Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Efficacy and Safety of Hydrogen Therapy in Patients with Early-Stage Interstitial Lung Disease: A Single-Center, Randomized, Parallel-Group Controlled Trial
Authors Tang C, Wang L, Chen Z, Yang J, Gao H, Guan C, Gu Q , He S, Yang F, Chen S, Ma L, Zhang Z, Zhao Y, Tang L, Xu Y, Hu Y, Luo X
Received 1 September 2023
Accepted for publication 28 November 2023
Published 11 December 2023 Volume 2023:19 Pages 1051—1061
DOI https://doi.org/10.2147/TCRM.S438044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Chang Tang,1,2,* Lanting Wang,1,2,* Zihua Chen,1,2,* Jin Yang,1,2 Haiqing Gao,1,2 Chenggong Guan,1,2 Qiaozhi Gu,1,2 Shan He,1,2 Fanping Yang,1,2 Shengan Chen,1,2 Li Ma,1,2 Zhen Zhang,1,2 Ying Zhao,1,2 Lin Tang,1,2 Yu Xu,1,2 Yue Hu,3 Xiaoqun Luo1,2
1Department of Allergy & Immunology, Huashan Hospital Affiliated to Fudan University, Shanghai, People’s Republic of China; 2Department of Dermatology, Huashan Hospital Affiliated to Fudan University, Shanghai, People’s Republic of China; 3Department of Clinical Laboratory, Huashan Hospital Affiliated to Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoqun Luo, 12 Wulumuqi Zhong Road, Jing’an District, Shanghai, People’s Republic of China, Tel +86 13918825230, Email [email protected]
Purpose: Several in vivo experiments have shown that molecular hydrogen is a promising therapeutic agent for interstitial lung diseases (ILD). In this study, hydrogen therapy was investigated to determine whether it is superior to N-Acetylcysteine (NAC) for the treatment of patients with early-stage ILD.
Patients and Methods: A prospective, single-center, randomized, controlled clinical trial was conducted in 87 patients with early-stage ILD. Hydrogen or NAC therapy was randomly assigned (1:1 ratio) to the eligible patients. The primary endpoint was the change in the high-resolution computed tomography (HRCT) and composite physiologic index (CPI) scores from baseline to week 48. Pulmonary function was evaluated as a secondary endpoint, and adverse events were recorded for safety analysis.
Results: The rate of HRCT image improvement from the baseline in the HW group (63.6%) was higher than that in the NAC group (39.5%). A significant decrease in CPI and improvement in DLCO-sb were observed in the hydrogen group compared with those in the control group. Changes in other pulmonary function parameters, including FVC, FEV1, FEV1/FVC%, and TLC, were not significantly different between the two groups. Adverse events were reported in 7 (15.9%) patients in the HW group and 10 (23.3%) patients in the NAC group, but the difference was not significant (P=0.706).
Conclusion: Hydrogen therapy exhibits superior efficacy and acceptable safety compared with NAC therapy in patients with early-stage ILD.
Keywords: hydrogen, N-acetylcysteine, interstitial lung disease, therapeutic effects
Introduction
Interstitial lung disease (ILD) is a heterogeneous group of non-neoplastic and non-infectious diseases that affect the lung parenchyma. It is characterized by an initial inflammation of the pulmonary alveoli that extends to the interstitium and beyond, leading to diffuse pulmonary fibrosis.1 These lesions could cause a decrease in lung capacity, diffusing function, and disturbance of the ventilation–perfusion ratio, ultimately resulting in hypoxemia and respiratory failure. ILD can be divided into known-causes ILD (including pneumoconiosis and connective tissue disease-associated ILD) and unknown causes (including idiopathic pulmonary fibrosis).2
It is well established that oxidative stress plays a key role in ILD pathogenesis. The increase in reactive oxygen species (ROS) in the lung could induce an inflammatory response, which in turn activates the proliferation of lung fibroblasts to produce collagen deposition and ultimately leads to pulmonary fibrosis.3 Since pulmonary fibrosis is irreversible, effective intervention in the early inflammatory stage of ILD is considered an important treatment modality. N-Acetylcysteine (NAC) is a precursor of reduced glutathione (GSH), which can scavenge oxygen free radicals in the body.4 As a commonly used antioxidant, NAC monotherapy is effective for stopping cognitive decline in forced vital capacity (FVC) in patients with early-stage ILD.5–8 However, oral administration of NAC exhibits poor bioavailability in lung tissue, even when given in high doses.9,10 Without high bioavailability, NAC could not increase the antioxidant capacity of the lungs associated with the inability to maintain steady state of GSH levels. Unlike specific binding drugs, NAC nonspecifically binds to oxygen free radicals, which may remove ROS with important signaling effects, resulting in an imbalance in the redox status in the body and limiting the therapeutic effect. Recently, two randomized controlled trials did not observe a significant survival benefit with NAC monotherapy.11,12
Molecular hydrogen (H2), which is formed by two hydrogen atoms tightly connected by covalent bonds, is the lightest and smallest gas molecule. Benefiting from its low density, strong permeability, and fast diffusion speed, H2 can directly reach the vicinity of the mitochondria responsible for producing ROS in cells and selectively reduce the levels of the most cytotoxic ROS, such as hydroxyl radicals (·OH) and peroxynitrite (ONOO−), without interfering with metabolic redox reactions.13 Consequently, it has been accepted to treat many diseases as an efficient and harmless antioxidant.14,15 Several in vivo experiments have validated the protective effects of H2 in ILD.16–18 By establishing a mouse model of rheumatoid arthritis (RA)‐associated ILD, Yasuhiro demonstrated that H2 can reduce oxidative stress and the level of lung inflammation and fibrosis in RA-ILD mice.19 Here, we propose that H2 may be a more effective treatment for early-stage ILD owing to its special advantages. Thus, we conducted a randomized controlled trial to compare the efficacy and safety of hydrogen therapy and NAC in patients with early-stage ILD.
Materials and Methods
Trial Design
A prospective, single-center, randomized, controlled trial comparing hydrogen therapy and NAC in patients with early-stage ILD. Patients were recruited from January 1, 2019, to December 31, 2021, in the Department of Dermatology and Department of Allergy & Immunology, Huashan Hospital Affiliated to Fudan University in Shanghai, China. The trial was approved by the local ethics committee and was registered as ChiCTR-ONC-17013055. Informed consent was obtained from all recruited patients in accordance with the Declaration of Helsinki.
Participants Eligibility
Eligible patients met the following criteria: patients aged 30–85 years fulfill the diagnosis of ILD.1 The diagnosis of ILD requires multidisciplinary cooperation and evaluation by clinicians and radiologists. High-resolution computed tomography (HRCT), which was evaluated by two professors of radiology, revealed ground glass, fine mesh, and honeycombing. Pulmonary function, which can be used as an auxiliary test, is characterized by restrictive ventilatory dysfunction and decreased diffusion. ILD is divided into early-stage ILD and advanced-stage ILD.2 Those with more than one of the following will be classified as advanced-stage ILD: shortness of breath, pulmonary hypertension, diffusing capacity of lung for carbon monoxide (DLCO) less than 40% of the expected, oxygen saturation of the six-minute walk test less than 88%, and high-resolution CT showing honeycomb pneumonia.2 All eligible patients with early-stage ILD belonged to GAP stage I.20
Patients were ineligible if they had primary lung diseases, including 1) lung diseases caused by various microbial infections, such as community-acquired pneumonia and tuberculosis; 2) previous history of lung diseases, such as COPD; 3) silicon lung and silicon work contact history; and 4) long-term use of pulmonary toxic drugs.
Intervention
The patients were randomly assigned to receive either hydrogen or NAC therapy in a 1:1 ratio. For the hydrogen therapy group, patients received an oral administration of 350 mL 1.6 ppm hydrogen-rich water (HW, provided by Shanghai Yiqingquan Health Technology Co., Ltd.) twice a day. In the control group, the patients received oral administration of 600 mg NAC three times a day. The follow-up period was 48 weeks.
Outcomes and Assessment
Change from baseline in HRCT images was the primary efficacy endpoint, including improvement, stability, and worsening, which were defined as reduction, no change, and progression in the extent of fibrosis (honeycombing and reticular opacity), and ground-glass opacity compared with baseline, respectively. The composite physiologic index (CPI) is another primary indicator. The calculation formula of CPI: CPI = 91-(0.65 × DLCO%predicted) − (0.53 × FVC%predicted) + (0.34 × FEV1%predicted). The secondary endpoints were the change in pulmonary function from baseline, which included forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC%, total lung capacity (TLC), and DLCO single-breath method (DLCO-sb). Any adverse events (AEs) were recorded during the trial period in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, and two independent samples t-test was used to compare continuous variables between groups. Classification data were presented as numbers (percentages) and analyzed by χ2 test. P < 0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics (RRID:SCR_019096), and all figures were mapped using GraphPad Prism (RRID:SCR_002798). ITT analysis was performed on all patients who were randomly assigned, and PP analysis was performed on patients who were treated according to the regimen and completed follow-up.
Results
Patient Characteristics
A total of 139 subjects were screened and 87 were randomly assigned to the treatment group (Figure 1). Hydrogen therapy was administered to 44 patients, and NAC therapy was administered to 43 patients. A total of 87 patients were included in the ITT analysis as full analysis set (FAS) population. Furthermore, 75 patients completed the study and were included in the per-protocol set (PPS) analysis (HW group: 37; NAC group: 38). The two groups were well balanced in terms of baseline characteristics including age, sex, smoking, comorbidities, CPI, and pulmonary function parameters (Table 1).
 |
Table 1 Baseline Variables of Patients in FAS Population |
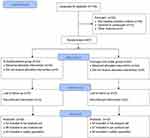 |
Figure 1 Patient flow diagram. |
Primary Endpoint
Although no significant difference was observed in the primary endpoint in the FAS population, the rate of improvement in the HW group (63.6%) was higher than that in the NAC group (39.5%), whereas the rate of worsening in each group was similar (Table 2). Notably, in the PPS population, patients who received hydrogen therapy showed a more significant improvement in HRCT images than those who received NAC (P = 0.008). More importantly, in both the FAS (P < 0.05, Figure 2a) and PPS populations (P < 0.01, Figure 2b), a significant reduction in CPI was observed in the HW group compared to that in the NAC group.
 |
Table 2 Primary Endpoint in FAS and PPS Population |
 |
Figure 2 Changes from baseline in CPI in FAS (a) and PPS (b) population. CPI composite physiologic index, FAS full analysis set, PPS per-protocol set, *P<0.05, **P<0.01. |
Secondary Endpoints
With regard to pulmonary function parameters, changes in FVC (Figure 3), FEV1 (Figure 4), FEV1/FVC% (Figure 5), and TLC (Figure 6) from the baseline did not differ significantly between the HW and NAC groups. These pulmonary function parameters were consistent between the FAS and PPS groups. However, a significant improvement in DLCO-sb was observed when hydrogen therapy was compared with NAC in the FAS (P < 0.01, Figure 7a) and PPS populations (P < 0.001, Figure 7b).
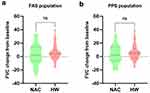 |
Figure 3 Changes from baseline in FVC in FAS (a) and PPS (b) population. FVC forced vital capacity, FAS full analysis set, PPS per-protocol set, ns no significance. |
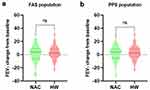 |
Figure 4 Changes from baseline in FEV1 in FAS (a) and PPS (b) population. FEV1 forced expiratory volume in one second, FAS full analysis set, PPS per-protocol set, ns no significance. |
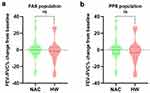 |
Figure 5 Changes from baseline in FEV1/FVC% in FAS (a) and PPS (b) population. FAS full analysis set, PPS per-protocol set, ns no significance. |
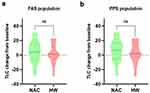 |
Figure 6 Changes from baseline in TLC in FAS (a) and PPS (b) population. TLC total lung capacity, FAS full analysis set, PPS per-protocol set, ns no significance. |
 |
Figure 7 Changes from baseline in DLCO-sb in FAS (a) and PPS (b) population. DLCO-sb DLCO single-breath method, FAS full analysis set, PPS per-protocol set, **P<0.01, ***P<0.001. |
Safety Analysis
As summarized in Table 3, 7 (15.9%) adverse events were reported in the HW group and 10 (23.3%) in the NAC group, with no significant difference (P = 0.706). Common AEs in the two groups included cough (4.5% vs 4.7%), upper respiratory tract infection (4.5% vs 7.0%), and diarrhea (6.8% vs 4.7%). Elevated blood pressure, arrhythmias, and deep venous thrombosis were observed in the NAC group. No deaths occurred during the study.
 |
Table 3 Summary of Adverse Events |
Discussion
An HRCT scan of the chest was performed to determine whether the patient had ILD and to identify the progress of the disease.21 The primary endpoint was divided into improving, stable, and worsening based on the comparison of HRCT images before and after treatment. Consequently, the improvement in HRCT images from baseline was greater in patients who received HW than in those who received NAC. More exhilaratingly, >60% of patients receiving hydrogen therapy could avoid developing irreversible pulmonary fibrosis. In 2003, Wells et al developed CPI that combines multiple lung function parameters to assess the severity of ILD, and confirmed that it is superior to a single lung function index in assessing the degree of pulmonary fibrosis and predicting survival.22 Here, our study showed a greater degree of decline in CPI in the HW group. Both HRCT and CPI results may indicate a better prognosis in patients receiving hydrogen therapy.
With regard to the secondary endpoints, FVC, FEV1, FEV1/FVC%, and TLC were comparable between the two groups during the treatment period. However, regarding DLCO-sb, our study showed a statistically significant increase in the HW group compared to the NAC group in both FAS and PPS populations. Among ILDs, DLCO-sb correlates better with the extent of disease on HRCT scans than lung volumes or spirometry.23–25 More importantly, several investigative groups have identified a baseline decrease in DLCO-sb to be highly predictive of mortality in ILDs.26–28 The better improvement in DLCO-sb after oral administration of HW supported our primary outcomes, indicating the superiority of hydrogen therapy. A previous study in RA-ILD found that drinking H2-rich water reduces inflammation and fibrosis of the lungs, further supporting the findings of this study19 Actually, a growing number of studies have shown that hydrogen may protect the lungs from various diseases, including acute lung injury, chronic obstructive pulmonary disease, asthma, lung cancer, pulmonary arterial hypertension, and COVID-19.29 In conclusion, hydrogen therapy appears to have the potential to be a novel and effective treatment for patients with early-stage ILD.
Inhalation of hydrogen gas is a typical method for treating pulmonary diseases. This often requires a hydrogen manufacturing machine and inhaler, which is inconvenient and expensive. In addition, there is a risk of an explosion that limits the hydrogen concentration in the device.14 Compared with inhalation, the oral intake of hydrogen-rich water is cheaper, safer, and more portable. A study investigating the pharmacokinetics of H2 in pigs proved that drinking a highly concentrated H2-rich solution within a short time is also an effective way to supply H2 directly to the lung.30
The pathology of ILD includes chronic lung inflammation in the early stage and irreversible alveolar fibrosis in the late stage.1 ROS in the inflammatory stage of ILD can enhance transforming growth factor-β1 (TGF-β1) production and modulate extracellular matrix (ECM) deposition, both of which induce epithelial–mesenchymal transition (EMT).31–34 EMT can activate myofibroblasts, which play a central role in the pathogenesis of pulmonary fibrosis.35 H2 effectively suppresses oxidative stress by inhibiting TGF-β1, increasing E-cadherin (an epithelial marker), and decreasing vimentin (a mesenchymal marker) in lung tissues, thereby blocking alveolar EMT and fibrosis.16,17
Safety is a critical requirement in the development of new treatments. In this trial, approximately 20% of the patients experienced treatment-related AEs. All AEs were mild or moderate in severity and have been reported in other publications.8,14 Overall, both hydrogen and NAC therapy have acceptable safety and tolerability. Additionally, fewer AEs occurred in the hydrogen group, indicating that hydrogen therapy has a more favorable safety profile than NAC. We speculate that hydrogen therapy may be an alternative to NAC in treating ILD in early-stage ILD.
Despite the significant findings of this study, it has several limitations. First, it lacks blindness owing to the differences in pharmaceutical formulations. Second, all patients in this trial were diagnosed with early-stage ILD after HRCT and pulmonary function tests due to autoimmune skin diseases. Therefore, differences in etiology may need to be considered when hydrogen therapy is administered to patients with unknown causes of ILD. Finally, the relatively small sample size of this trial may have decreased the power of the conclusions. A larger-scale clinical trial is needed to validate this conclusion based on the findings of this study.
Conclusion
For the first time, we verified the efficacy and safety of hydrogen molecules in the treatment of early-stage ILD in a randomized controlled clinical trial. This provides a new perspective for the treatment of ILD, as an effective block in the early inflammatory stage, where hydrogen molecules can prevent irreversible pulmonary fibrosis and improve the prognosis.
Abbreviations
ILD, interstitial lung disease; ROS, reactive oxygen species; NAC, N-acetylcysteine; GSH, glutathione; FVC, forced vital capacity; H2, molecular hydrogen; OH, hydroxyl radicals; ONOO−, peroxynitrite; RA, rheumatoid arthritis; DLCO, diffusing capacity of the lung for carbon monoxide; HW, hydrogen-rich water; HRCT, high-resolution computed tomography; CPI, composite physiological index; FEV1, forced expiratory volume in one second; TLC, total lung capacity; DLCO-sb, DLCO single-breath method; AEs, adverse events; PP, Per protocol; ITT, intention-to-treat; FAS, full analysis set; PPS, per protocol set; TGF-β1, transforming growth factor-β1; ECM, modulate extracellular matrix; EMT, epithelial–mesenchymal transition.
Data Sharing Statement
Data in this study can be made available upon request to the corresponding author.
Ethics Approval and Informed Consent
The trial was approved by the Ethics Committee of Huashan Hospital affiliated with Fudan University and registered as ChiCTR-ONC-17013055. Informed consent was obtained from all the recruited patients in accordance with the Declaration of Helsinki (revised in 2013).
Acknowledgments
We would like to thank Shanghai Yiqingquan Health Technology Co., Ltd. for hydrogen-rich water support.
Funding
This study was supported by the National Key Clinical Specialty Construction Project in 2021 (3030294001): Construction of a multidisciplinary treatment team for the Department of Allergy and Immunology at Huashan Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. The Lancet. 2022;400(10354):769–786. doi:10.1016/S0140-6736(22)01052-2
2. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: an Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205(9):e18–e47. doi:10.1164/rccm.202202-0399ST
3. Bast A, Weseler AR, Haenen GR, den Hartog GJ. Oxidative stress and antioxidants in interstitial lung disease. Curr Opin Pulm Med. 2010;16(5):516–520. doi:10.1097/MCP.0b013e32833c645d
4. Gillissen A, Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med. 1998;92(4):609–623. doi:10.1016/S0954-6111(98)90506-6
5. Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156(6):1897–1901. doi:10.1164/ajrccm.156.6.9706065
6. Muramatsu Y, Sugino K, Ishida F, et al. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir Investig. 2016;54(3):170–178. doi:10.1016/j.resinv.2015.11.004
7. Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. doi:10.1056/NEJMoa042976
8. Homma S, Azuma A, Taniguchi H, et al. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology. 2012;17(3):467–477. doi:10.1111/j.1440-1843.2012.02132.x
9. Bridgeman MM, Marsden M, Selby C, Morrison D, MacNee W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax. 1994;49(7):670. doi:10.1136/thx.49.7.670
10. Cotgreave IA, Eklund A, Larsson K, Moldéus PW. No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluid. Eur J Respir Dis. 1987;70(2):73–77.
11. Sakamoto S, Kataoka K, Kondoh Y, et al. Pirfenidone plus inhaled N-acetylcysteine for idiopathic pulmonary fibrosis: a randomised trial. Eur Respir J. 2021;57(1):2000348. doi:10.1183/13993003.00348-2020
12. Martinez FJ, de Andrade JA, Anstrom KJ, King TJ, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093–2101.
13. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi:10.1038/nm1577
14. Yang M, Dong Y, He Q, et al. Hydrogen: a Novel Option in Human Disease Treatment. Oxid Med Cell Longev. 2020;2020:8384742. doi:10.1155/2020/8384742
15. Zhu Q, Wu Y, Li Y, et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci Rep. 2018;8(1):8051. doi:10.1038/s41598-018-26388-3
16. Gao L, Jiang D, Geng J, Dong R, Dai H. Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition. Exp Physiol. 2019;104(12):1942–1951. doi:10.1113/EP088028
17. Dong WW, Zhang YQ, Zhu XY, et al. Protective Effects of Hydrogen-Rich Saline Against Lipopolysaccharide-Induced Alveolar Epithelial-to-Mesenchymal Transition and Pulmonary Fibrosis. Med Sci Monit. 2017;23:2357–2364. doi:10.12659/MSM.900452
18. Aokage T, Seya M, Hirayama T, et al. The effects of inhaling hydrogen gas on macrophage polarization, fibrosis, and lung function in mice with bleomycin-induced lung injury. BMC Pulm Med. 2021;21(1):339. doi:10.1186/s12890-021-01712-2
19. Terasaki Y, Terasaki M, Kanazawa S, et al. Effect of H(2) treatment in a mouse model of rheumatoid arthritis-associated interstitial lung disease. J Cell Mol Med. 2019;23(10):7043–7053. doi:10.1111/jcmm.14603
20. Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi:10.7326/0003-4819-156-10-201205150-00004
21. Baratella E, Ruaro B, Giudici F, et al. Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis. Diagnostics. 2021;11(5):56.
22. Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167(7):962–969. doi:10.1164/rccm.2111053
23. Wells AU, Hansell DM, Rubens MB, et al. Fibrosing alveolitis in systemic sclerosis: indices of lung function in relation to extent of disease on computed tomography. Arthritis Rheum. 1997;40(7):1229–1236. doi:10.1002/1529-0131(199707)40:7<1229::AID-ART6>3.0.CO;2-W
24. Wells AU, King AD, Rubens MB, et al. Lone cryptogenic fibrosing alveolitis: a functional-morphologic correlation based on extent of disease on thin-section computed tomography. Am J Respir Crit Care Med. 1997;155(4):1367–1375. doi:10.1164/ajrccm.155.4.9105081
25. Xaubet A, Agustí C, Luburich P, et al. Pulmonary function tests and CT scan in the management of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158(2):431–436. doi:10.1164/ajrccm.158.2.9709008
26. Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172(4):488–493. doi:10.1164/rccm.200412-1756OC
27. Jegal Y, Kim DS, Shim TS, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171(6):639–644. doi:10.1164/rccm.200403-331OC
28. Mogulkoc N, Brutsche MH, Bishop PW, et al. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med. 2001;164(1):103–108. doi:10.1164/ajrccm.164.1.2007077
29. Fu Z, Zhang J. Molecular hydrogen is a promising therapeutic agent for pulmonary disease. J Zhejiang Univ Sci B. 2022;23(2):102–122. doi:10.1631/jzus.B2100420
30. Ichihara G, Katsumata Y, Moriyama H, et al. Pharmacokinetics of hydrogen after ingesting a hydrogen-rich solution: a study in pigs. Heliyon. 2021;7(11):e08359. doi:10.1016/j.heliyon.2021.e08359
31. Ishikawa F, Kaneko E, Sugimoto T, et al. A mitochondrial thioredoxin-sensitive mechanism regulates TGF-β-mediated gene expression associated with epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014;443(3):821–827. doi:10.1016/j.bbrc.2013.12.050
32. Hecker L, Vittal R, Jones T, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15(9):1077–1081. doi:10.1038/nm.2005
33. Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L525–L534. doi:10.1152/ajplung.00163.2007
34. Domvri K, Organtzis I, Apostolopoulos A, et al. Prognostic Value of Serum Biomarkers in Patients with Idiopathic Pulmonary Fibrosis in Relation to Disease Progression. J Pers Med. 2023;13(9):1307. doi:10.3390/jpm13091307
35. Zhang YQ, Liu YJ, Mao YF, et al. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-β1 signaling. Clin Nutr. 2015;34(4):752–760. doi:10.1016/j.clnu.2014.08.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
