Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Efficacy and Tolerability of a Sunscreen Containing Licochalcone a and L-Carnitine as an Adjunct to Retinoids in the Management of Acne and Post-Acne Pigmentation Among Malaysian Patients
Authors How KN , Ho WC, Sundaran M, Wan Ahmal Kammal WSL, Lim PY , Chew W
Received 25 May 2023
Accepted for publication 8 September 2023
Published 23 December 2023 Volume 2023:16 Pages 3719—3729
DOI https://doi.org/10.2147/CCID.S422898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Kang Nien How,1,2,* Wen Chung Ho,1,2,* Meroshini Sundaran,1,2,* Wan Syazween Lyana Wan Ahmal Kammal,1,2,* Poh Ying Lim,3 Wilson Chew4
1Dermatology Unit, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia; 2Hospital Sultan Abdul Aziz Shah, Universiti Putra Malaysia, Serdang, Selangor, Malaysia; 3Department of Community Health, Universiti Putra Malaysia, Serdang, Selangor, Malaysia; 4Beiersdorf (Malaysia) Sdn Bhd, Petaling Jaya, Selangor, Malaysia
*These authors contributed equally to this work
Correspondence: Kang Nien How, Dermatology Unit, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Selangor, 43400, Malaysia, Tel +603 97695601, Email [email protected]
Purpose: We aim to evaluate the effectiveness and tolerability of a sunscreen formulation containing licochalcone A (LicA) and L-carnitine (LC) as an adjuvant to adapalene in the management of acne and post-acne pigmentation (PAH).
Patients and Methods: A randomized, double-blind, active comparator-controlled trial of 51 patients aged 18 years or older with a clinical diagnosis of mild-to-moderate acne vulgaris was conducted at the Hospital Sultan Abdul Aziz Shah, Universiti Putra Malaysia. The efficacy and tolerability of once-daily adapalene 1.0% were assessed during the 2-week run-in period. Subsequently, patients were randomized to receive either an add-on investigational LicA-containing sunscreen or niacinamide-containing comparator sunscreen every 4 hourly during daytime for 4 weeks. Patients were followed up at Weeks 2 and 4 to assess for improvement in acne severity, PAH, calorimetric parameters and cutaneous tolerability.
Results: Two weeks of adapalene usage significantly improved acne severity; however, up to 52% of patients experienced dryness, burning and stinging. Adding LicA-containing or comparator sunscreens was associated with further improvement in acne severity, PAH and calorimetric parameters at the study endpoint. No significant differences in the cutaneous tolerability profiles were observed between treatment groups. Notably, significantly fewer patients receiving LicA-containing sunscreen developed scaliness at Week 4 compared with those in the comparator group. In addition, more patients receiving LicA-containing sunscreen reported less dryness, burning and stinging reactions than the comparator group. Importantly, more patients receiving LicA-containing sunscreen agreed that their treatment led to excellent improvement than the comparator group; of note, one patient reported that their condition worsened with the receipt of the comparator product.
Conclusion: The concurrent use of LicA–containing sunscreen with adapalene may improve the cutaneous tolerance to adapalene among Malaysian patients.
Keywords: acne vulgaris, cosmeceuticals, retinoids, sunscreen agents, skin pigmentation
Introduction
Acne vulgaris is a common chronic inflammatory pilosebaceous disorder estimated to affect 9.38% of the global population across all age groups, with almost all adolescents affected at some point in their life.1 Of those with acne, 66% were categorized as mild, 33% as moderate, and less than 10% as severe.2 Acne has a significant psychological impact on patients that can adversely affect their quality of life (QoL).3
Acne is attributed to hyperseborrhea, abnormal follicular keratinization, proliferation of Cutibacterium acnes and an inflammatory reaction. Briefly, acne management is aimed at reducing sebum production, reducing bacterial content, inflammation and unclogging pores. This includes the usage of benzoyl peroxide (BPO), sulfur, azelaic acid, retinoids and alpha- or beta-hydroxyl acids.4
Acne is commonly treated according to its severity levels, with various clinical guidelines recommending topical retinoids as the first-line treatment and maintenance for mild-to-moderate acne.5–8 However, despite the effectiveness of the combination of topical retinoids and BPO for mild-to-moderate acne, the high discontinuation rate due to side effects (SEs) during the early treatment phase limits their clinical use.9 Additionally, the fear of retinoid-induced dermatitis, especially among Asians, further contributes to their underutilization.10 Strategies to improve cutaneous tolerability (through patient education, moisturizer use, short-contact therapy and alternate-day application method) have all been shown to enhance adherence to topical retinoids.10–12
Post-acne hyperpigmentation (PAH) is a significant acne sequelae and a common cosmetic concern that can persist for at least 5 years in 22.3% of patients.13 PAH is even more prominent among patients with darker skin color14 Notably, one-third of patients regard PAH as more problematic than acne lesions.13,15 While topical retinoids are effective in addressing PAH, they can also lead to cutaneous intolerance similar to that observed in acne management.16
The role of cosmeceuticals, a subclass within the domain of drugs and cosmetics, has gained significant traction for acne management. Therapeutic treatment and a proper skincare routine are integral components of holistic acne management. The four fundamental skincare routine includes cleansing, moisturizing, medicating and sun protection.17 However, the increased sebum production that results in oily and greasy skin appearance, common in patients with acne, could contribute to reduced sunscreen use.18 This can be problematic as many acne therapies (including retinoids) can compromise the skin barrier and increase susceptibility to ultraviolet damage, thereby contributing to the formation of free radicals that can cause acne flares.19 Therefore, the use of light and non-occlusive sunscreen instead of moisturizers for oily skin could provide a dual benefit of moisturizing while providing sun protection.18 Furthermore, some sunscreens with opacifying agents could also help conceal acne red spots.
Research has shown that C. acnes induces the secretion of major proinflammatory cytokines implicated in acne pathogenesis via an inflammasome pathway, specifically the NACHT, LRR and PYD domain-containing protein 3 (NLRP3).20 Licochalcone A (LicA), a chalconoid isolated from the root of Glycyrrhiza inflata, was demonstrated the ability to suppress the activation of NLRP3 inflammasome and reactive oxygen species both in vitro and in a murine model.21 Moreover, LicA has exhibited antibacterial effects against C. acnes.21 The combination of LicA, L-carnitine (LC) and other active ingredients in a topical emulsion has been found to be effective in managing mild-to-moderate acne and preventing relapse, improving retinoid efficacy and tolerability, enhancing photodynamic therapy efficacy, and suppressing and modifying lipid secretion.22–26
However, the role of sunscreen containing these active substances for acne vulgaris has not been evaluated. Thus, our aim is to compare the efficacy and safety of a sunscreen formulation containing LicA and LC with a comparator sunscreen as an adjuvant to adapalene in managing mild-to-moderate acne vulgaris and PAH among Malaysian patients.
Materials and Methods
Study Design
We conducted a 4-week, prospective, double-blind, randomized, active comparator-controlled trial at the Hospital Sultan Abdul Aziz Shah, Universiti Putra Malaysia, between October 2021 and July 2022. All test formulations were provided in identical packages to ensure the integrity of the blinding. In addition, an independent third party was responsible for study randomization, dispensary of study formulations and education on how to apply the formulations. The study was conducted in accordance to the Declaration of Helsinki. It is registered with the ISRCTN registry (ISRCTN31328643) and was approved by the Universiti Putra Malaysia Ethics Committee for Human Research Malaysia (NMRR-21-793-59045). All participants provided written, informed consent prior to study enrolment.
Study Participants
Fifty-six male and female participants aged 18 years or older with clinically diagnosed mild-to-moderate acne vulgaris as defined by the Comprehensive Acne Severity Scale (CASS) were included. Participants were ineligible if they have other forms of acne (such as acne excoriee, acne conglobata and acne fulminans), severe acne and a history of using oral antibiotic (in the past month), oral isotretinoin (in the last 6 months), or topical antimicrobial or tretinoin (in the previous 2 weeks). Pregnant and lactating women were also excluded (Full details are available as Supplementary Data).
Treatment Regimens
All patients were prescribed adapalene gel 0.1% (T3 Ada, Hoe Pharma) once daily at bedtime during the 2-week run-in period to assess the efficacy and cutaneous tolerance to adapalene. Subsequently, patients were randomly assigned in a 1:1 ratio using a chit method to receive either an add-on investigational sunscreen (Eucerin® Sun Dry Touch Oil Control, Beiersdorf) or niacinamide-containing comparator sunscreen every 4 hourly during daytime for 4 weeks. Patients were given a cleansing gel and instructed to cleanse their face twice daily before applying a pea-sized amount of adapalene gel to the whole face. The efficacy and tolerability of investigational and comparator sunscreens were assessed at Weeks 2 and 4. Patients were advised against using other skincare/cosmetic products or acne medications throughout the study.
Methods of Assessment
All assessments were performed by two investigators (KNH & WSL). Acne severity was rated by clinical evaluation using the Global Acne Grading System (GAGS), CASS and lesion-counting methods at each visit. The GAGS score considered five locations of the face (forehead, nose, left cheek, right cheek and chin), with a factor based roughly on the surface area, distribution and density of pilosebaceous units. Each of the five locations was graded separately from 0 to 4, with the most severe lesion within that location determining the local score. The global score is a summation of all local scores.27 The CASS graded acne severity at each region into six different categories, from “clear” to “very severe acne”. The scale also considers the number of comedones, papules, pustules, nodules and cysts and the visibility of lesions from 2.5 m away.28 For the lesion-counting method, the number of inflammatory and non-inflammatory lesions (ILs and NILs) are individually counted.
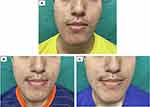
Colorimetric melanin and erythema indices (MI and EI) were tested using a skin analysis system (DermaLab Combo, Cortex Technology). The three most pigmented and erythematous macules were measured at every study visit; the average of the three most pigmented lesions was then considered for the final analysis. PAHPI was measured using previously validated methods.29 Standard digital photography was taken using a smartphone without flash (Huawei Mate 20); the photographs were taken from the left, right and center of the face using the same angle and lighting.
Evaluation of QoL impairment using the Cardiff Acne Disability Index (CADI) was also carried out at every study visit, with a total score ranging from 0 to 15. Furthermore, patient satisfaction with their treatment was assessed using a visual analogue scale (VAS) that has a total score ranging from −1 to 2. The presence of solicited cutaneous reactions (such as erythema, scaling, dryness, burning/stinging and pruritis) was recorded at Weeks 0, 2 and 4.
Outcome Measures
The primary outcome measure was the improvement in acne severity between the two treatment arms at Week 4, evaluated using CASS, GAGS and total lesion counts. The secondary outcome measures were objective skin assessment, tolerability and acceptability evaluation at the study endpoint.
Statistical Analysis
Statistical analysis was performed using SPSS version 26 (Chicago, IL) for Windows. Efficacy analyses were performed in the per-protocol population. Mean and standard deviation (SD) were used to describe continuous variables, whereas categorical variables were expressed in counts and percentages. Independent Student’s T-test was used to determine baseline variability for continuous variables. Statistical differences between different time points and arms for continuous variables were calculated using repeated measures ANOVA. The chi-square test was used to compute differences between treatment arms for categorical variables. A p-value less than 0.05 was considered statistically significant.
Results
Baseline Demographics and Clinical Characteristics
Participant flow through the study is shown in Figure 1. A total of 51 patients who completed the 2-week run-in period were randomized and treated (interventional group, n = 26; active comparator group, n = 25), and 49 patients were included in the final per-protocol analysis. Baseline patient demographic and clinical characteristics are shown in Table 1. The mean age was 25.5 ± 5.32 years; 35 patients were female. Apart from gender, other patient characteristics at baseline did not differ significantly between the two groups. In addition, there were no differences in baseline dermatologic scores between groups (Table 2).
 |
Table 1 Baseline Patient Demographic and Clinical Characteristics in the per-Protocol Analysis |
 |
Table 2 Baseline Dermatologic Scores in the per-Protocol Analysis |
 |
Figure 1 Participant flow through the study. |
Primary Outcome Measures
Acne Severity: CASS, GAGS, IL Count and NIL Count
The mean scores of CASS, GAGS, IL count and NIL count were significantly reduced 2 weeks after adapalene use. Patients in both the intervention and comparator arms continued to demonstrate statistically significant improvements in their acne severity at Week 4 compared with the baseline period (Figures 2A–D and 3A–C). Additionally, mean scores of GAGS and ILs of both treatment groups were also significantly reduced at Week 2 compared with Week 0 (Figure 2B and C). However, there were no significant interarm differences in the mean scores of CASS, GAGS, IL count and NIL count.
Secondary Outcome Measures
Skin Color: MI and EI
Similarly, the mean MI score was significantly reduced after 2 weeks of adapalene use; however, the mean EI score of both groups did not differ significantly. At the study endpoint, the mean scores of MI and EI were significantly reduced in both arms versus Week 0. The mean MI and EI scores did not show significant interarm differences.
PAH: PAHPI
Although the mean PAHPI remained relatively unchanged after 2 weeks of the adapalene run-in period, there was a significant reduction in PAHPI observed with both interventional and comparator formulations at Week 4 versus Week 0 (Figures 3A–C and 4). The difference in the mean PAHPI between both treatment arms also did not reach statistical significance.
QoL Impairment
Patients in the intervention and comparator arms reported significant improvement in their mean CADI scores after the run-in period to Week 0 and continued to Week 4 of treatment (Figure 5). Nonetheless, the degree of improvements in CADI observed with both treatment modalities was not significantly different.
Acceptability and Tolerability Profiles
At Week 4, more patients receiving LicA-containing sunscreen agreed that their treatment led to excellent improvement compared with those receiving a comparator product (13 versus 10 patients). Similar number of patients reported mild improvement with investigational and comparator sunscreens at the study endpoint. Interestingly, one patient claimed their condition worsened after receiving a comparator sunscreen.
Erythema, dryness, scaliness, burning/stinging, and pruritus emerged as the prevailing adverse events reported during the initial 2-week trial phase involving adapalene. Additional adverse events associated with the utilization of adapalene encompassed acne eruption (n = 5) and oily skin (n = 2). Notably, following a 4-week period of employing sunscreen, a statistically significant disparity in the reduction of erythema, stinging/burning, scaliness, and dryness was observed within the investigator group (Figure 6). These discernible changes were not mirrored in the comparator group. However, upon comparing these two groups, only with regards to scaliness (p = 0.017), were significant distinctions detected. Intriguingly, at the culmination of the study, one patient each within the comparator group exhibited occurrences of urticaria and eczema. Nevertheless, establishing a direct correlation between these incidents and the treatment remains uncertain.
Discussion
Retinoids are effective in managing acne vulgaris, making them the mainstay in acne treatment. However, local irritations are common in the first few weeks of treatment regardless of which generation of retinoids is used, often leading to treatment discontinuation.30 In our study, more than 50% of our patients experienced at least one cutaneous intolerance during the first 2 weeks of the adapalene run-in period. Cutaneous irritations are thought to occur as a result of retinoid normalizing desquamation, during which corneocyte arrangement is disrupted, leading to the loss of cohesion. Irritations generally resolve within 1–2 weeks of continuous treatment after the completion of epidermal rearrangement.30
Several approaches, such as short contact therapy and alternate-day application have been found to improve cutaneous tolerability.11,12 In addition, moisturizer use (alongside adapalene therapy) has been shown to yield greater skin tolerability without compromising treatment efficacy. Chularojanamontri et al conducted an 8-week randomized controlled trial evaluating the tolerability and efficacy of a moisturizer formulation containing LicA and LC in Asian patients with mild-to-moderate acne receiving adapalene treatment. They showed that patients receiving LicA-containing moisturizer had significantly better skin tolerance than those using adapalene alone at Week 8 (0.60 ± 0.39 vs 0.85 ± 0.52; p = 0.048).26
A well-formulated, water-in-oil emulsion sunscreen with the dual benefit of hydration and sun protection could be a promising alternative for oily skin. Our study showed that receipt of LicA-containing sunscreen for 4 weeks was equally as effective as the comparator sunscreen in improving acne severity across all parameters, including CASS, GAGS, IL count and NIL count. Nevertheless, despite significant improvement in acne severity with adapalene, our study revealed no significant interarm differences between the intervention and comparator groups.
In contrast, previous studies showed that the application of active formulation containing LicA and LC resulted in a significant reduction in acne severity compared with the respective control groups.24,26,31 This may be explained by the fact that our study has a relatively shorter follow-up period than the previous three studies, with a follow-up duration ranging from 8 to 12 weeks.24,26,31 The difference in the study design employed in our study could also account for the conflicting results. For example, the three prior studies used a placebo vehicle without active ingredients as control,24,26,31 whilst we utilized an active comparator sunscreen containing niacinamide – a potent whitening and anti-inflammatory agent.
In general, sunscreen use in our study was associated with significant overall improvements in cutaneous tolerability. Specifically, patients receiving LicA-containing sunscreen consistently reported better skin tolerability than those receiving the comparator product at Week 4. Notably, significantly more patients who used LicA-containing sunscreen showed a reduction in scaliness than those in the comparator group at the study endpoint, corroborating with findings by Kulthanan et al.24 In their study, patients receiving LicA-containing moisturizer reported significantly less erythema at the study endpoint.24 Of equal importance, our trial revealed that more patients in the intervention group agreed that receipt of LicA-containing sunscreen led to excellent improvement compared with the comparator group.
Retinoids not only stimulate desquamation and melanocyte disbursal, but they also directly affect pigmentation by blocking melanosome transfer to the keratinocyte while increasing epidermal turnover. Moreover, irritation due to retinoid use may potentially worsen post-inflammatory hyperpigmentation (PIH). Therefore, regular sunscreen use is recommended to minimize retinoid-induced photodermatitis.17,19 A study by Puaratanaarunkon and Asawanonda demonstrated that LicA-containing sunscreen for 6 weeks effectively reduced PIH after picosecond laser treatment.32 Specifically, our study demonstrated the effectiveness of LicA-containing sunscreen in reducing PAH. In addition, two pilot studies conducted in Thailand showed that LicA-containing sunscreen use for 4 weeks was associated with a significant reduction in PAH (unpublished data). Together, these findings support the role of LicA-containing sunscreen in reducing PAH – a major consideration when treating acne in Asian patients, as they are at higher risk of developing PAH and often regard PAH as more bothersome than acne itself.14
Our study is limited by a small sample population, the inclusion of only patients with mild-to-moderate acne and a short follow-up period. Besides, our study excluded patients who are on standard of care, such as adapalene, benzoyl peroxide, doxycycline and oral isotretinoin. This might potentially affects the diversity of our samples. Lastly, this study was conducted only among Malaysian patients. Therefore, caution should be exercised when applying our findings to populations outside of Malaysia. Despite these limitation, our study lays the groundwork for future research. Larger-scale multi-center studies involving diverse populations, with longer follow-up periods could provide more comprehensive insights into the potential benefits and limitations of LicA-containing sunscreen in acne management.
Conclusion
Although adapalene is efficacious in acne management, intolerance is common. The concurrent use of adapalene and LicA-containing sunscreen effectively reduced acne severity and PAH among Malaysian patients. Crucially, receipt of LicA-containing sunscreen improved the tolerability of adapalene, thus representing an important adjunct to the therapeutic management of mild-to-moderate acne.
Data Sharing Statement
No further data will be shared from this article. Kindly contact the corresponding author if more information is required.
Acknowledgments
This research was funded by Beiersdorf (Malaysia) Sdn. Bhd.
Author Contributions
All authors made substantial contributions to the conception and design of the study, collection of data, or analyses or interpretation of data; took part in the writing of the manuscript; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of work.
Disclosure
WC is an employee of Beiersdorf (Malaysia) Sdn. Bhd. Dr KNH received an honorarium from Beiersdorf as the study’s principal investigator; personal fees from Beiersdorf (M) Sdn Bhd, Loreal Malaysia Sdn Bhd, Janssen, Novartis and Hyphens Pharma; Zuellig Pharma, grants, personal fees from Lipidware Sdn Bhd, Chua Sin Sdn Bhd, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
1. Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10(1):1–29. doi:10.1038/s41598-020-62715-3
2. Tan JKL, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(Suppl 1):3–12. doi:10.1111/BJD.13462
3. Revol O, Milliez N, Gerard D. Psychological impact of acne on 21st-century adolescents: decoding for better care. Br J Dermatol. 2015;172(Suppl 1):52–58. doi:10.1111/BJD.13749
4. Baumann L. Understanding and treating various skin types: the Baumann skin type indicator. Dermatol Clin. 2008;26(3):359–373. doi:10.1016/J.DET.2008.03.007
5. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. doi:10.1016/J.JAAD.2015.12.037
6. Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne – update 2016, short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261–1268. doi:10.1111/JDV.13776
7. Layton AM, McDonald BS, Mohd Mustapa MF, Levell NJ. National institute for health and care excellence (NICE) acne guideline: what’s the latest for dermatologists? Br J Dermatol. 2022;186(3):426–428. doi:10.1111/BJD.20888
8. Asai Y, Baibergenova A, Dutil M, et al. Management of acne: Canadian clinical practice guideline. CMAJ. 2016;188:118–126. doi:10.1503/cmaj.140665
9. Mavranezouli I, Daly CH, Welton NJ, et al. A systematic review and network meta-analysis of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Br J Dermatol. 2022;187(5):639–649. doi:10.1111/BJD.21739
10. See JA, Goh CL, Hayashi N, Suh DH, Casintahan FA. Optimizing the use of topical retinoids in Asian acne patients. J Dermatol. 2018;45:522–528. doi:10.1111/1346-8138.14314
11. Bertolani MB, Rodighiero E, Gandolfi M, et al. Efficacy and tolerability of short contact therapy with tretinoin, clindamycin, and glycolic acid gel in acne: a randomized, controlled, assessor-blinded two-center trial: the MASCOTTE study. Dermatol Ther. 2021:
12. Leyden J, Lowe N, Kakita L, Draelos Z. Comparison of treatment of acne vulgaris with alternate-day applications of tazarotene 0.1% gel and once-daily applications of Adapalene 0.1% gel: a randomized trial. Cutis. 2001;67(6 Suppl):10–16.
13. Abad-Casintahan F, Chow SKW, Goh CL, et al. Frequency and characteristics of acne-related post-inflammatory hyperpigmentation. J Dermatol. 2016;43(7):826–828. doi:10.1111/1346-8138.13263
14. Gorelick J, Daniels SR, Kawata AK, et al. Acne-related quality of life among female adults of different races/ethnicities. J Dermatol Nurses Assoc. 2015;7(3):154–162. doi:10.1097/JDN.0000000000000129
15. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7(7):19–31.
16. Callender VD, Baldwin H, Cook-Bolden FE, Alexis AF, Stein Gold L, Guenin E. Effects of topical retinoids on acne and post-inflammatory hyperpigmentation in patients with skin of color: a clinical review and implications for practice. Am J Clin Dermatol. 2022;23(1):69–81. doi:10.1007/S40257-021-00643-2
17. Goh CL, Wu Y, Welsh B, et al. Expert consensus on holistic skin care routine: focus on acne, rosacea, atopic dermatitis, and sensitive skin syndrome. J Cosmet Dermatol. 2023;22(1):45–54. doi:10.1111/JOCD.15519
18. Kang S, Amagai M, Bruckner AL, et al. eds. Fitzpatrick’s Dermatology.
19. Bowe WP, Kircik LH. The importance of photoprotection and moisturization in treating acne vulgaris - PubMed. J Drugs Dermatol. 2014;13(8):s89–s94.
20. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134(2):310–313. doi:10.1038/JID.2013.505
21. Yang G, Lee HE, Yeon SH, et al. Licochalcone A attenuates acne symptoms mediated by suppression of NLRP3 inflammasome. Phytother Res. 2018;32(12):2551–2559. doi:10.1002/PTR.6195
22. Wongtada C, Pewlong P, Asawanonda P, et al. Influence of moisturizer containing licochalcone A, 1,2-decanediol, L-carnitine, and salicylic acid on facial skin lipidome among seborrhea participants. J Cosmet Dermatol. 2022;21(12):7081–7089. doi:10.1111/JOCD.15381
23. Wanitphakdeedecha R, Tavechodperathum N, Tantrapornpong P, et al. Acne treatment efficacy of intense pulsed light photodynamic therapy with topical licochalcone A, L-carnitine, and decanediol: a spilt-face, double-blind, randomized controlled trial. J Cosmet Dermatol. 2020;19(1):78–87. doi:10.1111/JOCD.13178
24. Kulthanan K, Trakanwittayarak S, Tuchinda P, Chularojanamontri L, Limphoka P, Varothai S. A double-blinded, randomized, vehicle-controlled study of the efficacy of moisturizer containing licochalcone A, decanediol, L-carnitine, and salicylic acid for prevention of acne relapse in Asian population. Biomed Res Int. 2020;2020:2857812. doi:10.1155/2020/2857812
25. Dall’oglio F, Fabbrocini G, Tedeschi A, Donnarumma M, Chiodini P, Micali G. Licochalcone A in combination with salicylic acid as fluid-based and hydroxy-complex 10% cream for the treatment of mild acne: a multicenter prospective trial. Clin Cosmet Invest Dermatol. 2019;12:961–967. doi:10.2147/CCID.S206935
26. Chularojanamontri L, Tuchinda P, Kulthanan K, Varothai S, Winayanuwattikun W. A double-blinded, randomized, vehicle-controlled study to access skin tolerability and efficacy of an anti-inflammatory moisturizer in treatment of acne with 0.1% Adapalene gel. J DermatolTreat. 2016;27(2):140–145. doi:10.3109/09546634.2015.1079298
27. Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36(6):416–418. doi:10.1046/J.1365-4362.1997.00099.X
28. Tan JKL, Tang J, Fung K, et al. Development and validation of a comprehensive acne severity scale. J Cutan Med Surg. 2007;11(6):211–216. doi:10.2310/7750.2007.00037
29. Savory SA, Agim NG, Mao R, et al. Reliability assessment and validation of the post-acne hyperpigmentation index (PAHPI), a new instrument to measure post-inflammatory hyperpigmentation from acne vulgaris. J Am Acad Dermatol. 2014;70(1):108–114. doi:10.1016/J.JAAD.2013.09.017
30. Baldwin H, Webster G, Stein Gold L, Callender V, Cook-Bolden FE, Guenin E. 50 years of topical retinoids for acne: evolution of treatment. Am J Clin Dermatol. 2021;22(3):315–327. doi:10.1007/S40257-021-00594-8
31. Angelova-Fischer I, Rippke F, Fischer TW, Neufang G, Zillikens D. A double-blind, randomized, vehicle-controlled efficacy assessment study of a skin care formulation for improvement of mild to moderately severe acne. J Eur Acad Dermatol Venereol. 2013;27(Suppl 2):6–11. doi:10.1111/JDV.12168
32. Puaratanaarunkon T, Asawanonda P. A randomized, double-blinded, split-face study of the efficacy of using a broad-spectrum sunscreen with anti-inflammatory agent to reduce post-inflammatory hyperpigmentation after picosecond laser. Clin Cosmet Invest Dermatol. 2022;15:331–337. doi:10.2147/CCID.S355329
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.