Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy and Safety of Regorafenib Plus Immune Checkpoint Inhibitors with or Without TACE as a Second-Line Treatment for Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis
Authors Yang X, Deng H, Sun Y, Zhang Y, Lu Y, Xu G, Huang X
Received 25 November 2022
Accepted for publication 14 February 2023
Published 27 February 2023 Volume 2023:10 Pages 303—313
DOI https://doi.org/10.2147/JHC.S399135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Jörg Trojan
Xuegang Yang,1,2 Heping Deng,3 Yanyuan Sun,1 Yi Zhang,3 Yujie Lu,3 Guohui Xu,1,* Xiaoqi Huang2,*
1Department of Interventional Therapy, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Chengdu, People’s Republic of China; 2Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 3Department of Radiology, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guohui Xu, Department of Interventional Therapy, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, 55 Renmin South Road 4th Section, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86-13708010123, Fax +86-02885420195, Email [email protected] Xiaoqi Huang, Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital, Sichuan University, No. 37 Guo Xue Xiang, Chengdu, 610041, People’s Republic of China, Tel +86-18980605806, Fax +86-02885420195, Email [email protected]
Purpose: We compare the efficacy and safety of regorafenib plus immune checkpoint inhibitors (ICIs) with transarterial chemoembolization (R+ICIs+TACE) versus regorafenib plus ICIs (R+ICIs) as the second-line treatment for patients with advanced hepatocellular carcinoma (HCC).
Methods: This retrospective study included patients with advanced HCC who received R+ICIs+TACE or R+ICIs as the second-line treatment from January 2019 to April 2022. Objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and treatment-related adverse events (TRAEs) were compared between the two groups. The propensity score matching (PSM) was used to reduce the influence of confounding factors on the outcomes. Factors affecting PFS and OS were analyzed using a Cox proportional-hazards regression model.
Results: In total, 52 patients were included in this study, of whom 28 patients received R+ICIs+TACE and 24 patients received R+ICIs. After PSM (n=23 in each group), patients who received R+ICIs+TACE had a higher ORR (34.8% vs 4.3%, P=0.009), a longer PFS (5.8 vs 2.6 months, P< 0.0001), and a longer OS (15.0 vs 7.5 months, P=0.014) than those who received R+ICIs. Age ≤ 50 years old, Child-Pugh class A6 and B7, and R+ICIs were found as independent prognostic factors for poor PFS. R+ICIs, α-fetoprotein > 400 ng/mL, and platelet-to-lymphocyte ratio > 133 were noted as independent prognostic factors for poor OS. The difference in the incidence of TRAEs between the two groups was not statistically significant (P> 0.05).
Conclusion: Compared to regorafenib plus ICIs, regorafenib plus ICIs with TACE was tolerated and improved survival as the second-line treatment for patients with advanced HCC.
Keywords: advanced hepatocellular carcinoma, regorafenib, immune checkpoint inhibitors, transarterial chemoembolization, second-line treatment
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer and the third leading cause of cancer-related deaths worldwide.1 Surgical resection and liver transplantation are potential radical treatments for HCC. However, the majority of patients with HCC are diagnosed in advanced stage.2
Sorafenib is a first-line therapy that was approved for advanced HCC through SHAEP trial and the Asia Pacific trial in 2007.3,4 Lenvatinib was approved as a first-line therapy for advanced HCC through REFLECT trial.5 Sorafenib and lenvatinib only prolonged progression-free survival (PFS) of patients with advanced HCC for 3.7–7.3 months.3,5 Thus, regorafenib,6 cabozantinib,7 and ramucirumab8 were approved as second-line therapies for advanced HCC.9 Nonetheless, these second-line treatments only resulted in a median PFS of 2.8–5.2 months and a median overall survival (OS) of 8.5–10.6 months versus a median OS of 7.3–8.0 months for patients treated with placebo, and regorafenib was reported as the most appropriate option to prolong OS.10 However, the outcome is unsatisfactory and a more effective second-line treatment is needed.
Immune checkpoint inhibitors (ICIs) are a second-line treatment option for advanced HCC. Pembrolizumab and nivolumab have been approved by the US Food and Drug Administration (FDA) as second-line therapies for HCC, in which a previous sorafenib treatment failed based on the results of a Phase 1/2 trial.11,12 Camrelizumab was reported as a second-line therapy for advanced HCC, in which a previous systemic treatment approved by the China FDA (CFDA) had failed based on the results of a Phase 2 trial.13
Transarterial chemoembolization (TACE) was recommended to treat intermediate-stage HCC.14,15 TACE has local anticancer effects and promotes antitumor immunity,16 while it inevitably induces post-TACE angiogenesis.17 Regorafenib inhibits angiogenesis, which can promote “tumor vascular normalization” to alleviate hypoxia and therefore enhance the efficacy of TACE and immunotherapy.18 ICIs combined with other antitumor therapies can stimulate the immune system and increase intratumoral CD8+ T-cell infiltration, thereby improving antitumor immunomodulatory effect.19,20
In the present study, it was hypothesized that the comprehensive therapy of regorafenib combined with ICIs plus TACE might improve the survival of patients with advanced HCC. Therefore, the efficacy and safety of the regorafenib+ICIs+TACE (R+ICIs+TACE) regimen were compared with those of the regorafenib+ICIs (R+ICIs) regimen as a second-line treatment for advanced HCC.
Materials and Methods
Patients’ Selection
This retrospective study was performed in compliance with the Declaration of Helsinki, it was approved by the Ethics Committee of the Sichuan Cancer Hospital (Chengdu, China), and written informed consent was obtained from all patients prior to treatment. Patients’ data were retrieved from a prospectively maintained HCC database of the Sichuan Cancer Hospital. Between January 2019 and April 2022, consecutive patients with HCC received R+ICIs or R+ICIs+TACE.
HCC was diagnosed according to guidelines of the European Association for the Study of Liver or histological confirmation. Tumors were staged according to the Barcelona Clinic Liver Cancer (BCLC) system using magnetic resonance imaging (MRI) or computed tomography (CT) scan.
The inclusion criteria were as follows: 1) age ≥18 years old; 2) Eastern Cooperative Oncology Group performance status (ECOG PS) score ≤1 point; 3) Child-Pugh class ≤7. 4) BCLC stage C HCC; 5) radiographic disease progression of BCLC B stage HCC after local therapy (surgery, TACE, surgery + TACE or surgery + radiofrequency ablation + TACE) and first-line treatment (sorafenib or lenvatinib). The exclusion criteria were as follows: 1) R+ICIs or R+ICIs+TACE as the first-line therapy; 2) regorafenib combined with ICIs plus another therapy (chemotherapy, radiotherapy, or radiofrequency ablation); 3) history of receiving immunotherapy before regorafenib administration; 4) incomplete data.
Data Collection
Patients’ baseline characteristics were collected within 7 days before administration of regorafenib. Clinical, laboratory, and radiological data were collected from medical record systems, including age, gender, BCLC stage, ECOG PS score, hepatitis B surface antigen (HBsAg), Child-Pugh class, alpha-fetoprotein (AFP) level, tumor size, tumor number, extrahepatic metastasis, previous treatment procedures, and hematological and biochemical indices. Neutrophil-to-lymphocyte ratio (NLR) was calculated as neutrophil count divided by the lymphocyte count, and platelet-to-lymphocyte ratio (PLR) was calculated as the platelet count divided by the lymphocyte count.21 NLR and PLR were divided into low and high values according to the mean NLR (mean, 4) and PLR (mean, 133) in the present study.
Treatment
TACE Procedure
Hepatic arteriography and superior mesenteric arteriography were performed to assess the feeding arteries of the tumor. Subsequently, a microcatheter was inserted into the tumor-feeding arteries.
Conventional TACE (cTACE) was an intra-arterial injection of 40–60 mg of epirubicin (Pharmorubicin; Pfizer, Wuxi, China) mixed with 5–20 mL of lipiodol (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China). When needed, embosphere (100–300 μm) was used for further embolization to achieve stasis.
Drug-eluting bead TACE (DEB-TACE) was performed by CallSpheres® (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) beads (100–300 μm) loaded with doxorubicin (40–60 mg). CalliSpheres® beads and non-ionic contrast agent were mixed by 1:1 and injected at a speed of 1 mL/min. The injection was completed during the stasis flow of contrast agent.
TACE was repeated “on demand” after our multidisciplinary team (MDT) discussion depending on the results of examinations, including MRI/CT, AFP level, hematological and biochemical indices.
Administration of Regorafenib and ICIs
The dose of regorafenib was 120 mg/day in the first week, which could be adjusted to 80 or 160 mg/day according to the patient’s tolerance, orally for 3 weeks on 1 week off. Intravenous administration of 200 mg sintilimab (Innovent Biologics Co., Ltd., Suzhou, China) or camrelizumab (Hengrui Medical, Suzhou, China) was conducted every 21 days.
Regorafenib or ICIs were discontinued if patients experienced relevant intolerable toxicity. The treatment was terminated if disease progressed, which was confirmed by contrast-enhanced CT or MRI.
Follow-Up
All patients were regularly followed up at intervals of 3–6 weeks after initiation of regorafenib administration. The follow-up included physical examination, laboratory tests, contrast-enhanced CT or MRI, and other examinations. The selection of follow-up therapy was performed based on discussions between MDT and the patient’s requirements. The last follow-up time was July 31, 2022.
Evaluation of Treatment Response
Two radiologists with more than 10 years of experience were employed to evaluate the treatment response. The treatment response was evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).22 The objective response rate (ORR) was defined as the proportion of patients achieving complete response (CR) and partial response (PR). Disease control rate (DCR) was defined as the proportion of patients with CR, PR, or stable disease (SD). Adverse events (AEs) were recorded and assessed based on the Common Terminology Criteria for Adverse Events (CTCAE, Version 5.0).
PFS was defined as the time interval between the initiation of regorafenib and the time of disease progression due to any cause. OS was defined as the time interval between the initiation of regorafenib and the time of death or the last date of follow-up.
Statistical Analysis
The propensity score matching (PSM) model included age, gender, BCLC stage, ECOG PS, and tumor size. As previously described,23 the model provided a 1:1 match between the two groups, with a caliper width of 0.3 of the standard deviation of the logit of the propensity score. Quantitative data were presented as median value (interquartile range [IQR]), and categorical data were expressed as number (percentage). Chi-square test or the Fisher’s exact test was used to compare categorical data (patients’ baseline characteristics, treatment responses, and AEs) between the two groups. Independent-samples t-test or Mann–Whitney U-test was used to compare quantitative data (baseline characteristics) between the two groups. Survival curves with 95% confidence intervals (CIs) were analyzed by the Kaplan–Meier method using the Log rank test. Univariate and multivariate analyses used a Cox proportional-hazards regression model to determine the prognostic factors. All statistically significant (P<0.05) factors identified by the univariate analysis were entered into a Cox proportional-hazards regression model to identify independent predictors. All tests were two-sided, and P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS 25.0 software (IBM, Armonk, NY, USA).
Results
Patients’ Characteristics
Totally, 52 patients with advanced HCC were involved in the present study, including 24 patients who received R+ICIs and 28 patients who received R+ICIs+TACE (Figure 1). In addition, 22 patients received cTACE and 6 patients received DEB-TACE.
Patients’ baseline characteristics are summarized in Table 1. Before PSM, patients in the R+ICIs group had worse BCLC stage (P=0.029) and ECOG PS (P=0.029) compared with those in the R+ICIs+TACE group. Performing PSM resulted in matched cohorts of 23 patients in each group with well-balanced baseline characteristics (Table 1).
 |
Table 1 Baseline Characteristics of Patients Before and After Propensity Score Matching |
Treatment Outcomes
Tumor Response Evaluation
No patient achieved CR in the present study. The ORR was higher in the R+ICIs+TACE group than that in the R+ICIs group before PSM (35.7% vs 4.2%, P=0.005) and after PSM (34.8% vs 4.3%, P=0.009) (Table 2). There was no significant difference in DCR between the two groups before PSM (67.9% vs 41.7%, P=0.058) and after PSM (69.6% vs 43.5%, P=0.074) (Table 2).
 |
Table 2 Summary of Response Rates Before and After Propensity Score Matching |
Survival Analysis
The median follow-up time was 8.0 months (95% CI, 8.0–11.4). In addition, 53.6% (15/28) of patients in the R+ICIs+TACE group and 62.5% (15/24) of patients in the R+ICIs group died.
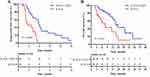
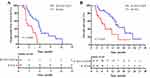
Before PSM, the median PFS was 5.8 months (95% CI, 5.2–6.4) in the R+ICI+TACE group and 2.6 months (95% CI, 0.5–4.7) in the R+ICIs group (P<0.0001, Figure 2A); the median OS was longer in the R+ICIs+TACE group than that in the R+ICIs group (13.6 months (95% CI, 10.7–16.5) vs 7.5 months (95% CI, 4.3–10.7)) (P=0.011, Figure 2B).
After PSM, the median PFS was 5.8 months (95% CI, 5.3–6.3) in the R+ICIs+TACE group and 2.6 months (95% CI, 0.6–4.6) in the R+ICIs group (P<0.0001, Figure 3A); the median OS was longer in the R+ICIs+TACE group than that in the R+ICIs group (15.0 months (95% CI, 12.0–18.0) vs 7.5 months (95% CI, 4.3–10.7)) (P=0.014, Figure 3B).
Analysis of Prognostic Factors
The prognostic factors were analyzed in the matched cohort (Table 3). The Cox proportional-hazards regression model showed that age (≤50 vs >50 years old) (hazard ratio [HR] = 0.260, 95% CI, 0.112–0.605, P=0.002), Child-Pugh class (A5 vs A6+B7) (HR=3.125, 95% CI, 1.293–7.555, P=0.011), and treatment option (R+ICIs vs R+ICIs+TACE) (HR=0.244, 95% CI, 0.096–0.622, P=0.003) were independent prognostic factors for PFS (Table 3).
 |
Table 3 Univariate and Multivariate Predictors of Progression-Free Survival and Overall Survival After PSM |
Multivariate analysis showed that AFP (≤400 vs >400 ng/mL) (HR=2.625, 95% CI, 1.194–5.770, P=0.016), PLR (low vs high) (HR=2.384, 95% CI, 1.006–5.648, P=0.048), alanine transaminase (ALT) (≤35 vs >35 U/L) (HR=0.405, 95% CI, 0.176–0.932, P<0.034), and treatment option (R+ICIs vs R+ICIs+TACE) (HR=0.410, 95% CI, 0.170–0.988, P=0.047) were independent prognostic factors for OS (Table 3).
Safety
Treatment-related AEs (TRAEs) after PSM are presented in Table 4. No more than grade 4 TRAEs occurred, and no treatment-related mortality was detected. TRAEs were similar between the R+ICIs group and the R+ICIs+TACE group (any grade, 87.0% vs 95.7%, P=0.295; grade 3/4, 30.4% vs 34.8%, P=0.753) (Table 4). There was a high incidence of fever in the R+ICIs+TACE group compared with that in the R+ICIs group (21.7% vs 4.3%, P=0.080).
 |
Table 4 Treatment-Related Adverse Events (TRAE) |
Due to grade 3/4 thrombocytopenia, 4.3% (1/23) of patients in the R+ICIs group and 13.0% (3/23) of patients discontinued treatment in the R+ICIs+TACE group. Because of hand-foot syndrome, 13.0% (3/23) of patients in the R+ICIs+TACE group and 8.7% (2/23) of patients in the R+ICIs group discontinued regorafenib therapy. Regorafenib was discontinued because of gastrointestinal hemorrhage in 4.3% (1/23) of patients in the R+ICIs group.
Discussion
The present study showed that R+ICIs+TACE improved survival benefits compared with R+ICIs as the second-line treatment for patients with advanced HCC who had previously failed in the first-line treatment. The median OS was prolonged from 7.5 to 15.0 months. The multivariate analysis suggested that R+ICIs+TACE was an independent predictor for higher PFS and OS. This survival benefit might be related to the higher ORR and longer PFS in patients who received R+ICIs+TACE compared with those who underwent R+ICIs. Thus, R+ICIs+TACE, as a second-line treatment, may be a promising option for patients with advanced HCC who experienced failure in treatment with sorafenib or lenvatinib.
In a phase 1b trial, regorafenib combined with pembrolizumab as the first-line treatment for advanced HCC showed an ORR of 28%,24 which was higher than that in the R+ICIs group (4.2%) achieved in the present study. The reasons could be summarized as follows: 1) tumors in all patients progressed after surgery or locoregional treatment in the present study; 2) regorafenib was combined with ICIs as the second-line treatment. However, ORR (34.8%) in the R+ICIs+TACE group was higher than that in patients who received regorafenib combined with pembrolizumab. Huang et al25 reported regorafenib combined with programmed cell death protein 1 (PD-1) blockade as the second-line treatment for advanced HCC, and a median OS of 13.4 months was achieved. In the present study, OS was 15.0 months in the R+ICIs+TACE group, which was longer than that in the R+ICIs group.
TACE embolizes the tumor-feeding arteries, which can induce “immunogenic cell death” by releasing tumor antigens from dying cancer cells and eliciting damage-associated molecular patterns, such as calreticulin and adenosine triphosphate (ATP) release and type I interferon response, in order to facilitate antitumor immunity,26,27 which may improve the efficacy of immunotherapy. Regorafenib can inhibit JAK1/2-STAT1 and MAPK signaling pathways, increasing intratumoral CD8+ T cell infiltration through vasculature normalization by targeting VEGFR2/3.20 Collectively, regorafenib combined with ICIs plus TACE as a second-line treatment could further improve the therapeutic efficacy for advanced HCC.
In the present study, age ≤50 years old, Child-Pugh class A6 and B7, and R+ICIs were found to be independent risk factors for PFS. Patients with a satisfactory liver function before the second-line treatment achieved a higher PFS from the sequential treatment, which was consistent with previously reported findings.6,28 AFP > 400 ng/mL, high PLR, ALT > 35 U/L, and R+ICIs were noted as independent risk factors for OS. AFP and PLR may be used as clinical indicators for screening patients who may benefit from second-line treatment. PLR is an inflammatory marker that may be used in several diseases for predicting inflammation and mortality, and a high PLR has been previously reported to be correlated with the poor survival of patients with HCC.29,30 A high platelet count stimulates angiogenesis and tumor proliferation,31 and a low lymphocyte count is commonly referred to “cold tumors” without significant T cell infiltration.32
No unexpected TRAEs were found in the present study. All TRAEs detected in the present study were consistent with those reported previously.6,25,33 Regorafenib combined with ICIs plus TACE did not increase the overall incidence of any grade or 3/4 grade TRAEs compared with regorafenib combined with ICIs.
There were several limitations in the present study. First, this was a single-center, small-scale retrospective study, which might be subjected to selection bias, and the PSM model was used to eliminate the effects of confounding factors. Moreover, no subgroup analysis was performed due to the small sample size. A multicenter, randomized clinical trial is therefore required to validate the findings of this study.
In conclusion, regorafenib combined with ICIs plus TACE, as a second-line treatment, was tolerated and yielded promising survival for patients with advanced HCC. These patients achieved a greater treatment response, a longer PFS, and a longer OS in comparison with those who were treated with regorafenib combined with ICIs.
Funding
This study was financially supported by the Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC0837), the Science and Technology Program of Chengdu, China (Grant No. 2022-YF05-01811-SN), Wu Jieping Medical Foundation (Grant No. 320.6750.2020-10-122), Beijing Medical Award Foundation (Grant No. YXJL-2020-0972-0424), Beijing Xisike Clinical Oncology Research Foundation (Grant No. Y-HR2020MS-0484), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC21041), the National Key R&D Program of China (grant number 2022YFC2404105).
Disclosure
The authors declare that there is no conflict of interest for this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Fitzmaurice C, Allen C, Barber RM, et al.; Global Burden of Disease Cancer C. Global, Regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. doi:10.1001/jamaoncol.2016.5688
3. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
4. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
5. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
6. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
7. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
8. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
9. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J Hepatol. 2021. doi:10.1016/j.jhep.2021.11.018
10. Wang D, Yang X, Lin J, et al. Comparing the efficacy and safety of second-line therapies for advanced hepatocellular carcinoma: a network meta-analysis of phase III trials. Therap Adv Gastroenterol. 2020;13:1756284820932483. doi:10.1177/1756284820932483
11. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Clinical Trial, Phase II Multicenter Study Research Support, Non-U.S. Gov’t. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
12. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Clinical Trial, Phase I Clinical Trial, Phase II Multicenter Study. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
13. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/S1470-2045(20)30011-5
14. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
15. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
16. Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74(4):2264–2276. doi:10.1002/hep.31840
17. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529. doi:10.1080/02841850801958890
18. Personeni N, Pressiani T, Santoro A, Rimassa L. Regorafenib in hepatocellular carcinoma: latest evidence and clinical implications. Drugs Context. 2018;7:212533. doi:10.7573/dic.212533
19. Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153(4):1107–1119 e10. doi:10.1053/j.gastro.2017.06.017
20. Shigeta K, Matsui A, Kikuchi H, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e001435. doi:10.1136/jitc-2020-001435
21. Suner A, Carr BI. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict tumor size and survival in HCC patients: retrospective study. Ann Med Surg. 2020;58:167–171. doi:10.1016/j.amsu.2020.08.042
22. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
23. Li S, Lyu N, Han X, et al. Hepatic artery infusion chemotherapy using fluorouracil, leucovorin, and oxaliplatin versus transarterial chemoembolization as initial treatment for locally advanced hepatocellular carcinoma: a propensity score-matching analysis. J Vasc Interv Radiol. 2021;32(9):1267–1276 e1. doi:10.1016/j.jvir.2021.06.008
24. El-Khoueiry AB, Kim RD, Harris WP, et al. Updated results of a phase 1b study of regorafenib (REG) 80 mg/day or 120 mg/day plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(15_suppl):4078. doi:10.1200/JCO.2021.39.15_suppl.4078
25. Huang J, Guo Y, Huang W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2022;9:157–170. doi:10.2147/JHC.S353956
26. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi:10.1038/nri.2016.107
27. Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235–260. doi:10.1159/000488035
28. Kim HD, Bang Y, Lee MA, et al. Regorafenib in patients with advanced Child-Pugh B hepatocellular carcinoma: a multicentre retrospective study. Liver Int. 2020;40(10):2544–2552. doi:10.1111/liv.14573
29. Schobert IT, Savic LJ, Chapiro J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol. 2020;30(10):5663–5673. doi:10.1007/s00330-020-06931-5
30. Zhang L, Yan ZP, Hou ZH, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of outcomes in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization plus sorafenib. Front Mol Biosci. 2021;8:624366. doi:10.3389/fmolb.2021.624366
31. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249. doi:10.1111/j.1538-7836.2010.04131.x
32. van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM. Migrating into the Tumor: a Roadmap for T Cells. Trends Cancer. 2017;3(11):797–808. doi:10.1016/j.trecan.2017.09.006
33. Han Y, Cao G, Sun B, et al. Regorafenib combined with transarterial chemoembolization for unresectable hepatocellular carcinoma: a real-world study. BMC Gastroenterol. 2021;21(1):393. doi:10.1186/s12876-021-01967-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.