statistically significant vs baseline, all P ≤ 0.049; paired t-test. |
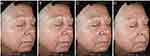
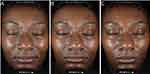
Standardized digital photographs provided further evidence of the improvement in skin health observed by investigators and in bioinstrumental analyses. In a Caucasian female aged 65, with Fitzpatrick skin type III, the combined treatment demonstrated changes in the appearance of perioral fine line/wrinkles and hyperpigmentation (Figure 4). In a 32-year-old African American female with Fitzpatrick skin type V, the skin appeared more radiant and with a more even texture at weeks 2 and 12 compared to baseline (Figure 5).
Self-Assessment Questionnaires
Participant-reported outcomes mirrored the positive results of clinically-graded parameters, with a greater proportion of participants selecting favorable responses vs unfavorable for a series of inquiries regarding treatment efficacy on their facial and neck skin (Figures 6 and 7). Immediately after the first DG procedure and HA5 DG infusion, 100% of participants agreed that their facial skin looked more hydrated, and above 85% reported a reduction in roughness, and improvements in the facial skin texture and smoothness. Regarding the neck skin, where participants received only the in-clinic treatment, 88.8% of the participants responded that the treatment did not make their skin feel dry or uncomfortably tight, and 85.1% highlighted that their skin looked and felt softer.
The long-term effects of the in-clinic and take-home treatments on facial skin were highly rated (all ≥87%), with over 90% of participants describing their skin as more radiant after 12 weeks of treatment, and 100% of participants reporting improved skin texture and hydration at the end of the study. Regarding the neck skin, ≥73.9% of participants rated the treatment efficacy favorably. Among the treatment effects, 95.7% of participants reported a reduction of neck skin dryness, 95.7% observed improved neck skin look and texture, and 91.3% reported that their neck skin looked rejuvenated.
Participants were highly satisfied with their skin appearance after the in-clinic procedure with or without the take-home HA5 serum (Figure 8); 95.7% and 82.6% of participants described the satisfaction with their facial and neck treatments as good or excellent, respectively.
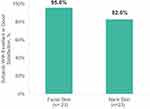 |
Figure 8 Percent of participants with excellent or good satisfaction with facial and neck treatments at week 12. |
Tolerability Results
Following each visit, objective and subjective tolerability was evaluated by assessing for signs and symptoms of facial irritation. Participants experienced a significant improvement in erythema from week 6 through week 12 (P ≤ 0.039) when compared with baseline. No other investigator- or participant-assessed tolerability issues arose during the study. One participant reported a serious adverse event consisting of back muscle spasms of moderate severity that was deemed unlikely to be related with the study treatment and resolved.
Discussion
It is well known that the levels of HA decrease drastically as skin ages,1,5 leading to a marked loss of skin hydration that lowers the elasticity of the skin and promotes the formation of wrinkles. Changes in photodamaged skin are also associated with lower levels of HA and an abnormal distribution of the HA polymers that limit their biological functions.1,5 It is therefore of utmost importance to restore the HA levels to maintain the properties of a youthful skin. However, external application of HA poses significant challenges given the high turnover of HA in the skin6 and the limited permeation of HA molecules, especially those with high molecular weights.8 Microdermabrasion has been used to improve skin permeability to certain drugs with promising results.13,14 This technique is most often used for facial rejuvenation, since it can soften fine lines and wrinkles, decrease hyperpigmentation, and improve skin texture.10–12 However, clinicians are now leveraging the effects of microdermabrasion by combining it with other procedures or products to optimize results and meet participants’ expectations. In this study, we demonstrate that combining a proprietary diamond-tip microdermabrasion procedure with HA serums provides immediate and long-term improvements in overall skin appearance in individuals with signs of aging skin.
The administration of the take-home HA5 and in-clinic HA5 DG serums in combination with DG induced a significant drop in skin dryness which reached −48.3% at weeks 10 and 12, as assessed by the investigators; these effects were further substantiated by a significant increase in epidermal hydration measured by the corneometer. The changes in skin hydration were associated with a significant reduction in fine lines and wrinkles, improved tactile skin smoothness, firmness, and radiance. The clinical improvements seen with this combined skincare regimen can be attributed in part to the unique blend of 5 HA forms in the serums which includes: 1) low molecular weight HA, which is able to penetrate deeper into the skin than high molecular weight HA, providing greater hydration effects;18 2) nanoparticles of HA, which have demonstrated improved infusion into the skin compared to HA,19 and provide skin hydration, firmness, and elasticity, as well as a decrease in wrinkles depth;20 3) time-release encapsulated HA which ensures a controlled and sustained HA release to deliver enduring effects; 4) cross-linked HA polymers that guarantee an extended duration of action given their increased resistance to biodegradation;21 5) and uncross-linked HA which hydrates and smooths the skin, but has a shorter half-life compared to cross-linked forms. In previous studies, the HA5 blend increased the expression of HA synthases and decreased the levels of hyaluronidases in vitro, and increased endogenous HA levels in ex vivo human skin, suggesting that the serums not only deliver HA but also stimulate its endogenous synthesis and slow down its degradation.6 Furthermore, administration of HA5 twice daily induced a significant improvement in periocular and forehead lines, skin hydration, and tactile roughness that lasted for 8 weeks.6 The characteristics of the HA molecules included in the serums along with the in vitro and in vivo results support the role of the HA5 formulation not only in the immediate effects observed in skin appearance, but also in the cumulative and increased improvements seen over the 12 weeks of this study.
The participants of this study experienced a transitional increase in TEWL at day 3, that is likely due to the removal of the stratum corneum by the DG procedure. At later timepoints, in contrast, TEWL values were markedly reduced, which indicates a healthy skin barrier state. Previous studies in 3D human skin models showed that the HA5 blend enhanced the expression of keratinocyte differentiation markers and increased the levels of claudin-1 and −4,6 which have been linked to the formation of tight junctions between keratinocytes.22 These tight junctions along with the stratum corneum are responsible for the epidermal barrier properties. Since the DG procedure removes the stratum corneum to a great extent, the TEWL decrease is likely due to the new formation of tight junctions which suggests that the benefits of the combined treatment go beyond skin moisturization. Indeed, both HA5 serums contain a flower stem cell extract in combination with marine micro-organism polysaccharides and a peptide complex, which was previously shown to have anti-inflammatory and antioxidant properties in addition to supporting the internal HA restoration and the recovery of epidermal homeostasis.6
With the increasing number of available procedures for facial rejuvenation, patients seek meaningful data to select the treatment that is right for them. Thus, to support an informed decision, it is essential for clinicians to capture patient satisfaction, preferences, and perceived efficacy following aesthetic interventions. In the present study, participants perceived increased hydration, smoothness, and better texture of the facial and neck skin as being the most improved aspects of their skin appearance immediately post-DG and at week 12. Remarkably, 95.7% of participants agreed that they would like to continue receiving the combined treatment on their facial skin. It is well known that neck skin shows more severe aging patterns than facial skin.23 However, the perceived treatment effectiveness and satisfaction with neck skin was high among the participants of the study, which further supports the benefits of the DG+HA5 DG combined treatment in skin rejuvenation.
Moreover, the treatment was well-tolerated, with no signs of edema, burning, itching, or stinging on the face. Symptoms of erythema were found at baseline and decreased significantly after 6 weeks of treatment. Photoaging and excessive skin dryness could explain the presence of facial erythema before the beginning of the study. In addition, microdermabrasion has been associated with a temporary mild to moderate erythema that lasts approximately 1 to 2 days after the procedure.24 However, the reduction of facial erythema at later timepoints suggests the combined treatment has healing effects that build up over time. Evidence of the anti-inflammatory and wound healing effects of HA can be found throughout the literature.25, Additionally, the serums contain other ingredients that may enhance the HA anti-inflammatory and wound healing properties.
This study showcases a comprehensive approach of in-clinic and at-home treatments to improve the appearance of aged skin. Together, DG with both HA5 serums produced significant immediate and long-term improvements in skin dryness, fine lines and wrinkles, skin smoothness, radiance, and texture that were supported clinically by bioinstrumental analysis and digital photography. These clinical findings were further corroborated by participants, who in a great majority reported high treatment effectiveness and satisfaction. Overall, the combined use of DG with professional grade HA5 serums is a well-tolerated and effective strategy to replenish skin moisture and restore a youthful glow and texture.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.html.
Ethics Approval
This study was conducted per all applicable guidelines for the protection of human subjects for research as outlined in 21 CFR 50, the accepted standards for Good Clinical Practice (GCP), and was compliant with the guidelines in the Declaration of Helsinki. All participants completed the informed consent process before participating in the study.
Acknowledgments
Writing assistance was provided to the authors by Ana Vicente-Sanchez, PhD, of AbbVie Inc, and funded by AbbVie Inc. Editorial assistance was provided by Angela Hadsell, of AbbVie Inc, and funded by AbbVie Inc.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
This study was sponsored by Allergan Aesthetics, an AbbVie company. All authors meet the ICMJE criteria for authorship, and neither honoraria nor other forms of payment were made for authorship.
Financial arrangements of the authors with companies whose products may be related to the present report are as follows, as declared by the authors:
Ms. Makino, Mrs. Huang, and Dr. Mehta are employees of AbbVie Inc and may own stock/stock options in the company.
Dr. Jiang reports grants from AbbVie/SkinMedica, during the conduct of the study.
The authors report no other conflicts of interest in this work.
References
1. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258.
2. Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241–251. doi:10.1002/path.2098
3. Rittie L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. doi:10.1101/cshperspect.a015370
4. Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi:10.1177/0963689717725755
5. Meyer LJ, Stern R. Age-dependent changes of hyaluronan in human skin. J Invest Dermatol. 1994;102(3):385–389. doi:10.1111/1523-1747.ep12371800
6. Narurkar VA, Fabi SG, Bucay VW, et al. Rejuvenating hydrator: restoring epidermal hyaluronic acid homeostasis with instant benefits. J Drugs Dermatol. 2016;15(1 Suppl 2):s24–s37.
7. Bernstein EF, Underhill CB, Hahn PJ, Brown DB, Uitto J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br J Dermatol. 1996;135(2):255–262. doi:10.1111/j.1365-2133.1996.tb01156.x
8. Juncan AM, Moisa DG, Santini A, et al. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules. 2021;26(15):4429. doi:10.3390/molecules26154429
9. Fabi SG, Zaleski-Larsen L, Bolton J, Mehta RC, Makino ET. Optimizing facial rejuvenation with a combination of a novel topical serum and injectable procedure to increase patient outcomes and satisfaction. J Clin Aesthet Dermatol. 2017;10(12):14–18.
10. Coimbra M, Rohrich RJ, Chao J, Brown SA. A prospective controlled assessment of microdermabrasion for damaged skin and fine rhytides. Plast Reconstr Surg. 2004;113(5):1438–43; discussion 1444. doi:10.1097/01.PRS.0000113026.94292.0B
11. Karimipour DJ, Karimipour G, Orringer JS. Microdermabrasion: an evidence-based review. Plast Reconstr Surg. 2010;125(1):372–377. doi:10.1097/PRS.0b013e3181c2a583
12. Shim EK, Barnette D, Hughes K, Greenway HT. Microdermabrasion: a clinical and histopathologic study. Dermatol Surg. 2001;27(6):524–530. doi:10.1046/j.1524-4725.2001.01001.x
13. Lee WR, Shen SC, Kuo-Hsien W, Hu CH, Fang JY. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J Invest Dermatol. 2003;121(5):1118–1125. doi:10.1046/j.1523-1747.2003.12537.x
14. Lee WR, Tsai RY, Fang CL, Liu CJ, Hu CH, Fang JY. Microdermabrasion as a novel tool to enhance drug delivery via the skin: an animal study. Dermatol Surg. 2006;32(8):1013–1022. doi:10.1097/00042728-200608000-00006
15. Ashtikar M, Matthaus C, Schmitt M, Krafft C, Fahr A, Popp J. Non-invasive depth profile imaging of the stratum corneum using confocal Raman microscopy: first insights into the method. Eur J Pharm Sci. 2013;50(5):601–608. doi:10.1016/j.ejps.2013.05.030
16. Heinrich U, Koop U, Leneveu-Duchemin MC, et al. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). Int J Cosmet Sci. 2003;25(1–2):45–53. doi:10.1046/j.1467-2494.2003.00172.x
17. Antonov D, Schliemann S, Elsner P. Methods for the assessment of barrier function. Curr Probl Dermatol. 2016;49:61–70.
18. Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10(9):990–1000.
19. Shigefuji M, Tokudome Y. Nanoparticulation of hyaluronic acid: a new skin penetration enhancing polyion complex formulation: mechanism and future potential. Materialia. 2020;14:100879. doi:10.1016/j.mtla.2020.100879
20. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7(3):27–29.
21. Choi SC, Yoo MA, Lee SY, et al. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J Biomed Mater Res A. 2015;103(9):3072–3080. doi:10.1002/jbm.a.35437
22. Sugawara T, Iwamoto N, Akashi M, et al. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J Dermatol Sci. 2013;70(1):12–18. doi:10.1016/j.jdermsci.2013.01.002
23. Perez MI. An anatomic approach to the rejuvenation of the neck. Dermatol Clin. 2001;19(2):387–396. doi:10.1016/S0733-8635(05)70276-X
24. Fernandes M, Pinheiro NM, Crema VO, Mendonca AC. Effects of microdermabrasion on skin rejuvenation. J Cosmet Laser Ther. 2014;16(1):26–31. doi:10.3109/14764172.2013.854120
25. Marinho A, Nunes C, Reis S. Hyaluronic acid: a key ingredient in the therapy of inflammation. Biomolecules. 2021;11(10):1–34. doi:10.3390/biom11101518
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.