Back to Journals » International Journal of Women's Health » Volume 15
Efficacy and Safety of Acupuncture for Cyclic Mastalgia: Study Protocol for a Randomized, Sham-Controlled Trial
Authors Gao S, Sun Y, Shi H, Fang J, Liu Z
Received 24 February 2023
Accepted for publication 16 May 2023
Published 29 May 2023 Volume 2023:15 Pages 845—855
DOI https://doi.org/10.2147/IJWH.S410000
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Shuai Gao,1,* Yuanjie Sun,1,* Hangyu Shi,1,2 Jiufei Fang,1 Zhishun Liu1
1Department of Acupuncture, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2Graduate School, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhishun Liu, Department of Acupuncture, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, No. 5 Beixiange Street, Xicheng District, Beijing, People’s Republic of China, Email [email protected]
Purpose: Cyclic mastalgia is prevalent among women and negatively impairs their daily life and work. There is still a lack of effective therapies for mastalgia, and acupuncture may be a promising method. We design this multicenter randomized trial to evaluate the efficacy and safety of acupuncture on cyclic mastalgia.
Study Design and methods: Sixty participants with moderate-to-severe cyclic breast pain (with a duration of 5– 21 days and the worst pain scoring 5 points or more on Numerical Rating Scale [NRS]) will be recruited at three hospitals in China. They will be randomly assigned to acupuncture group or sham-acupuncture group at 1:1 ratio to receive 16-session treatment during 3 consecutive menstrual cycles, and follow-up for 6 menstrual cycles after treatment. The primary outcome is the change from baseline in the NRS score on the worst breast pain during the third cycle of treatment period. All statistical tests will be two-sided and P value < 0.05 will be considered statistically significant.
Keywords: cyclic breast pain, complementary and alternative medicine, RCT, sham acupuncture
Introduction
Mastalgia is one of the most common breast diseases complained by women and the leading reason to consult primary care doctors.1,2 It can be sub-categorized as cyclic, non-cyclic and extra-mammary pain according to different onset time and area, with the first two types referred to as true mastalgia.3 Approximately 70% of the women will suffer breast pain during their lives, and for 10% to 20% of them, the intensity of the pain can reach moderate-to-severe degree,4,5 which will substantially impact their social activities, interpersonal relationship, sexual behaviour, and quality of life.6,7
Cyclic mastalgia accounts for two-thirds in true breast pain. It typically occurs during 1–2 weeks prior to menstruation and relieves by the onset of menses.8,9 The pain can also be accompanied by tenderness, swelling, dull heaviness, lump and/or nodularity. Many women may fall into extreme anxiety and concern worrying about the potential malignance.5,10 Previous studies have shown that acknowledgement of the natural course of cyclic mastalgia will help to relieve their symptoms. Unfortunately, there are still about 15% to 20% people needing treatment, especially for patients whose intensity reached moderate to severe.8
The pathogenesis of cyclic mastalgia remains indefinite. It might be associated with the periodic change of hormone levels, stress, dietary preferences, living habits, improper size of underwear and other factors.11,12 In clinic, patients are therefore advised to alter their lifestyles in the first step, such as exercise more, take multiple vitamins, and consume less caffeine and fat. Besides, pharmacological therapies, such as non-steroidal anti-inflammatory drugs (NSAIDs), either oral or topical, tamoxifen, danazol are all frequently recommended.3,13 NSAIDs can be effective in reducing breast pain in a short time. External topical preparation is associated with fewer side effects than oral drugs, but the inconvenience of it may reduce patients’ compliance13. Tamoxifen is widely used in endocrine therapy, and more than 70% of the women’s conditions have been alleviated.3 However, the high risk of venous thromboembolism and relapse rate must be taken into consideration when using tamoxifen.14 As the only drug approved by US Food and Drug Administration (FDA) for treating cyclic mastalgia, danazol can be considered in relieving severe and sustained pain condition.8 Nevertheless, the usage of danazol is limited out of its androgen-like side effects, such as menstrual disorder, hirsutism, weight gain, acne, and so on.15,16 Therefore, a great number of patients seek help from complementary and alternative therapeutic methods.
Acupuncture has been widely employed in relieving pain around the world.17 It might have the potential to reduce breast pain and ease psychological burden among patients with breast pain.18,19 However, previous clinical trials of acupuncture on breast pain are without appropriate comparison and of relatively low quality.20 Still no conclusion can be elicited whether acupuncture is effective for cyclic mastalgia. For this reason, we plan to design and conduct this randomized controlled trial, whose aim is to evaluate the efficacy of acupuncture on breast pain, in comparison with sham control, among women with moderate-to-severe cyclic mastalgia.
Methods
Study Design, Setting, and Recruitment
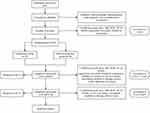
This is a prospective, multicenter, parallel-group, patient- and assessor-blinded randomized trial, projected to enrol 60 patients with cyclic breast pain. The duration of trial for each participant is 10 menstrual cycles: 1-menstrual-cycle baseline assessment, 3-menstrual-cycle treatment and 6-menstrual-cycle follow-up after acupuncture. The whole study procedures and time frame are shown in Figure 1 (Study flowchart) and Table 1 (Study schedule). The protocol is designed according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) and the Standards for Reporting Interventions in Clinical Trials of Acupuncture guidelines (STRICTA).21,22 It has been registered at ClinicalTrials.gov on September 21, 2022 (Identifier: NCT05548374). The trial will be conducted at three hospitals in China. The protocol has been approved by the Institute Review Board of individual sites and will be conducted according to the Declaration of Helsinki.
 |
Table 1 Study Schedule |
Patients will be recruited from both hospitals and communities through public advertisements such as posters, hospital networks, WeChat public accounts and Internet from March 2023 to March 2024. Qualified participants will be required to sign the written informed consent form voluntarily, and they can withdraw from the study at any time.
Patient and Public Involvement
Patients or the public are not involved in the design, implementation, report or dissemination of our study.
Randomization and Blinding
Eligible participants will be randomly assigned to acupuncture group or sham acupuncture (SA) group at a ratio of 1:1. The random numbers will be generated by SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and sealed in sequence-numbered opaque envelopes, which are kept by a staff not participating the trial. Once the eligibility of participants is confirmed, the envelopes will be opened in sequence, and only the acupuncturist will be informed of the patient’s group assignment. Participants, outcome assessors and data analysts will be blinded to the group allocation throughout the trial.
Participants
Diagnosis
The main symptom of patient is breast pain related to menstruation, which usually occurs during the luteal phase. The pain may be accompanied by swelling, tenderness, dull heaviness, lump or nodularity, or a radiating feeling to the upper arm and armpit.23
Inclusion Criteria
- Meeting the diagnosis criteria of cyclic breast pain.
- Female patients aged between 18 and 55.
- Relatively regular menstrual cycle. (The length of menstrual cycle between 25 and 35 days).24
- Cyclic breast pain lasting for 3 consecutive menstrual cycles or more.
- The score of worst pain ≥5 on Numerical Rating Scale (score range: 0–10, higher score indicates severe pain, 0 refers to no pain and 10 refers to the worst possible pain) of Cardiff Breast Pain Chart and any level of breast pain lasting 5–21 days during the run-in period of one menstrual cycle.25
- No previous experience of acupuncture for breast diseases.
- Willing to use non-hormonal contraceptives if any risk of pregnancy.
- Volunteer to the trial and signing written informed consent.
The above eight criteria should be met at the same time.
Exclusion Criteria
- Noncyclic breast pain.
- Extramammary pain only.
- A history of breast cancer or suspicious of malignancy breast disease by examinations.
- The Breast Imaging Reporting and Data System (BI-RADS) category 4–6 in ultrasound or mammography examinations.
- Combined with mastitis.
- Breast pain following injury, surgery, hormones, and/or other drugs.
- Usage of hormones (including hormonal contraceptives) in the past three months.
- Combined with severe diseases in the cardiac, respiratory, renal, liver, and hematopoietic systems, psychiatric disorder and/or cognitive disorders.
- A history of bilateral ovariectomy or premature ovarian failure.
- Pregnancy, lactation, or wishing to conceive before the end of the trial.
- Poor adherence.
Participants who meet any of the above criteria should be excluded.
Interventions
Participants will be randomized to receive true acupuncture or SA therapy twice a week, in a succession of 3 consecutive menstrual cycles. In the first two menstrual cycles, 6 sessions will be given for 3 weeks before the onset of menstruation, and in the last menstrual cycle, 4 sessions will be given for 2 weeks before the onset of menstruation, 16 sessions in total.
In both groups, the acupoints of Shangyintang, Danzhong (RNl7), Juque (RN14), Liangmen (ST21), Zhongwan (RNl2), Hegu (LI4), Waiguan (SJ5), Zusanli (ST36), Sanyinjiao (SP6), Taichong (LR3) are selected as primary acupoints and used each treatment (for acupoint locations, refer to Table 2). The other three acupoints will be chosen one each session according to the syndrome differentiation: Taixi (KI3) for liver and kidney deficiency, Fenglong (ST40) for spleen deficiency and phlegm coagulation, and Xuehai (SP10) for qi-stagnation and blood-stasis. To be more specific, acupuncturist will determine a Traditional Chinese Medicine (TCM) syndrome pattern based on the symptoms and manifestations of the patient. Liver and kidney deficiency pattern refers to insufficiency of liver blood and kidney essence in TCM, characterized by scanty menstrual volume, dizziness, tinnitus and dry eyes. Spleen deficiency and phlegm coagulation pattern refers to insufficiency of spleen coupled with internal generation of phlegm dampness, characterized by body heaviness, fatigue, abdominal distension, soft and slack pulse and enlarged tongue with a greasy coating. Qi-stagnation and blood-stasis pattern refers to qi stagnation affecting the flow of blood, characterized by stabbing pain on breast in a fixed location, delayed menstrual period, dysmenorrhea and/or with blood clots, purple tongue or with ecchymosis and wiry and hesitant pulse.
 |
Table 2 Acupoint Location |
Acupuncture Group
Sterile adhesive pads will be placed on the acupoints after skin disinfection. Hwato-brand disposable acupuncture needles (size 0.30×40mm) will be inserted into the acupoints through the adhesive pads. For acupoint Shangyintang, the needle will be inserted downward at an angle of 15 under the periosteum with a depth of 10–25mm. The needles will be inserted downward at an angle of 15 to a depth of 10–25mm for acupoint RNl7 and a depth of 10mm for RN14. The needle will be inserted vertically to a depth of 20–30mm for acupoint of ST21, 25–40mm for acupoints of RNl2, ST36, SP6, LR3, ST40 and SP10, and 10–25mm for acupoints of LI4, SJ5 and KI3. Afterwards, the acupuncture will be lifted, thrusted and twirled gently for 3 times to achieve deqi sensation and manipulated every 10 minutes during 30-minute maintenance.
Sham Acupuncture Group
Sterile adhesive pads will be placed on the acupoints after skin disinfection. The needles (with the handle identical to the needles in the acupuncture group and the body at a size 0.30×25mm) will be inserted into adhesive pads without skin penetration. Acupoints used in the sham acupuncture group are the same as those in the acupuncture group. Needles will be lifted, thrusted and twirled gently for 3 times to mimic real acupuncture. No manipulation will be conducted during 30-minute maintenance.
Other Intervention
During the process of the trial, patient can take the NSAIDs orally or smear on the pain area locally if the breast pain is unbearable. Except NSAIDs, any other concomitant treatment method specific for breast pain will be discouraged. Patients using other concomitant therapies will not be excluded from the trial, but the details of the usage will be recorded on the case report form and analyzed.
Outcome Measurement Tools
Patients will be required to keep a Cardiff Breast Pain Chart (Supplementary Figure 1) for 6 menstrual cycles: cycle 0 before randomization, cycles 1–3 over the treatment period and cycle 6 and cycle 9 during the post-treatment follow-up period. Cardiff Breast Pain Chart is a diary graph for monitoring mastalgia, reporting severity and duration of pain, which has been widely utilized for assessing pain and therapeutic effect in clinic.26,27 The intensity of daily worst and average pain will be assessed by Numerical Rating Scale (NRS), with a range of 0–10, 0 refers to no pain and 10 refers to the worst pain. The highest NRS score on the worst breast pain during the cycle is defined as the final score on the worst pain. The average NRS score on the average breast pain is defined as the mean of the average pain score during the cycle.
Short-Form Health Survey 12 (SF-12) investigates the patient’s state of health via 8 different dimensions of general health perception, physical health, limited physical role function, physical pain, vitality, mental health, limited emotional role function and social function.28 The sores of SF-12 range from 0 to 100, with higher scores indicating better physical and mental health functioning.
Patient Global Impression of Change (PGIC) is a 7-point scale reflecting patient’s self-rating of overall improvement: “very much improved”, “much improved”, “minimally improved”, “no change”, “minimally worse”, “much worse” or “very much worse”.29,30 The selection of much improved or very much improved usually indicates a clinically important improvement.31
Hospital Anxiety and Depression Scale (HADS) consists of 14 items, which are divided into two 7-item subscales to reflect a state of generalized anxiety and depression.32 The respondent rates each item on a 4-point scale ranging from 0 (absence) to 3 (extreme presence). The total scores of HADS ranges from 0 to 42, with higher scores indicating worse symptoms.
Primary Outcome and Measurements
The primary outcome of this study is the change from baseline in the NRS score on the worst breast pain during the third cycle of treatment period.
Secondary Outcome and Measurements
- The changes from baseline in the NRS score on the worst breast pain through Cycle 1, Cycle 2, Cycle 6 and Cycle 9.
- The changes from baseline in the NRS score on the average breast pain through Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- Proportions of participants with at least 50% reduction of NRS score on the worst breast pain through Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- Proportions of participants with at least 50% reduction of NRS score on the average breast pain through Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- The changes from baseline in the number of days with an NRS score of 5 or over on the worst breast pain through Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- The changes from baseline in the number of days with breast pain through Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- The proportions of patients reported “very much improved” or “much improved” per the PGIC at the end of the Cycle 3, Cycle 6 and Cycle 9.
- The impact of breast pain on sexual life, daily life, mood and sleep rated by NRS at the end of the Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- The changes from baseline in the SF-12 at the end of the Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- The changes from baseline in the total and sub-scores of HADS at the end of the Cycle 1, Cycle 2, Cycle 3, Cycle 6 and Cycle 9.
- Patients’ expectation will be assessed at baseline.
- Patients’ belief towards the effectiveness of acupuncture will be assessed both at baseline and at the end of cycle 3.
- The success of blinding will be assessed at the end of the Cycle 3.
Safety Assessment
Any adverse events (AE) will be recorded on the case report form (CRF), and will be further divided into acupuncture-related AEs and non-treatment-related AEs. Acupuncture-related AEs include but are not limited to broken needles, dizziness and unbearable acupuncture pain, local infection or hematoma. Once serious adverse events occur, the researcher will report to the Medical Ethics Committee of Guang’anmen Hospital within 24 hours.
Sample Size Calculation
To detect a 2-point difference between the true acupuncture and SA groups, with an assumed 2.5-point standard deviation at 6 weeks, a total of 60 eligible patients will be required to achieve 80% statistical power using two-sided tests at a significance level (α) of 0.05, while accounting for a 10% dropout rate.
Statistical Analysis
Data analysis will be conducted using SAS software (version 9.4), adhering to the intention-to-treat (ITT) principle, which includes all randomized participants. A two-sided P-value of less than 0.05 will be considered statistically significant.
The primary outcome will be assessed by employing a mixed effects model for repeated measures (MMRM). The dependent variable will be the observed change from baseline scores at each scheduled post-baseline visit. This approach will also be utilized for analyzing other continuous outcomes, such as changes from baseline in average breast pain NRS scores and alterations in the number of days with breast pain.
The proportions of participants experiencing at least a 50% reduction in NRS scores for worst breast pain will be compared using a generalized linear model with a binomial distribution and identity link, incorporating the same covariates as the MMRM model. Other categorical variables will be analyzed using an analogous approach.
Descriptive data on patients’ beliefs regarding acupuncture effectiveness, blinding, participants taking rescue medicine and adverse events will be provided for informational purposes only. Missing data for secondary outcomes will not be imputed, as they are considered exploratory in nature.
Data Management and Quality Control
Before the start of the trial, a unified training will be conducted concerning the aim of the experiment, recruitment criteria, screening process, intervention procedure, and outcome measurement. The acupuncture therapists at each centre will be the only person aware of the subject group allocation, and thus cannot serve as the outcome assessors. Independent assessors will evaluate the outcomes and fill in the CRF, and then the data will be input to an electronic platform by clinical research coordinator for remote monitoring. During the whole trial process, dropouts and withdrawals and their reasons will be recorded in detail.
Discussion
For mastalgia patients, two-thirds of them experience pain in the luteal phase for 1–2 weeks, which is defined as cyclic breast pain.9 Swelling and minor discomfort lasting less than 5 days prior to menstruation are considered normal and requires no intervention. However, moderate-to-severe cyclic mastalgia adversely affects women’s daily activities, reduces their quality of life, and brings an additional psychological burden.23,33 Consequently, this study is targeted at patients with cyclic mastalgia lasting for 5 days or more and scoring 5 points or higher of worst pain based on NRS. The diagnosis and inclusion criteria reflect the severity of pain in a comprehensive and integrity way, considering both duration of days and the degree of pain.
The complex and unclear pathophysiology factors in related to psychology, metabolism and nutrition make it difficult to handle moderate-to-severe cyclic breast pain.34 There is also evidence suggesting that patients with cyclic mastalgia have greater anxiety and lower quality of life than healthy group.1,10 Considering the side effects accompanied with the pharmacological therapies, more and more women tend to choose complementary and alternative therapy, which usually features good safety profile. The unique advantage of acupuncture lies in that it not only relieves pain but also have the potential to alleviate anxiety and depression with fewer side effects.
This research aims to explore the efficacy and safety of acupuncture in relieving breast pain among patients with moderate-to-severe cyclic mastalgia. For clinical trials of acupuncture, it is quite a challenge to design an ideal sham control method with scarce effects. In this study, blunt needles with identical appearance to true needles and no skin penetration are adopted as sham control, with similar perception of needle penetration with the real needle,35 might produce minimum physiological effects while maintaining blinding at the same time. It will be lifted, thrusted, and twirled gently at skin to mimic real acupuncture. The preliminary hypothesis of this trial is that acupuncture is superior to sham acupuncture in relieving breast pain, which is mainly reflected in the change from baseline in the NRS score on the worst breast pain during the third cycle of treatment period. Women with mastalgia often complain that breast pain negatively interfere with their daily lives, especially the worst evoked by exercise, touch and other conditions, which is consistent with the peak-end effect that the apex of pain is critical in the assessment of the pain conditions.36 Therefore, changes on the worst pain can accurately reflect the alleviation of symptom and be used as the primary outcome. Apart from the first three menstrual cycles during treatment, breast pain at cycle 6 and 9 will also be assessed for the possible durable effects of acupuncture on cyclic mastalgia after stimulation stopped. In order to evaluate the curative effect more comprehensively, other aspects should also be taken into consideration, such as pain duration time, physical and mental health conditions.
Based on the guidance of Huangdi Canon and clinical experiences, diseases in the area where meridian passes can be treated by the acupoints of this meridian themselves,37 and that is why the acupoints in the liver meridian (LR) and stomach meridian (ST) are selected to alleviate breast pain. Ren meridian (RN), one of the eight extra-channels, is often used to regulate the metabolic system of female patients. This study adopts a semi-standardized acupuncture scheme: primary acupoints will be used in every session to relieve the pain and ease the psychological burden and adjunct acupoints will be selected according to TCM theory for accompanied deficiency symptoms, heaviness or stabbing pain. This intervention scheme aims not only in symptoms relief but also overall health status improvement. The demographic characteristics, including age, education level, occupation, menstrual history, marriage status, childbearing history, caffeine taken, and fast food or fat consume will all be collected at baseline to picture the population included. To better capture the outburst of breast pain, the intervention started 3 weeks before the menses, that is 1 week prior to the onset of cyclic breast pain. Considering most participants are at a relatively young age and have a busy schedule, the treatment in the third cycle will be restricted to 2 weeks before menses after the acupuncture intervention has taken some effects.
Breast pain is a subjective feeling and cannot easily be quantified. The severity of pain is mainly evaluated by NRS in Cardiff Breast Pain Chart in this trial. NRS is a standard instrument of high validity and sensitivity in evaluating the degree of pain.38,39 It can properly convey their perception of pain and response to treatment. A reduction of 2 points in NRS has been determined as the minimal clinically important difference (MCID) in chronic pain evaluation, and widely adapted in the interpretation of clinical trials.40,41 However, considering that each patient’s original pain situation is different, those with higher pain level tend to have a more obvious response to treatment.42 Thus, a 2-point change may not consistently reflect the relationship between pain intensity and pain relief. A reduction of 50% in NRS on pain from baseline, as a more rigorous standard to indicate clinically important improvement, is coherent to the rating of “very much improved” in the PGIC.39,43 In our trial, the change from baseline in worst pain score is applied as the primary outcome, and the change from baseline in the average pain score and the proportion of participants with at least 50% reduction of pain are applied as secondary outcomes to assess the efficacy of acupuncture on cyclic breast pain in a more comprehensive way.
This study has some limitations. Firstly, acupuncturists cannot be blinded during the operation, which may lead to potential bias. Secondly, although sham needles will not penetrate the skin, the tiny stimulation on the skin may still produce certain biological effects. In addition, this study is conducted in Chinese population, which may limit its universality in patients of other races.
Ethic Statement
This study has been approved by the Medical Ethics Committee of Guang’anmen Hospital (approval number: 2022-007-KY) and will be in accordance with the Declaration of Helsinki. Participants will provide written informed consent to participate in this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Shuai Gao and Yuanjie Sun contributed equally to this work and are co-first authors for this study.
Funding
This study is supported by the Young Elite Scientists Sponsorship Program by CAST (2021QNRC001) and Fundamental Research Funds for Central Public Welfare Research Institutes (ZZ15-YQ-027).
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Padden DL. Mastalgia: evaluation and management. Nurse Pract Forum. 2000;11(4):213–218.
2. Kataria K, Dhar A, Srivastava A, Kumar S, Goyal A. A systematic review of current understanding and management of mastalgia. Indian J Surg. 2014;76(3):217–222. doi:10.1007/s12262-013-0813-8
3. Cornell LF, Sandhu NP, Pruthi S, Mussallem DM. Current management and treatment options for breast pain. Mayo Clin Proc. 2020;95(3):574–580. doi:10.1016/j.mayocp.2019.12.014
4. Ader DN, South-Paul J, Adera T, Deuster PA. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22(2):71–76. doi:10.3109/01674820109049956
5. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288. doi:10.1136/bmj.f3288
6. Khan SA, Apkarian AV. The characteristics of cyclical and non-cyclical mastalgia: a prospective study using a modified McGill Pain Questionnaire. Breast Cancer Res Treat. 2002;75(2):147–157. doi:10.1023/A:1019685829799
7. Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20(5):508–513. doi:10.1111/tbj.12305
8. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79(3):353–372. doi:10.4065/79.3.353
9. Hubbard TJ, Sharma A, Ferguson DJ. Breast pain: assessment, management, and referral criteria. Br J Gen Pract. 2020;70(697):419–420. doi:10.3399/bjgp20X712133
10. Hafiz SP, Barnes NLP, Kirwan CC. Clinical management of idiopathic mastalgia: a systematic review. J Prim Health Care. 2018;10(4):312–323. doi:10.1071/HC18026
11. Goodwin PJ, Miller A, Del Giudice ME, Singer W, Connelly P, Ritchie JW. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179(2):430–437. doi:10.1016/S0002-9378(98)70375-8
12. Samoli E, Trichopoulos D, Lagiou A, et al. The hormonal profile of benign breast disease. Br J Cancer. 2013;108(1):199–204. doi:10.1038/bjc.2012.493
13. Colak T, Ipek T, Kanik A, Ogetman Z, Aydin S. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525–530. doi:10.1016/S1072-7515(02)01893-8
14. Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg. 1988;75(9):845–846. doi:10.1002/bjs.1800750905
15. Mansel RE, Wisbey JR, Hughes LE. Controlled trial of the antigonadotropin danazol in painful nodular benign breast disease. Lancet. 1982;1(8278):928–930. doi:10.1016/S0140-6736(82)91932-8
16. Sutton GL, O’Malley VP. Treatment of cyclical mastalgia with low dose short term danazol. Br J Clin Pract. 1986;40(2):68–70. doi:10.1111/j.1742-1241.1986.tb07914.x
17. Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA. 2018;320(2):167–176. doi:10.1001/jama.2018.8907
18. Xuecheng Z. A Preliminary Evaluation on the Efficacy of Acupuncture in Relieving Moderate and Severe Cyclic Breast Pain. Beijing University of Chinese Medicine Thesis of Master’s Degree; 2020.
19. Muyan S. Acupuncture in relieving periodic mastodynia--a randomized control trial; 2018.
20. Jiazi L. Systematic Review and Meta-analysis of Acupuncture and Moxibustion for the Treatment of Hyperplasia of Mammary Glands. Guangzhou University of Chinese Medicine Thesis of Master’s Degree; 2016.
21. MacPherson H, Altman DG, Hammerschlag R, et al. Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. Acupunct Med. 2010;28(2):83–93. doi:10.1136/aim.2009.001370
22. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
23. Ader DN, Browne MW. Prevalence and impact of cyclic mastalgia in a United States clinic-based sample. Am J Obstet Gynecol. 1997;177(1):126–132. doi:10.1016/S0002-9378(97)70450-2
24. Munro MG, Critchley HOD, Fraser IS. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018;143(3):393–408. doi:10.1002/ijgo.12666
25. Tavaf-Motamen H, Ader DN, Browne MW, Shriver CD. Clinical evaluation of mastalgia. Arch Surg. 1998;133(2):211–213; discussion 214. doi:10.1001/archsurg.133.2.211
26. Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med. 1992;85(1):12–15. doi:10.1177/014107689208500105
27. Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res. 2015;20(6):723–727. doi:10.4103/1735-9066.170003
28. Soh SE, Morello R, Ayton D, et al. Measurement properties of the 12-item Short Form Health Survey version 2 in Australians with lung cancer: a Rasch analysis. Health Qual Life Outcomes. 2021;19(1):157. doi:10.1186/s12955-021-01794-w
29. Perrot S, Lantéri-Minet M. Patients’ Global Impression of Change in the management of peripheral neuropathic pain: clinical relevance and correlations in daily practice. Eur J Pain. 2019;23(6):1117–1128. doi:10.1002/ejp.1378
30. Guy W. ECDEU assessment manual for psychopharmacology; 1976.
31. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
32. Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--A review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi:10.1016/S0022-3999(96)00216-4
33. Katar MK, Başer M. Relationship between mastalgia and anxiety-depression: an observational study. Cureus. 2021;13(1):e12734. doi:10.7759/cureus.12734
34. Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275–285. doi:10.1056/NEJMra035692
35. Liu B, Xu H, Ma R, Mo Q, Yan S, Liu Z. Effect of blinding with a new pragmatic placebo needle: a randomized controlled crossover study. Medicine. 2014;93(27):e200. doi:10.1097/MD.0000000000000200
36. Redelmeier DA, Kahneman D. Patients’ memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66(1):3–8. doi:10.1016/0304-3959(96)02994-6
37. Veith I, Pauley GB; Association IJBotML. The Yellow Emperor’s classic of internal medicine. J Invertebr Pathol. 1968;12(4):425. doi:10.1016/0022-2011(68)90350-9
38. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi:10.1111/j.1365-2702.2005.01121.x
39. Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi:10.1016/S0304-3959(01)00349-9
40. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. doi:10.1016/j.ejpain.2003.09.004
41. Johannsen M, Sørensen J, O’Connor M, Jensen AB, Zachariae R. Mindfulness-based cognitive therapy (MBCT) is cost-effective compared to a wait-list control for persistent pain in women treated for primary breast cancer-Results from a randomized controlled trial. Psychooncology. 2017;26(12):2208–2214. doi:10.1002/pon.4450
42. Sherman KJ, Cherkin DC, Ichikawa L, et al. Characteristics of patients with chronic back pain who benefit from acupuncture. BMC Musculoskelet Disord. 2009;10:114. doi:10.1186/1471-2474-10-114
43. Rowbotham MC. What is a “clinically meaningful” reduction in pain? Pain. 2001;94(2):131–132. doi:10.1016/S0304-3959(01)00371-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.