Back to Journals » Journal of Asthma and Allergy » Volume 15
Efficacy and Safety of Masitinib in Corticosteroid-Dependent Severe Asthma: A Randomized Placebo-Controlled Trial
Authors Davidescu L , Ursol G, Korzh O , Deshmukh V, Kuryk L, Nortje MM, Godlevska O, Devouassoux G, Khodosh E, Israel E , Moussy A, Mansfield CD , Hermine O, Chanez P
Received 18 September 2021
Accepted for publication 19 May 2022
Published 7 June 2022 Volume 2022:15 Pages 737—747
DOI https://doi.org/10.2147/JAA.S337284
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Lavinia Davidescu,1 Grigoriy Ursol,2 Oleksii Korzh,3 Vikranth Deshmukh,4 Lesia Kuryk,5 Monja-Marie Nortje,6 Olga Godlevska,3 Gilles Devouassoux,7 Eduard Khodosh,8 Elliot Israel,9,10 Alain Moussy,11 Colin D Mansfield,11 Olivier Hermine,11– 13 Pascal Chanez14
1Department of Pulmonology, University of Oradea, Oradea, Romania; 2Medical and Diagnostic Center of Private Enterprise of Private Production Company “Acinus”, Kropyvnytskyi, Ukraine; 3Department of General Practice - Family Medicine, Kharkiv Medical Academy of Postgraduate Education, Kharkiv, Ukraine; 4Department of Pulmonary Medicine, Respira Hospital, Nagpur, Maharashtra, India; 5National Institute of Phthisiology and Pulmonology Named After F.G. Yanovsky of National Academy of Medical Sciences of Ukraine, Kyiv, Ukraine; 6Moriana Clinical Research, Brandfort, South Africa; 7Department of Pulmonology, Hôpital de la Croix Rousse, GHN, HCL and Université Claude Bernard Lyon 1, Lyon, France; 8Department of Pulmonology, Municipal Nonprofit Enterprise, City Clinical Hospital #13, Kharkiv, Ukraine; 9Harvard Medical School, Boston, MA, USA; 10Division of Pulmonary and Critical Care Medicine and Allergy and Immunology, Brigham and Women’s Hospital, Boston, MA, USA; 11AB Science, Paris, France; 12Imagine Institute, INSERM UMR 1163 and CNRS ERL 8254, Laboratory of Cellular and Molecular Mechanisms of Hematological Disorders and Therapeutic Implication, Hôpital Necker, Paris, France; 13Université Paris Descartes, Sorbonne Paris Cité, Paris, France; 14Clinique des Bronches, Allergie et Sommeil, APHM Hôpital Nord, C2VN Center INSERM INRAE UMR1062, Aix-Marseille Université, Marseille, France
Correspondence: Pascal Chanez, Clinique des Bronches, Allergie et Sommeil, APHM Hôpital Nord, C2VN Center INSERM INRAE UMR1062, Aix-Marseille Université, Marseille, France, Tel +33 6 50 71 07 05, Email [email protected]
Background: Masitinib is an oral tyrosine kinase inhibitor that selectively targets mast cell activity and platelet-derived growth factor receptor (PDGFR) signaling, both of which are implicated in various mechanisms of asthma pathogenesis.
Objective: Assessment of masitinib as an add-on to standard maintenance therapy as compared with placebo in the treatment of oral corticosteroid-dependent severe asthma.
Methods: We conducted a randomized (2:1), placebo-controlled study of masitinib (6 mg/kg/d) in adults with severe asthma uncontrolled by high dose inhaled corticosteroids and long-acting beta-adrenoreceptor agonists plus oral corticosteroids (OCS) (≥ 7.5 mg/d). No minimum baseline blood eosinophil count was specified. Following a protocol amendment, the primary endpoint was reduction of annualized severe asthma exacerbation rate adjusted for the overall time on treatment (SAER). Subgroup analysis according to yearly cumulative OCS intake was also performed, a higher OCS dose indicating more severe asthma that is harder to control.
Results: Following an average exposure of approximately 13 months, masitinib (n = 240) reduced the SAER by 35% relative to placebo (n = 115) (rate ratio (RR) 0.65 (95% CI [0.47– 0.90]; P = 0.010)). For patients with eosinophil ≥ 150 cell/μL, masitinib (n = 181) reduced SAER by 38% relative to placebo (n = 87); RR 0.62 (95% CI [0.42– 0.91]; P = 0.016). Benefit of masitinib was shown to increase in the most severely affected patients (OCS intake of > 1000 mg/year), with a significant (P < 0.01) reduction in SAER of 50%– 70%. Safety was consistent with the known masitinib profile.
Conclusion: Orally administered masitinib reduces the risk of asthma exacerbations in severe asthma patients, with an acceptable safety profile. Masitinib may potentially provide a new treatment option for oral corticosteroid-dependent severe asthma.
Keywords: asthma clinical trials, asthma medication, mast cells, tyrosine kinases, severe asthma
Plain Language Summary
Masitinib is an orally administered, small molecule drug that selectively targets mast cell activity and protein kinase signaling, which are implicated in various mechanisms of asthma pathogenesis. We conducted a randomized controlled trial of masitinib in 419 adults with severe asthma that was uncontrolled by standard treatments, including oral corticosteroids. Results showed that patients treated with masitinib had a lower risk of severe asthma exacerbations compared with those in a placebo-control group that did not receive masitinib. Benefit of masitinib was shown to increase in patients that required a higher oral corticosteroid dose, corresponding to the most severely affected patients. Safety results from this study were consistent with the known profile for masitinib, with no new safety concerns. Masitinib may provide a new effective and well-tolerated treatment option for oral corticosteroid-dependent severe asthma, including severe asthmatics that are ineligible to receive or in failure to registered biologics. This represents an entirely different treatment approach to that which is associated with currently registered Type-2 targeted biologics.
Introduction
Corticosteroid-dependent severe asthma, also referred to as severe uncontrolled asthma, is a difficult-to-treat asthma that remains uncontrolled despite adherence to maximal optimized therapy (eg, high dose inhaled corticosteroids with a second controller and/or systemic corticosteroids) or that worsens when high-dose treatment is decreased.1,2 Patients can be broadly divided into two phenotypes, Type-2-high or Type-2-low, which have distinct inflammatory signatures, clinical characteristics and treatment management strategies. Monoclonal antibodies targeting type-2 cytokines are now an established therapeutic strategy for severe Type-2-high asthma; however, failure to respond or suboptimal response is still a relatively common occurrence and there are currently no targeted therapies available for severe Type-2-low asthmatics.3 Overall, patients with severe asthma are still recognized as a population having a major unmet medical need with a high burden of costs and reduced quality-of-life.
A growing body of research implicates mast cells as being a crucial factor for initiating, promoting and sustaining pathophysiological processes that drive asthma exacerbations and structural changes of the airway in severe asthmatics.4–9 This occurs directly via intercellular cross-talk and indirectly through mediator release; moreover, increased mast cell activity is associated with both Type-2-high and Type-2-low asthma, suggesting it represents a steroid insensitive pathway.10 Hence, there is a strong rationale to target mast cells in severe asthma.
Masitinib is an oral tyrosine kinase inhibitor that selectively targets mast cell activity via its action on the c-Kit (stem cell factor receptor), Lyn, and Fyn protein kinases.11 Masitinib is also a potent inhibitor of platelet-derived growth factor receptor (PDGFR) signaling, which is associated with pathologic airway smooth muscle cell proliferation and airway remodeling.12 In preclinical models of asthma, masitinib significantly improved airway inflammation and lung mechanics in cats.13 Likewise, imatinib, which is also an inhibitor of c-Kit and PDGFR, has been reported to effect airway smooth muscle thickening in mice, and airway hyperresponsiveness and mast cell counts in patients with severe asthma.14,15 Proof-of-concept that masitinib may improve the control of severe corticosteroid-dependent asthma with respect to placebo was previously demonstrated in a small (n = 44) placebo-controlled study.16
Our hypothesis was that masitinib as an add-on to standard maintenance therapy would significantly reduce asthma-related symptoms (eg, rate of exacerbations and pulmonary function) as compared with placebo in the treatment of oral corticosteroid-dependent severe asthma.
Methods
Trial Design and Oversight
Study AB07015 was a randomized, double-blind, placebo-controlled, Phase 3 trial, assessing the efficacy and safety of oral masitinib (6 mg/kg/day) in severe asthma uncontrolled despite the use of high dose inhaled corticosteroids (ICS) and long-acting beta-adrenoreceptor agonists (LABA) plus the need for daily oral corticosteroids (OCS). No minimum baseline blood eosinophil count was specified.
Major protocol amendments were implemented during the study with an aim of improving the study’s benefit/risk balance and to enhance the clinical relevance of response. To ensure enrolment was restricted to severe corticosteroid-dependent asthmatics, and consistent with revised GINA guidance,17 the inclusion criterion for stable baseline OCS dose (prednisone-equivalent) was raised from a minimum of 5 mg/day to a prolonged exposure of ≥7.5 mg/day (protocol version 9.0, after about 27% of patients had been randomized). At this time, exposure for the primary endpoint was also changed from assessment of person-time exposure at week 36 to an overall person-time exposure, ie, the full exposure period incorporating both initial 36-week period plus blinded extension period (protocol version 9). Enhanced clinical relevance was further achieved by modifying the primary endpoint to assess only severe asthma exacerbations, as opposed to moderate/severe asthma exacerbations, the latter of which being reclassified as a secondary endpoint (protocol version 10, after about 34% of patients had been randomized), and to perform a subgroup analysis based on patients with a baseline blood eosinophil count of ≥150 cell/µL (protocol version 12, after about 52% of patients had been randomized). Adjustments to sample size and statistical analysis were also required to accommodate these changes.
A 2-week, single-blind placebo treatment run-in period served as a reference for evaluating symptoms, rescue medication requirements, OCS dose stability, and study treatment compliance. Upon satisfactory completion of the run-in period, patients were randomly assigned (2:1) to receive masitinib at 6 mg/kg/day (administered orally as two daily intakes) or matching placebo for an initial treatment period of 36 weeks, which was followed by a blinded extension period during which patients continued their assigned treatments. During the initial treatment period, pulmonary function was assessed by spirometry every 4 weeks, while patient-reported outcome questionnaires were assessed at week-12 and every 8 weeks thereafter. During the blinded extension period, assessments were performed every 12 weeks.
Study AB07015 was conducted according to the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines, and national regulations. An independent data monitoring committee periodically reviewed blinded patient safety and efficacy data. Relevant health authorities and local ethics committees or institutional review boards (including the Comité de Protection des Personnes Ouest VI, Centre Hospitalier Universitaire Morvan, Brest, France) approved the study protocol and amendments. All patients provided written informed consent before trial participation. Data were collected by study investigators and analyzed by the sponsor. This trial was registered to the European Clinical Trials Database (EudraCT #2010-020803-63) prior to enrollment of the first patient on 26 January, 2011. Registration to the ICMJE-compliant ClinicalTrials.gov database (#NCT01449162) occurred about 8 months later, following recruitment of 6% (27/419) of the study population. No protocol amendment occurred during this period and all data remained blinded to patients, physicians, and study staff.
Patients and Study Measurements
Primary analysis was performed on patients with severe asthma in a cohort referred to hereafter as the “primary population”. Eligible patients were aged 18–75 years with severe uncontrolled asthma despite being treated with high-dose ICS/LABA plus OCS at ≥7.5 mg/day (prednisone or equivalent) for at least 3 months prior to screening, with no significant change in the regular asthma medication and no severe asthma exacerbation for at least 4 weeks prior to screening. Additionally, patients had experienced a minimum of two exacerbations in the previous year (including one severe asthma exacerbation as per protocol definition), had a prebronchodilator forced expiratory volume in 1 s (FEV1) of 35%−80% predicted normal value (demonstrated at least 6 hours after short-acting β2-agonist or 12 hours after LABA), were non-smokers for at least 1 year and with a prior tobacco consumption <10 pack-years, and uncontrolled asthma symptoms in the week prior to screening (defined as at least 2 of the following ACQ items: daytime symptoms at least twice/week, need for reliever medication at least twice/week, any nighttime symptoms or any activity limitation). Patients who had continuous exposure to allergens or other asthma trigger factors were excluded, as were patients receiving OCS at <7.5 mg/day. Sensitivity analyses were performed in the intention-to-treat (ITT) population and “full analysis set” (FAS), the latter cohort comprising all patients with documented OCS treatment at baseline, including randomized patients that did not comply with the aforementioned protocol amendment (ie, patients with baseline OCS dose <7.5 mg/day). The safety dataset comprised all patients that received at least one dose of study medication (Figure 1).
The primary endpoint was the annualized severe asthma exacerbation rate (SAER) in each treatment group adjusted for the overall time on treatment. A severe asthma exacerbation was defined as a worsening in asthma symptoms that required an increase in the stable maintenance dose of systemic corticosteroids for at least 3 days, with or without hospital admission. A key secondary endpoint was the overall (moderate/severe) annualized rate of asthma exacerbations (adjusted for the overall time on treatment). This endpoint included both moderate and severe asthma exacerbations; a moderate asthma exacerbation being defined as a worsening in asthma symptoms and/or an increase in rescue medication use that lasted for 2 or more days and required a change in asthma treatment without hospitalization. Assessment of pulmonary function was performed according to change from baseline in prebronchodilator FEV1, forced vital capacity (FVC), and FEV1/FVC ratio. Evaluation of asthma disease control was according to change from baseline in the 7-question version of the Asthma Control Questionnaire (ACQ-7) score (with a change of 0.5 points considered the minimum clinically significant difference);18 and quality-of-life assessment was according to change from baseline in the Asthma Quality of Life Questionnaire score (AQLQ).19
Patients were monitored for safety from the date of informed consent until 28 days after discontinuing the study drug. Adverse events (AE) were coded according to the MedDRA dictionary (version 20), and severity of AE was graded according to Common Terminology Criteria for Adverse Events (version 4). In the event of severe toxicity related to masitinib, treatment interruption or dose reduction was permitted according to predefined criteria.
Statistical Analysis
We estimated that masitinib would reduce the SAER by 33% relative to placebo (0.08 versus 0.12 severe exacerbations per month, respectively). Detection of this difference, with a two-sided 0.025 significance level and a power of 80% in the primary population, would require a minimum sample size of 339 patients (226 and 113 in the masitinib and placebo arms, respectively).
Patients were centrally randomized using a computerized central randomization system and minimization method according to the covariates of country, OCS dose at baseline (above or below 15 mg/day), concomitant asthma control treatment, and eosinophil count (above or below 150 cells/µL).
Estimation of between-group difference in the SAER (primary endpoint) was calculated using a Poisson regression model (log-link function and Poisson distribution) with factors of age, baseline FEV1, annual exacerbation rate, and above-mentioned stratification covariates. The primary endpoint analysis was performed sequentially, first on the primary population and then on the eosinophil (≥150 cell/µL) subgroup, using a hierarchical alpha-spending procedure with alpha set to 5% at each step. For the analysis of secondary endpoints, changes from baseline over 96 weeks in ACQ-7, AQLQ, FEV1, FVC, and FEV1/FVC ratio were estimated using a multivariate mixed model of repeated measures (MMRM) with treatment effect (masitinib versus placebo) reported as the between-group difference with corresponding 95% confidence intervals (CI). For FEV1, FVC, FEV1/FVC and AQLQ, a positive between-group difference favors masitinib; whereas for ACQ-7, a negative value favors masitinib. Sensitivity analyses and secondary endpoints were tested at the 0.05 significance level. All analyses and reporting procedures were performed using SAS version 9.4 (SAS Institute. Cary, NC).
Results
Patients
A total of 419 patients (279 and 140 in the masitinib and placebo arms, respectively), from 90 study sites in 18 countries (Supplementary Table 1), were randomized (ITT population) (Figure 1). Of these, 404 patients received at least one dose of study medication (271 masitinib and 133 placebo) and were eligible for safety analysis, and 402 patients (269 masitinib and 133 placebo) were included in the FAS dataset, which comprised all patients with documented OCS treatment at baseline (including 47 patients who were randomized prior to the aforementioned protocol amendment with a baseline OCS dose <7.5 mg/day). A total of 355 patients (240 masitinib and 115 placebo) were included in the primary population, of which 268 patients (181 masitinib and 87 placebo) were included in the eosinophil (≥150 cell/µL) subgroup (Figure 1).
Baseline characteristics were consistent with severe uncontrolled asthma and were balanced between treatment-arms (Table 1). Among patients receiving at least one dose of study medication, 154 (57%) patients from the masitinib arm and 84 (63%) from the placebo arm completed the initial (36-week) treatment period, with 130 (48%) and 70 (53%), respectively, continuing treatment during the blinded extension phase (Supplementary Table 2). Overall duration of exposure was an average of 13.2 ± 12.0 versus 13.1 ± 9.7 months, respectively, with 84 (31%) and 43 (32%) of patients continuing to receive treatment until data cutoff (23 October 2019). Treatment exposure and patient disposition were therefore similar between treatment-arms throughout the study.
Primary Efficacy Analysis
In the primary population, masitinib showed a statistically significant 35% reduction in the SAER relative to placebo, with a rate ratio of 0.65 (95% CI [0.47–0.90]; P = 0.010) (Table 2). This positive treatment effect was corroborated by predefined sensitivity analyses in the ITT and FAS datasets, with a significant 33% reduction in SAER for both (Table 2). For the eosinophil subgroup (sequential second step of the primary analysis), masitinib showed a significant 38% reduction in the SAER relative to placebo with a rate ratio of 0.62 (95% CI [0.42–0.91]; P = 0.016) (Table 2).
 |
Table 2 Summary of Primary Endpoint (SAER) and Associated Sensitivity Analyses |
Because greater cumulated (long-term) use of OCS is indicative of more severe asthma that is harder to control, a post-hoc sensitivity analysis was performed on the primary endpoint (ie, reduction of annualized severe asthma exacerbation rate adjusted for the overall time on treatment) with cohorts according to cumulative OCS intake (Supplementary Table 3). The magnitude of masitinib treatment effect is correlated with increasing disease severity (ie, higher cumulated use of OCS). For severe asthma patients receiving an annualized cumulative OCS intake of >500, >1000, and >1500 mg/year, masitinib showed a significant reduction in SAER compared with placebo of 41% (P < 0.01), 51% (P < 0.01), and 57% (P = 0.02), respectively. A similar but more pronounced treatment effect was observed in the eosinophil subgroup, with corresponding reductions of 49% (P < 0.01), 71% (P < 0.001), and 72% (P < 0.01), respectively.
An additional post-hoc sensitivity analysis was also performed using the European Respiratory Society and American Thoracic Society (ERS/ATS) task force recommended definition of severe exacerbations for clinical trials;20,21 ie, an increase in stable maintenance dose of OCS for at least 3 days, wherein said increase was defined for the purpose of this analysis as a dose of at least 40 mg/day (prednisone-equivalent).22 Masitinib showed a statistically significant reduction compared with placebo in the rate of severe asthma exacerbations according to this definition, across all time points tested (ie, overall time on treatment, weeks 36, 48, 52, 72, and 96) (Supplementary Table 4). In the primary efficacy population, there was a significant 39% to 54% reduction, with respective rate ratios of 0.61 (95% CI [0.38–0.96]; P = 0.033) to 0.46 (95% CI [0.31–0.68]; P = 0.0001). For the eosinophil (≥150 cell/µL) subgroup there was a significant 44% to 53% reduction, with respective rate ratios of 0.56 (95% CI [0.32–0.97]; P = 0.040) to 0.47 (95% CI [0.28–0.79]; P = 0.004), and for the FAS dataset there was a significant 40% to 53% reduction, with respective rate ratios of 0.60 (95% CI [0.38–0.93]; P = 0.023) to 0.47 (95% CI [0.32–0.68]; P < 0.0001).
Secondary Endpoint Analyses
Masitinib showed a statistically significant 36% reduction in the moderate/severe annualized rate of asthma exacerbations relative to placebo for the primary population, with corresponding rate ratio of 0.64 (95% CI [0.48, 0.84]; P = 0.001). Likewise, for the eosinophil (≥150 cell/µL) subgroup, with a significant 31% reduction and rate ratio of 0.69 (95% CI [0.49, 0.95]; P = 0.025) (Supplementary Table 5).
Evaluation of pulmonary function (FEV1, FVC, and FEV1/FVC ratio) over 96 weeks (MMRM methodology) also corroborated the observed masitinib treatment effect, with statistical significance reached in FEV1 (0.07 L; P = 0.016) and in FEV1/FVC ratio (1.6%; P = 0.049) for the primary population (Table 3). This advantage was further improved for the eosinophil subgroup, with significance reached in both FEV1 (0.11 L; P < 0.001) and FVC (0.1 L; P = 0.032), while the FEV1/FVC ratio was maintained at 1.6% (P = 0.071).
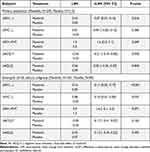 |
Table 3 Summary of Secondary Efficacy Endpoints (Changes in Asthma Characteristics from Baseline Over 96 Weeks, Mixed Model of Repeated Measures Methodology) |
A statistically significant difference in favor of masitinib was reached in ACQ-7 for the primary population (P = 0.050), with a decrease in score from baseline of 0.54 points and a between-group difference of 0.21 (Table 3).23 There was no discernable difference between treatment-arms in AQLQ.
Safety
The overall rates of adverse events (AE), severe AE, and serious non-fatal AE (SAE) were similar for both treatment-arms with the exception of patient discontinuation rate due to treatment-related AE, for which masitinib had a greater incidence (relative risk = 3.0) (Table 4). Adverse events (MedDRA preferred terms, regardless of severity) occurring more commonly for masitinib compared with placebo were vomiting, diarrhea, maculopapular rash, upper abdominal pain, nausea, and various asymptomatic abnormal laboratory results (Supplementary Table 6). No new masitinib-related safety signals were detected, and there was no evidence for increased risk of infection relative to placebo. During the study, there were 3 deaths (1.1%) in the masitinib group (pneumonia, ischemic stroke, pulmonary embolism), and 1 death (0.8%) in the placebo group (cardiopulmonary failure), none of which were deemed related to study treatment.
 |
Table 4 Summary of Adverse Events Over the Study Period (Safety Population) |
Discussion
Despite numerous drugs now being marketed for the treatment of severe asthma (ie, monoclonal antibody therapies targeting interleukin (IL)-5 and IL-4/IL-13 cytokine pathways), an unmet need persists for many patients. This includes severe Type-2-low asthmatics (estimated to be about 50% of the population), as well as severe Type-2-high asthmatics with sub-optimal response to anti-interleukin therapies (estimated to be as high as 50% for OCS-dependent asthmatics receiving the anti-IL-5 monoclonal antibodies of either mepolizumab or reslizumab).3,5,24
Efficacy results from study AB07015 significantly favored oral masitinib over placebo regarding reduced risk of severe asthma exacerbations, improved lung function and improved asthma control. The rate of severe asthma exacerbations was reduced by 35% relative to placebo in the primary population, rising to an improvement of at least 50% in the most severely affected patients (ie, those with a cumulative OCS intake of >1000 mg/year). A positive treatment effect was also evident for masitinib in terms of improved lung function, as measured by FEV1, with a between-group difference of 0.07 and 0.11 L for the primary population and eosinophil subgroup, respectively. Considering anti-IL-5 therapies for severe asthma, these are collectively associated with reduced clinically significant asthma exacerbation rates of approximately 50%, for example, subcutaneous mepolizumab (55%), intravenous mepolizumab (47%), intravenous reslizumab (58%), and subcutaneous benralizumab (38%).25 Likewise, in severe eosinophilic asthmatics, intravenous mepolizumab, subcutaneous mepolizumab, intravenous reslizumab, and subcutaneous benralizumab showed a FEV1 effect size of 0.08, 0.11, 0.12, and 0.13 L, respectively.25 We note, however, that comparison between masitinib AB07015 results and other treatments for severe asthma is complicated by differences in study design (eg, the use of overall person-time exposure), patient inclusion criteria and pharmacological action.
Masitinib has therefore achieved the main therapeutic objectives for severe asthma and notably does so through an entirely different mechanism to that which is associated with Type-2 targeted biologics. Indeed, masitinib’s observed benefit is not associated with a reduction in eosinophil levels, with levels remaining relatively stable throughout 36 weeks of treatment as evidenced from a timeseries of change in blood eosinophil concentration relative to baseline in the eosinophil subgroup (Supplementary Figure 1).
Safety results from this study were consistent with the known profile for masitinib (eg, diarrhea, vomiting, nausea, rash, and dyspepsia), and there were no new safety concerns. Regarding the higher rate of patient discontinuation due to a treatment-related AE in the masitinib arm as compared with placebo, a large proportion (60%) of this was attributable to AEs of mild or moderate severity that can be efficiently managed by dose reduction or temporary interruption.
It is possible that the aforementioned protocol amendments could limit the interpretation of results; however, predefined primary endpoint sensitivity analyses in the ITT and FAS populations (the latter of which included patients with a baseline OCS dose <7.5 mg/day) were both convergent with data from the primary population. Likewise, for assessment of the moderate/severe asthma exacerbation rate, the implication being that the study’s positive outcome cannot therefore be attributed to these changes. One limitation of the current study is that it did not evaluate masitinib’s potential OCS‐sparing properties, which is also considered an important therapeutic objective. However, should masitinib’s predominant mechanism of action in severe asthma be via modulation of steroid insensitive pathways, as is suspected, then complete cessation of OCS use is possibly infeasible.
Conclusions
Overall, these positive findings provide further clinical evidence implicating mast cells and/or PDFGR signaling to the pathophysiology of severe asthma, which could influence the future direction of drug development. In conclusion, orally administered masitinib, as used in the present randomized control study, may potentially provide a treatment option for oral corticosteroid-dependent severe asthma, including severe asthmatics that are either ineligible to receive or in failure to registered biologics.
Abbreviations
ACQ-7, 7-question version of the Asthma Control Questionnaire; AE, adverse event; AQLQ, Asthma Quality of Life Questionnaire score; EudraCT, European Clinical Trials Database; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GCP, Good Clinical Practice; ICS, inhaled corticosteroids; IL, interleukin; ITT, intention-to-treat; LABA, long-acting beta-adrenoreceptor agonists; MMRM, multivariate mixed model of repeated measures; OCS, oral corticosteroids; SAE, serious non-fatal adverse event; SAER, severe asthma exacerbation rate (annualized rate adjusted for the overall time on treatment).
Clinical Trial Registration
Study AB07015 was registered to the European Clinical Trials Database (EudraCT #2010-020803-63) prior to enrollment of the first patient on 26 January, 2011. For example, the record for the Bulgarian national competent authority (Bulgarian Drug Agency) was first entered in the EudraCT database on 20th January 2011, and the record for the Hungary national competent authority (National Institute of Pharmacy) was first entered in the EudraCT database on 6th December 2010, at least 2 months before the first participant was randomized to the study (9th February 2011). Registration to the ClinicalTrials.gov database (#NCT01449162) occurred about 8 months later, following recruitment of 6% (27/419) of the study population.
Data Sharing Statement
Masitinib is under clinical investigation and has not yet been approved in any sought-after indication by any health authority worldwide. As such, there is no plan for data-sharing at this point in time.
Ethics Approval and Informed Consent
Study AB07015 was conducted according to the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines, and national regulations. An independent data monitoring committee periodically reviewed blinded patient safety and efficacy data. Trial conduct was overseen by local ethics committees, who approved the study protocol and amendments. All patients provided written informed consent before trial participation.
Consent for Publication
All authors have read and approved the manuscript, which they have collectively written in its entirety.
Acknowledgements
We are grateful to the study participants and thank the AB07015 Study Group investigators (Supplementary Table 1).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
AB Science, Paris, France. Masitinib is under clinical development by the study funder, AB Science.
Disclosure
AM, OH and CDM are employees and shareholders of AB Science. AM reports patents WO2004/014903 issued to AB Science, PCT/EP2020/084251 pending to AB Science, US9078894B2 issued to AB Science, WO2003002106A2 issued to AB Science. PC reports grants/research support from Almirall, Boston Scientific, Boehringer Ingelheim, Centocor, GlaxoSmithKline, AstraZeneca, Novartis, and Teva; honoraria or consultation fees from Almirall, Boehringer Ingelheim, Johnson & Johnson, GlaxoSmithKline, Merck Sharp & Dohme, AstraZeneca, Novartis, Teva, Chiesi, Sanofi, AMU, SNCF, Centocor, Boston Scientific and ALK; served on advisory committees for Almirall, Boehringer Ingelheim, Johnson & Johnson, GlaxoSmithKline, AstraZeneca, Novartis, Teva, Chiesi, Schering Plough, and Sanofi. EI reports personal fees, non-financial support from AB Science, during the conduct of the study; and grants from AstraZeneca (Destination), Avillion - Mandala/Denali, Circassia, Gossamer Bio, NIH-SARP4, Novartis, PCORI; Personal fees for royalties or licenses from Wolters Kluwer; Consulting fees from Allergy and Asthma Network, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Avillion, Biometry, Equillium, Genentech, GlaxoSmithKline, Merck, NHLBI (CONNECTS), Novartis, Pneuma Respiratory, PPS Health, Regeneron, Sanofi Genzyme, Sienna Biopharmaceuticals, TEVA, Cowen; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events for Westchester Medical Center, Yale School of Medicine. Payment for expert testimony: Cambridge Medical Experts, Danaher Lagnese, SettlePou; Non-financial supports from Circassia (Equipment for PCORI-PREPARE Study), Genentech (Study Drug for NIH-Funded Study (PARK)), TEVA (Study Drug for PCORI-PREPARE Study), GSK (Background study medication for NIH PrecISE Trial); Data Safety Monitoring Board or Advisory Board for Novartis; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for NAEPP: National Asthma Education Prevention Program (Member, Coordinating Committee; Unpaid) Stock or stock options: Vorso (Stock Options; Unpaid). All remaining authors have no competing interests in this work.
References
1. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
2. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2020. Available from www.ginasthma.org.
3. Mukherjee M, Forero DF, Tran S, et al. Sub-optimal treatment response to anti-IL-5 monoclonal antibodies in severe eosinophilic asthmatics with airway autoimmune phenomena [published online ahead of print, 2020 May 22]. Eur Respir J. 2020;56(4):2000117. doi:10.1183/13993003.00117-2020
4. Penn RB. Mast cells in asthma: here I am, stuck in the middle with you. Eur Respir J. 2020;56(1):2001337. doi:10.1183/13993003.01337-2020
5. Hinks TS, Levine SJ, Brusselle GG. Treatment options in type-2 low asthma. Eur Respir J. 2020. doi:10.1183/13993003.00528-2020
6. Bradding P, Arthur G. Mast cells in asthma—state of the art. Clin Exp Allergy. 2016;46(2):194–263. doi:10.1111/cea.12675
7. Balzar S, Fajt ML, Comhair SA, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the severe asthma research program. Am J Respir Crit Care Med. 2011;183(3):299–309. doi:10.1164/rccm.201002-0295OC
8. Carter RJ, Bradding P. The role of mast cells in the structural alterations of the airways as a potential mechanism in the pathogenesis of severe asthma. Curr Pharm Des. 2011;17(7):685–698. doi:10.2174/138161211795428975
9. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):
10. Maun HR, Jackman JK, Choy DF, et al. An allosteric anti-tryptase antibody for the treatment of mast cell-mediated severe asthma [published correction appears in Cell. 2020 Jan 23;180(2):406]. Cell. 2019;179(2):417–431.e19. doi:10.1016/j.cell.2019.09.009
11. Dubreuil P, Letard S, Ciufolini M, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4(9):e7258. doi:10.1371/journal.pone.0007258
12. Kardas G, Daszyńska-Kardas A, Marynowski M, Brząkalska O, Kuna P, Panek M. Role of Platelet-Derived Growth Factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front Pharmacol. 2020;11:47. doi:10.3389/fphar.2020.00047
13. Lee-Fowler TM, Guntur V, Dodam J, Cohn LA, DeClue AE, Reinero CR. The tyrosine kinase inhibitor masitinib blunts airway inflammation and improves associated lung mechanics in a feline model of chronic allergic asthma. Int Arch Allergy Immunol. 2012;158(4):
14. Rhee CK, Kim JW, Park CK, et al. Effect of imatinib on airway smooth muscle thickening in a murine model of chronic asthma. Int Arch Allergy Immunol. 2011;155(3):243–251. doi:10.1159/000321261
15. Cahill KN, Katz HR, Cui J, et al. KIT inhibition by imatinib in patients with severe refractory asthma. N Engl J Med. 2017;376(20):1911–1920. doi:10.1056/NEJMoa1613125
16. Humbert M, de Blay F, Garcia G, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64(8):
17. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2014. Available from www.ginasthma.org.
18. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999a;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
19. Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the asthma quality of life questionnaire. Chest. 1999b;115(5):1265–1270. doi:10.1378/chest.115.5.1265
20. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi:10.1164/rccm.200801-060ST
21. Bourdin A, Bjermer L, Brightling C, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J. 2019;54(3):1900900. doi:10.1183/13993003.00900-2019
22. Global strategy for asthma management and prevention; 2020. Available from: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf.
23. Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi:10.1016/j.rmed.2004.10.008
24. Bakakos A, Loukides S, Bakakos P. Severe eosinophilic asthma. J Clin Med. 2019;8(9):1375. doi:10.3390/jcm8091375
25. Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9(9):CD010834. doi:10.1002/14651858.CD010834.pub3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


