Back to Journals » Research Reports in Clinical Cardiology » Volume 11
Efficacy and Safety Outcome of Angiotensin Receptor-Neprilysin Inhibitors (ARNIs) in Patients with Heart Failure and Preserved Ejection Fraction (HFpEF): Preliminary Results
Authors Elshaer F, Lawand S, Zeyad M, Al Ayoubi F , Hanfi Y , AlQarni A
Received 24 April 2020
Accepted for publication 12 June 2020
Published 7 July 2020 Volume 2020:11 Pages 39—47
DOI https://doi.org/10.2147/RRCC.S258978
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Kones
Fayez Elshaer,1,2 Samih Lawand,3 Mohamed Zeyad,3 Fakhr Al Ayoubi,1 Yassmin Hanfi,3 Abdullah AlQarni1,4
1King Fahad Cardiac Center, Department of Cardiac Sciences, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 2National Heart Institute, Cairo, Egypt; 3Dallah Hospital, Riyadh, Saudi Arabia; 4Department of Medicine, University of Bisha, Bisha, Saudi Arabia
Correspondence: Fayez Elshaer
King Fahad Cardiac Center (KFCC), King Khaled University Hospital (KKUH), King Saud University, Riyadh 7805, Saudi Arabia
Tel +4671161
Fax +4671158
Email [email protected]
Purpose: This study analyzes the safety and efficacy of LCZ696 (valsartan/sacubitril), a combination of angiotensin II receptor blocker and neprilysin inhibitor (ARNI), in patients with heart failure and preserved ejection fraction (HFpEF).
Patients and Methods: An observational pilot study was conducted using a prospective design. A sample of 50 HFpEF patients (27 females and 23 males) was included on LCZ696 (50 mg orally, twice daily), which was then titrated up to a maximum tolerated dose, and followed up in the outpatient clinic. Thirty-seven patients received LCZ696 during hospitalization for decompensated heart failure or before their discharge while same titration was followed for the remaining patients.
Results: Patients were classified as New York Heart Association (NYHA) class III (64%), NYHA class IV (22%), and NYHA class II (14%). Diabetes mellitus was found in 74% of patients, while hypertension in 94%. Rapid clinical improvement was found with significant reduction in NYHA class down to NYHA class II (p=0.018). Patients had cleared off the fine basal crackles (specific for the interstitial pulmonary disease) secondary to heart failure (p< 0.001) and improvement or disappearance of edema of the lower limbs (p< 0.001). Heart rate response and jugular venous pressure and NT-pro-BNP were reduced significantly (p-value < 0.001, 0.005, respectively). Echocardiographic criteria for diastolic LV dysfunction (primarily E/A ratio) improved (p=0.001). Serum sodium (NA) levels improved significantly (p=0.015), without worsening renal function or limiting hyperkalemia.
Conclusion: LCZ696 (sacubitril/valsartan; ARNI) led to significant clinical improvements in patients with HFpEF. Further, a randomized study is needed to test whether it leads to positive outcomes for a larger sample.
Ethical Approval: Project No. E-17-2414, King Saud University, Kingdom of Saudi Arabia.
Keywords: impaired diastolic relaxation, left ventricular hypertrophy, LVH, subtle left ventricular systolic dysfunction, ventricular-vascular
Introduction
Heart failure (HF) is diagnosed in about 37 million people.1 HF prevalence is found to increase due to an increase in life expectancy, improved acute cardiovascular treatment, and an increase in its risk factors.2–4 HF represents a health care cost, which is likely to increase to $70 billion in 2030.5 Mazurek et al6 report that heart failure with preserved ejection fraction (HFpEF) represents half of the HF patients with a prevalence ratio of 35%; however, HFpEF might be misdiagnosed due to its comorbidities (hypertension and diabetes).5,7
HFpEF was formally known as diastolic heart failure (EF ≥50%), increasing with age and is common in elderly hypertensive females.2 Though, a gap exists in contemporary understanding of its underlying pathophysiological mechanisms. Some of the pathophysiological mechanisms include left ventricular hypertrophy (LVH) and fibrosis, subtle left ventricular systolic dysfunction, impaired diastolic relaxation, ventricular-vascular coupling abnormalities, increased cardiomyocyte stiffness, and systemic inflammation.8,9
The primary endpoints were not met by the four outcome trials involving renin-angiotensin aldosterone system (RAAS) inhibitors,10,11 no successful or regulatory approved therapies were adopted for morbidity and mortality reduction.2,12,13 This led to the continuance of empiric and symptom-based treatment of HFpEF. LCZ696 (sacubitril/valsartan) is the angiotensin receptor neprilysin inhibitor, which leads to simultaneous blockage of RAAS and endopeptidase neprilysin.14 Recognized as a ubiquitous enzyme, neprilysin breaks down various peptides such as adrenomedullin, biologically active natriuretic peptides (NPs), endothelin-1, and angiotensin.
While there is a treatment for HFrEF, there is currently no successful therapy for HFpEF. Even though, natriuretic peptides are found to be beneficial for HF patients, also aldosterone secretion suppression, vasodilation, natriuresis and diuresis, however, the results presented in previous studies were found to be lacking.15 Although, a combination of Sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker, reduced the risk of cardiovascular death and hospitalization in patients with chronic HFrEF (NYHA Class II–IV).2 However, no evidence of incremental benefits is found in patients with HFpEF.
A protective mechanism of secretion of atrionatriuretic peptide (ANP) and brain natriuretic peptide (BNP) in response to cardiac myocyte stretching resulting from increased myocardial wall tension secondary to volume and pressure overload.2 Augmentation of natriuretic peptides could assist in HFpEF management. Also, neprilysin inhibits angiotensin II degradation and can increase both the circulating and tissue angiotensin II; therefore, simultaneous inhibition of the generation of action of angiotensin II is required.16–18
In PARAMOUNT trial (Prospective comparison of ARNI with ARB on Management of heart failure with preserved ejection fraction), LCZ696 reduced NT-pro-BNP more than valsartan at 12 weeks and was well tolerated in HFpEF patients. Though, these changes would transform into value-added outcomes still need to be verified prospectively.19,20 Therapy with LCZ696 for 36 weeks was associated with the maintenance of estimated glomerular filtration rate (eGFR) as compared with valsartan therapy, in spite of increased urine albumin creatinine ratio (UACR) in HFpEF patients. LCZ696 effects on NT-pro-BNP, left atrial volume, functional class, and eGFR were not necessarily associated with a reduction in systemic blood pressure (SBP).21
The heart failure guidelines provided by European Society of Cardiology (ESC) indicate research gap and need for better understanding of potential treatments in specific HF populations.12 Patients with chronic heart failure are recommended renin‐angiotensin‐aldosterone system (RAAS) with ARNI (Level of Evidence: B‐R) in conjunction with beta-blockers and aldosterone antagonists to reduce morbidity and mortality.22 One of the recent studies showed no significant difference in rates of worsened hyperkalaemia, angio‐oedema, renal function, and symptomatic hypotension.23 Moreover, Desai24 failed to investigate reductions of central aortic stiffness after administrating sacubitril/valsartan versus enalapril in patients with chronic HFpEF. Thereby, this study investigates the safety and efficacy of LCZ696 in patients with HFpEF.
Patients and Methods
Study Design
The study was a pilot observational prospective, event‐driven trial comparing efficacy and safety of LCZ696 among HFpEF diagnosed patients. Given the nature of this study, the selected design is appropriate as it helps identify the impact of study treatment on patients over some time. This study was conducted on hospitalized patients from October 2017 to April 2018. The protocol specified that an aldosterone antagonist should be observed, considering renal function, serum potassium, and tolerability in all patients.
Ethical Approval
The study received ethical approval from King Saud University, Saudi Arabia under the Project No. E-17-2414. Moreover, the study was conducted in accordance with the Declaration of Helsinki.
Study Sample and Procedure
The population comprised of HFpEF diagnosed patients who were treated at Dallah Hospital from October 2017 to April 2018. A sample of 50 HFpEF patients were recruited who were then prospectively followed initially every 2 weeks, 4 weeks, 8 weeks, and 3 months up to 6 months. LCZ696 (ARNI) was administered to 37 patients upon admission and pre-discharge for decompensated heart failure, who met the inclusion criteria for LCZ696 (Valsartan/Sacubitril), whereas, 13 patients started at an outpatient clinic follow-up visit. The inclusion and exclusion criteria are presented in Table 1.
 |
Table 1 Inclusion and Exclusion Criteria |
Monitoring Parameters
All patients had baseline and periodic blood pressure along with heart rate measurements in the clinic setting together with baseline renal profile and potassium levels. Patients were followed up in the heart failure clinic initially every 2 weeks after starting LCZ696 and with each dose change, up to 2 months, and then regularly. Patients were followed for symptomatic improvement NYHA class, resolution of edema, renal parameters, and development of angioedema for 6 months. ACUSON SC 2000 was used to obtain echocardiographic measurements. Analysis of Short- and long-axis two-dimensional views and Echo Doppler. Echocardiography at baseline and 6 months included LV and RV function, estimated systolic pulmonary artery pressure (SPAP) levels, LA size and LV diastolic dysfunction grades.
Precautions
Combined treatment with an angiotensin-converting enzyme inhibitor drug or angiotensin II blockers (ACE-I/AgIIb) and LCZ696 (sacubitril/valsartan, ARNI) was considered contraindicated. All participants signed an informed consent form that included the study purpose and participant’s rights to withdraw at any time without any obligation towards the study team. No incentives or payments were given to the participants.
Statistical Analysis
All the analysis was performed using [SAS/STAT] software (SAS Institute Inc., Cary, NC, USA). Categorical data were summarized with absolute numbers and percentages. Continuous data were summarized as means and Standard Deviations (SD) or Median. Comparisons between variables were performed using Chi-square test or Fisher’s exact test for categorical variables and t-test or Mann–Whitney U-test for continuous variables.
Results
Fifty patients were prospectively started on LCZ696. Patients were observed in the outpatient clinic for 6 months (27 females and 23 males). The median age was 70 ± 18. Medication was started pre-discharge for 37 patients (74%), while the remaining patients (n=13; 26%) started in the outpatient clinic after discharge. Seven (14%) patients were ACEI/ARB-naive. Calcium channel blockers were used in 39 patients (78%), beta-blockers in 36 patients (72%), and aldosterone blockers in 13 patients (26%). (Calcium channel blockers were more used for hypertension control in elderly patients due to valuable effect on cardiovascular outcome)
Most patients were NYHA class III (n=32; 64%), and NYHA IV (n=11; 22%), while the remaining patients (n=7; 14%) were NYHA class II. Comorbidities observed in patients included diabetes mellitus (DM) (n=37; 74%), hypertension (n=47; 94%), ischemic heart disease (IHD) (n=17; 34%), atrial fibrillation (AF) (n=14; 28%), hyperlipidemia (n=31; 62%), hypothyroidism (n=10; 20%), obesity (n=15; 30%), anemia (n=5; 10%), chronic obstructive pulmonary disease (COPD) (n=7; 14%), and cerebrovascular accident (CVA) (n=5; 10%). The clinical characteristics of DM in our patients account for the higher prevalence of DM II in Saudi population.
The median ejection fraction (EF) was 60% (Table 2). Patients showed clinical improvement with significant improvements in NYHA class (p<0.018), the disappearance of fine basal crackles secondary to heart failure (p<0.001), improvement of edema of the lower limbs (p<0.001), heart rate reduction (81 ± 16 beats/min before medication initiation vs 76 ± 13 beats/min; p<0.001), and a reduction of jugular venous pressure (12 ± 3 cm/water vs 7 ± 3 cm/water; p<0.001). There was no statistically significant reduction in systolic pressure (127.2 ± 17.2 mmHg at the first visit vs 124.1 ± 16.7 mmHg at the last visit; p=0.368) and diastolic blood pressure (68.3 ± 11.8 mmHg at the first visit vs 66.3 ± 10.2 mmHg at the last visit; p=0.740; Table 2).
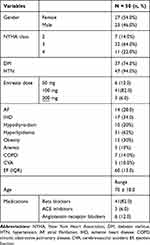 |
Table 2 Demographic Data |
NT-pro BNP was significantly reduced during the follow-up period (1500–800 after 6-month follow-up period; p=0.005). Sodium levels normalized during the follow-up period (124.2 ± 5.14 mmol at first visit vs 134.8 ± 4.7 mmol at the last visit; p=0.015), while there was no change in serum creatinine (86 mg at the first visit vs 87 at the last visit; p=0.197) and potassium (4.98 ± 0.5 mmol at the first visit vs 4.5 ± 0.3 mmol at the last visit; p=0.516). During the follow-up, re-hospitalization occurred in only 5 patients (10%) precipitated by the associated comorbidities and resulting in congestive heart failure, for which patients received temporary intensification of parenteral diuretics. The achieved LCZ696 dosage was 100 mg, 50 mg, and 200 mg, twice daily, in 41 (82%), 6 (12%), and 3 (6%) patients, respectively (Table 3). The same NT-proBNP cut off value was used for the diagnosis of heart failure as the majority were aged less than 75 years, also the patients with AF (14) had a clear clinical evidence of congestive heart failure.
 |
Table 3 Clinical Parameters |
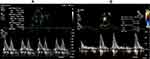
Echocardiography was performed twice in all patients, before drug administration and at the end of the study. After 6 months, treatment with LCZ696 improved the E/A ratio (p=0.001) and reduced systolic pulmonary artery pressure (sPAP) (54.5 ± 16.2 mmHg) at first visit vs 47 ± 13.8 mmHg at the last visit (p=0.001) (Table 4). Left atrium size, right ventricular size (RVD), inter-ventricular septum thickness (IVS), posterior wall thickness (PWT), and tricuspid regurgitation (TR) severity had no statistically significant differences (Figure 1–3).
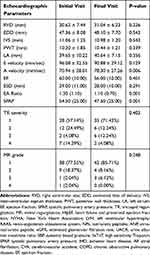 |
Table 4 Echocardiographic Parameters |
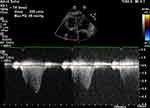 |
Figure 3 Echo Doppler of tricuspid valve inflow to assess right ventricular systolic pressure. |
Discussion
The traditional management schemes for HFpEF were approached by concentrating on the use of ACEI/ARB/diuretics without targeting the stress/stretch effects exerted by the different HFpEF comorbidities. The objective was to target patients with HFpEF whose treatment goals were not achieved previously, perhaps due to the heterogeneity of HFpEF patients.
Analysis of LCZ696 studies suggested that it provides improved protection from cardio-cerebrovascular events (eg, stroke and diastolic heart failure) due to reduction in pulse pressure (PP) (an independent predictor of cardiovascular events (myocardial infarction, heart failure, and cardiovascular death)).12 It was found that LCZ696 improved global circumferential but not longitudinal strain when compared to valsartan, an angiotensin II blocker, during 36 weeks.24 Also, LCZ696 offset the cardiac remodeling and dysfunction after myocardial infarction that might lead to superior inhibition of cardiac fibrosis and cardiac hypertrophy compared to either standalone neprilysin inhibitor or angiotensin receptor blocker.24,25
The rationale for the current study supported by the strong evidence from the PARADIGM-HF trial that demonstrated a bigger benefit of LCZ696 compared with enalapril in reducing cardiovascular (CV) mortality and morbidity in patients with HFrEF.26 Also, in the PARAMOUNT study, patients with HFpEF and NYHA class II–IV required ACEI or ARB for controlling symptoms of heart failure. LCZ696 was initiated to confirm tolerability at a minimum daily dose of 50 mg before titration up to 100 mg (PO bid) for 4 weeks, followed by up-titration to 200 mg (PO bid) for up to 12 weeks according to the clinical situation. LCZ696 reduced NT-pro-BNP to a greater extent than valsartan at 12 weeks and was well tolerated.12 Up-titration is not obligatory but rather, as per patients’ LCZ696 safety and tolerability, allowed for transitory dose breaks or dose drops. This dosing pattern is based on remarks of the TITRATION study (double-blind, randomized comparison of two up-titration regimens: Initiating sacubitril/valsartan in heart failure), which showed that a slow up-titration of LCZ696 from 50 mg to 200 mg (PO bid) over 6 weeks improved the likelihood of reaching target doses in patients with prior exposure to low doses of ACEI/ARB.27 Also, this regimen generated biomarker efficacy data such as changes in NT-pro-BNP at predefined time points (weeks 4 and 8, and month 6), assess the effect of 100 mg and 200 mg (bid) LCZ696 doses on HF-related biomarkers.22 Similarly, the present study used the same titration scheme aiming at a target dose of 200 mg (PO bid), which was maintained as long as tolerated. However, most of our patients continued on 100 mg po bid during the 6 months achieving the maximum clinical effect without adverse effect, it was generally well tolerated, as shown in the PARADIGM-HF17 and TITRATION studies for HFrEF patients.28
The primary endpoints in the current study included clinical effects, safety, and HF hospitalizations that are in line with the treatment goals for chronic HF, including improved morbidity, elimination of subjective symptoms, and improved quality of life.29 These outcomes best reflect the quality of life burden of chronic HF and have been used in trials such as PARADIGM-HF, SHIFT (Ivabradine and outcomes in chronic heart failure) EMPHASIS-HF (Eplerenone in patients with systolic heart failure and mild symptoms), and CHARM-Added (Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors).30
NT-pro-BNP is an effective biomarker to assess the effects of LCZ696 as it is not a substrate of neprilysin such as B-type natriuretic peptide (BNP).17 Stratification of patients by baseline NT-pro-BNP was applied to ensure proper patient risk assessment.31 Changes in NT-pro-BNP are of particular interest because natriuretic peptide (NP) levels have an essential predictive effect in patients with HF. Elevated levels of NPs are associated with adverse outcomes in these patients, and a reduction in their levels is associated with improvement in left ventricular wall stress.12
While mortality and HF hospitalizations have been usually used as endpoints in most HF trials, there is growing alertness that episodes of deteriorating HF should be acknowledged as an important morbidity event in the patient journey as they may suggest advancement of the underlying pathophysiology, decline of clinical status, and worse prognosis.32 In concordance with this observation, a recent posthoc analysis from the PARADIGM-HF study (Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure) showed that manifestations of worsening HF, such as outpatient intensification of HF therapy and emergency department visits, have serious predictive effects including an increased risk of all-cause mortality.33 The beneficial pleiotropic effects attributable to LCZ696 resulted in reducing stress/stretch effects along with its antifibrotic and anti-hypertrophic effects, which may have been of greater impact on patients’ outcomes than receiving either ACEI or ARB (RAAS inhibitors).34
However, contrary to the present study, Solomon et al35 showed that cardiovascular mortality accounts for 8.5% of the deaths. It showed that no significant benefits were found for sacubitril–valsartan concerning the heart failure patient’s low rehospitalization, with an ejection fraction of 45% or higher. However, Solomon et al36 study showed that sacubitril/valsartan provides a more effective results than renin-angiotensin-aldosterone–system inhibition for female HF patients hospitalization regardless of the ejection fraction, supporting the present study findings. The re-hospitalization rate in the present study cohort was 10% (5 patients) due to HF. The low hospitalization rate reflected improvements in patients’ quality of life, more frequent follow-up in the HF clinic and easy access to the medical care system.
The reason that our pilot study was positive contrary to PARAGON trial is simply because mortality was included in the primary combined outcome in PARAGON trial knowing that patients with 50% or more ejection fraction and HFpEF are more likely to die from comorbid conditions like infections, renal failure, stroke and less likely to die from cardiac causes.
Study Limitations
This prospective observational pilot study was limited due to its small cohort size, short follow-up duration, biomarker, and echocardiographic parameters. Moreover, it fails to address clinical improvement in the studied patients was due to the effect of LCZ696, due to the absence of control group. However, it is considered as the start of management redirection from traditional targets to new indications for using LCZ696 for management of HFpEF.
Conclusion
The present study has assessed the efficacy and safety of LCZ696 in these patients and showed that LCZ696 (ARNI) exerts a distinctive CV and renal effects in patients with HFpEF. These results have provided supportive evidence for an emerging outcome improving therapeutic approach than the currently used treatments in HFpEF patients. Following this pilot study, the researcher intends to continue gathering pre-specified patients together with large sample size and longer follow up. Moreover, future studies need to include control group to show clinical improvement in the patients was due to the effect of LCZ696 on a larger sample.
Acknowledgments
The authors are very thankful to all the associated personnel in any reference that contributed to this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat. 2016;13:368–378. doi:10.1038/nrcardio.2016.25
2. Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;2013(62):e147–e239. doi:10.1161/cir.0b013e31829e8776
3. Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
4. Maggioni AP. Epidemiology of heart failure in Europe. Heart Fail Clin. 2015;11(4):625–635. doi:10.1016/j.hfc.2015.07.015
5. Nielsen EE, Feinberg J, Raymond I, Olsen MH, Steensgaard-Hansen FV, Jakobsen JC. The effects of adding angiotensin receptor neprilysin inhibitors to usual care in patients with heart failure: a protocol for a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Syst Rev. 2019;8(1):251. doi:10.1186/s13643-019-1173-7
6. Mazurek JA, Jessup M. Understanding heart failure. Card Electrophysiol Clin. 2015;7(4):557–575. doi:10.1016/j.ccep.2015.08.001
7. Banerjee P. Heart failure with preserved ejection fraction: a clinical crisis. Int J Cardiol. 2016;204:198–199. doi:10.1016/j.ijcard.2015.11.170
8. Zile MR, Baicu CF. Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with a preserved ejection fraction. J Cardiovasc Transl. 2013;6(4):501–515. doi:10.1007/s12265-013-9472-1
9. Omar AM, Bansal M, Sengupta PP. Advances in echocardiographic imaging in heart failure with reduced and preserved ejection fraction. Circ Res. 2016;119(2):357–374. doi:10.1161/circresaha.116.309128
10. Pellicori P, Cleland JG. Heart failure with preserved ejection fraction. J Clin Med. 2014;14:s22–s28. doi:10.7861/clinmedicine.14-6-s22
11. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. doi:10.1056/nejmoa0805450
12. Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;2016(18):891–975. doi:10.1093/eurheartj/ehw128
13. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2017;376:897. doi:10.1056/nejmc1615918
14. Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail. 2014;2:663–670. doi:10.1016/j.jchf.2014.09.001
15. Sharkey AT, Ghafar MZ, O’Keeffe TS, Mulkerrin EC. Angiotensin receptor neprilysin inhibitors in older patients with heart failure. BMJ Evid Based Med. 2019;24(1):5–7. doi:10.1136/bmjebm-2018-110949
16. McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi:10.1056/nejmoa1409077
17. Langenickel TH, Dole WP. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today. 2012;9:e131–e139. doi:10.1016/j.ddstr.2013.11.002
18. Entresto. Summary of product characteristics. EMA Web Site; 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004062/WC500197536.pdf.
19. Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a Phase 2 double-blind randomised controlled trial. The Lancet. 2012;380(9851):1387–1395. doi:10.1016/s0140-6736(12)61227-6
20. Solomon SD, Janardhanan R, Verma A, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. The Lancet. 2007;369(9579):2079–2087. doi:10.1016/s0140-6736(07)60980-5
21. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail. 2001;7:176–182. doi:10.1054/jcaf.2001.25652
22. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;2017(70):776–803. doi:10.1016/j.jacc.2017.04.025
23. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi:10.1056/NEJMoa1812851
24. Desai AS, Solomon SD, Shah AM, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322(11):1077–1084. doi:10.1001/jama.2019.12843
25. Luepker RV, Steffen LM, Jacobs DR
26. McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail. 2013;15:1062–1073. doi:10.1093/eurjhf/hft052
27. Senni M, McMurray JJ, Wachter R, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double‐blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016;18:1193–1202. doi:10.1002/ejhf.548
28. Akahori M, Ayalasomayajula S, Langenickel T, Pal P, Zhou W, Sunkara G. Pharmacokinetics after single ascending dose, food effect, and safety of sacubitril/valsartan (LCZ696), an angiotensin receptor and neprilysin inhibitor, in healthy Japanese subjects. Eur J Drug Metab Pharmacokinet. 2017;42(3):407–416. doi:10.1007/s13318-016-0354-1
29. Japan Pharmaceutical and Medical Device Agency. Guidelines on Clinical Evaluation of Drugs to Treat Heart Failure. Japan: PMDA; 2011. Available from: https://www.pmda.go.jp/files/000208189.pdf.
30. McMurray JJ, Östergren J, Swedberg K, et al., CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. The Lancet. 2003;362(9386):767–771. doi:10.1016/s0140-6736(03)14283-3
31. Swedberg K, Komajda M, Böhm M, et al., SHIFT investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. The Lancet. 2010;376(376):875–885. doi:10.1016/s0140-6736(10)61198-1
32. Butler J, Braunwald E, Gheorghiade M. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA. 2014;312:789–790. doi:10.1001/jama.2014.6643
33. Okumura N, Jhund PS, Gong J, et al. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Circulation. 2016;133(23):2254–2262. doi:10.1161/circulationaha.115.020729
34. Tsutsui H, Momomura S, Saito Y, et al. Efficacy and safety of sacubitril/valsartan (LCZ696) in Japanese patients with chronic heart failure and reduced ejection fraction: rationale for and design of the randomized, double-blind PARALLEL-HF study. J Cardiol. 2017;70(3):225–231. doi:10.1016/j.jjcc.2016.11.011
35. Solomon SD, McMurray JJ, Anand IS, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. doi:10.1056/NEJMoa1908655
36. Solomon SD, Vaduganathan ML, Claggett B, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141(5):352–361. doi:10.1161/CIRCULATIONAHA.119.044586
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.