Back to Journals » Clinical Ophthalmology » Volume 16
Efficacy and Safety of 0.1% Cyclosporine versus 2% Cyclosporine in the Treatment of Severe Vernal Keratoconjunctivitis in Children
Authors Bourcier T, Dory A, Dormegny L, Alcazar J, Gaucher D, Sauer A
Received 6 May 2022
Accepted for publication 10 October 2022
Published 26 October 2022 Volume 2022:16 Pages 3589—3596
DOI https://doi.org/10.2147/OPTH.S370414
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tristan Bourcier,1 Anne Dory,2 Lea Dormegny,1 Joffrey Alcazar,1 David Gaucher,1 Arnaud Sauer1
1Department of Ophthalmology, Strasbourg University Hospital, University of Strasbourg, Strasbourg, France; 2Pharmacy, Strasbourg University Hospital, University of Strasbourg, Strasbourg, France
Correspondence: Arnaud Sauer, Department of Ophthalmology, Nouvel Hôpital Civil, Strasbourg University Hospital, BP426, Strasbourg, 67091, France, Email [email protected]
Introduction: Vernal keratoconjunctivitis (VKC) is an inflammatory condition in children that can cause severe eye complications. Treatment is based on corticosteroid therapy during flare-ups, then antihistamines and cyclosporine in calmer periods. The dosage and posology of cyclosporine are subject to debate.
Methods: The aim of the study is to compare the evolution in symptomatic and clinical scores, and need for topical corticosteroid treatment in a population of children with severe VKC treated with two dosages of cyclosporine treatment (0.1% and 2%). Data were compiled on inclusion then every three months from March, with a total follow-up duration of 12 months. Data concerning patient evolutions and complications were collected for the two treatment groups.
Results: The mean age of the 46 children was 8.8 ± 2.4 years with age at onset of symptoms of 5.1 ± 0.9 years. The cohort was predominantly (65%) male. Corticosteroid dependence on inclusion was present in 52% of the children included. A significant improvement in the various symptomatic and clinical scores was observed following treatment with cyclosporine (0.1% and 2%). Use of topical corticosteroid treatment reduced from 19 drops per month on inclusion to 4 drops per month at 12 months. Safety was comparable for the two groups.
Conclusion: Treatments with cyclosporine 0.1% and 2% lead to a favourable evolution in clinical and symptomatic scores and reduced corticosteroid use. Cyclosporine 0.1% is an interesting alternative to the 2% dosage, particularly due to its availability and ease of handling.
Keywords: cyclosporine, vernal keratoconjunctivitis
Introduction
Vernal keratoconjunctivitis (VKC) is a severe and complex chronic inflammatory pathology affecting the ocular surface. It occurs in young children around 6 years of age and mainly affects boys with a sex ratio of 3.5:1. Usually seasonal and characterised by periods of exacerbation during hot, sunny periods, VKC generally progresses between the ages of 2 and 10 years. While the disease most often resolves by the end of puberty, 10% of cases become chronic and take the form of adult atopic keratoconjunctivitis.1–3 A history of atopic diseases is only found in 50% of cases.4,5 The prevalence of VKC remains relatively rare in Europe (1 to 5 per 10.000), but severe cases can cause serious complications and a significant change in quality of life.6,7
Standardisation of the management of VKC is essential, particularly in order to limit the use of corticosteroids to severe flare-ups alone and prohibit them in stabilised forms.8,9 The most common iatrogenic complications associated with the long-term use of local corticosteroid treatment are infection of the ocular surface, development of glaucomatous optic neuropathy and cataract formation. The reduction of corticosteroid treatment is therefore a major element in the long-term management of patients with VKC.10,11
In severe corticosteroid-dependent forms of VKC, maintenance treatment with a local immunomodulator is therefore indicated, with first-line introduction of a cyclosporine-based eye drop.8,9 The efficacy and safety of different concentrations of cyclosporine (0.5%, 1% and 2%) with regard to symptoms and reduced corticosteroid use have been demonstrated in previous studies.12–14 In addition, several studies have proven the efficacy of cyclosporine 0.1% on symptoms with very good local tolerance. In one of the first studies in a large cohort of patients (596 patients included), Ebihara et al showed the efficacy of cyclosporine 0.1% on symptoms of VKC with follow-up of one year, discontinuation of corticosteroids in 30% of patients and a good safety profile with approx. 10% of adverse events (predominantly a burning sensation in half of patients reporting an adverse event).15 Bremond et al and Leonardi et al demonstrated the efficacy of cyclosporine 0.1% on symptoms and reduced corticosteroid use and also noted an improvement in quality of life in the children and adolescents treated.16,17
To date, no comparative studies of the efficacy and safety of low-dose cyclosporine (0.1% or 0.05%) versus cyclosporine 0.5%, 1% or 2% have been carried out. This study will compare the efficacy, safety and effect on corticosteroid use of cyclosporine 0.1% and cyclosporine 2% in a population of children with severe VKC.
Methods
Inclusion/Exclusion Criteria
In the context of a prospective mono-centre study, patients aged under 12 years consulting for a flare-up of severe VKC, with no previous treatment with cyclosporine or tacrolimus eye drops, were included. Severe VKC was defined as keratitis (superficial punctate keratitis, filamentary keratitis, ulcer or vernal corneal plaques) on slit-lamp examination, associated with the usual clinical signs of the disease (photophobia, ocular itching, presence of papillae on the tarsal conjunctiva or bilateral limbic inflammation). The level of severity was classified according to the scoring method put forward by Yücel.18 A follow-up of at least one year with the same practitioner was carried out for all patients.
The exclusion criteria included the presence of another active inflammatory ocular pathology, previous use of cyclosporine eye drops, systemic immunosuppressive or anti-inflammatory treatment, wearing of contact lenses and presence of severe or deep amblyopia.
All the children included benefited from minimal treatment combining environmental measures (wearing of tinted lenses, sun avoidance, saline solution eye wash), systematic local treatment with preservative-free antihistamine eye drops (ketotifen 0.25 mg/mL) applied four times per day and artificial tears (sodium hyaluronate 0.3%) applied three times per day. As the patients presented severe VKC, the initial treatment comprised application of dexamethasone eye drops (8 drops for one day, 3 drops for 2 days, 2 drops for two days, 1 drop for two days depending practitioner’s clinical habits. Then the following drops were instilled on the principle of self-medication as children were not able to avoid scratching with eyes wash and anti-histaminic drops or to open the eyes at wakening, with explanation to the parents of the need to limit use steroids). Treatment with cyclosporine was initiated in parallel. Two groups were defined: one receiving cyclosporine A 0.1% cationic emulsion (Santen Pharmaceutical®, posology 1 to 4 drops per day, depending on severity and predictable observance), and the other receiving cyclosporine 2% (hospital preparation, dilution of intravenous preparation 50 mg/mL cyclosporine in macrogolglycérol ricinoleate, 1 to 4 drops per day). Allocation to the groups was carried out using a randomisation grid taking into account age, sex and level of severity according to Yücel.18 Treatment was blinded: examiners did not know the group to which the patient was allocated.
The study and data collection were conducted in accordance with all local laws and were compliant with the principles of the Declaration of Helsinki. The study was approved by local institutional review board (Strasbourg University and Strasbourg University Hospital Center Ethics Committees).
Data Collected
The following data were collected systematically on inclusion and then every three months: age, sex, symptoms, refraction with visual acuity, slit-lamp examination, treatments, quality of life questionnaire, corticosteroid diary (in order to measure self-medication with corticosteroid eye drops), results of systematic allergy work-up (allergy interview, prick tests and measurement of total and specific blood IgE). A symptomatic score was defined based on the intensity of the following elements scored from 0 to 3 and totalled: itching, discomfort, tearing, discharge, photophobia (Table 1). A clinical score based on slit lamp examination was established using a score from 0 to 3 and the addition of the following elements: conjunctival hyperaemia, tarsal papillae, limbal involvement, keratitis, corneal neovascularisation.
 |
Table 1 Demographic and Clinical Data of Included Patients |
Judgement Criteria
The primary outcome of the study was to compare the efficacy of the two treatments by analysing the composite symptomatic and clinical score over the course of follow-up. The secondary outcomes were to compare safety, effect on use of corticosteroids (number of dexamethasone drops applied per month) and visual acuity across the two groups.
Informed consent was signed by the children and their representatives. The study was approved by a local ethics committee.
Statistical Analysis
Relative data are expressed in the form of mean ± standard deviation. The number of subjects to be included was calculated according to the criteria: number of cyclosporine drops instilled at 12 months. The expected average based on the studies published in the 2% cyclosporine group is 2. The hypothesis for the 0.01% Cyclosporine group was set at 1, with a power of 95% and an alpha risk of 0.05. The minimum number of subjects to include per group was 12. The statistical analysis was carried out using mixed linear models. A p<0.05 was considered statistically significant.
Results
In total, 46 children (30 boys and 16 girls) aged between 5 and 14 years with severe VKC were included consecutively. Of those, 24 children received treatment with cyclosporine 0.1% and 22 received treatment with cyclosporine 2% for a duration of 12 months. No premature discontinuation of treatment took place and no significant adverse events were reported during the study.
The groups were comparable in terms of age, sex, allergy history and clinical form of the disease. The demographic, clinical and allergy data are compiled in Table 1.
Treatment with cyclosporine 0.1% and 2% led to a significant reduction in symptomatic and clinical scores from inclusion to follow-up examinations at 3, 6 and 12 months. These scores are not significantly different with regard to the cyclosporine dosage at inclusion, 3, 6 and 12 months. No change in visual acuity was noted for either group over the 12-month follow-up period. Detailed data on the progression of symptomatic and clinical scores are compiled in Table 2.
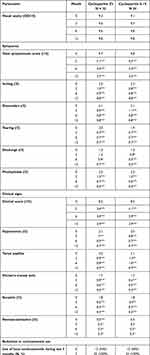 |
Table 2 Symptomatic and Clinical Scores in the Two Treatment Groups |
After an increase in corticosteroid use in the first three months (100% receiving corticosteroid treatment on inclusion), this was only necessary for three patients at six months, then two patients at twelve months in the cyclosporine 2% group and two patients at six and twelve months in the cyclosporine 0.1% group. The number of drops used also reduced substantially, from approx. 20 drops on inclusion and at three months to six then four drops at six and twelve months respectively in the cyclosporine 2% group and two then four drops at six and twelve months respectively in the cyclosporine 0.1% group. Use of corticosteroid treatment was not significantly different with regard to the cyclosporine dosage at inclusion, 3, 6 and 12 months. Discomfort on administration (considerable burning sensation) affected approx. 40% of children during the first three months of treatment, and less than 10% of children after six months. The results concerning posologies and tolerance of cyclosporine depending on dosage are detailed in Table 3.
 |
Table 3 Posology and Tolerance Based on Cyclosporine Dosage and Pharmaceutical Form |
Discussion
Cyclosporine has proven its efficacy in managing severe VKC in children. Studies of concentrations of 1% and 2% are most frequent.12–14 Some studies have investigated lower concentrations of 0.05% and 0.1%.16–19 All these studies have shown the efficacy of cyclosporine, particularly with regard to quality of life and reduced corticosteroid use. This study is the first to compare clinical progression in terms of efficacy, safety and corticosteroid use in children with severe VKC treated with cyclosporine 0.1% or 2%. As this is a cohort study for a rare disease, the number of patients is relatively limited (46 in total, with 24 and 22 patients in each group respectively). However, these 46 patients present demographic (average age 9 years and male) and clinical (history of allergy in 67% of cases, mixed VKC on slit-lamp examination in 50% of cases) characteristics comparable to those in other studies in the literature.15,16 The pharmaceutical form of the cyclosporines makes a blind study impossible. However, the design focussed on clinical follow-up makes this a real-life study. In addition, the study is based on current practices in the management of VKC and makes it possible to answer practical questions, such as the appropriate dosage of cyclosporine for children having difficulty instilling 4 drops per day, during a school day. Differences in judgement between examiners were avoided by ensuring a single examiner who collected data and prescribed the various treatments.
An improvement in symptomatic and clinical scores was observed, regardless of cyclosporine posology. There was no difference in progression between the two concentrations. These progressions have also been observed previously for cyclosporine 2%20 and cyclosporine 0.1%.16,17 The early efficacy is primarily secondary to the effects of the topical corticosteroid treatment prescribed to all patients with severe VKC on inclusion. The onset of action of cyclosporine and its association with topical corticosteroid treatment during treatment initiation are well-known data in the literature.21 This is based on simultaneous action on the various inflammatory pathways with reduced vascular and cellular permeability, reduced polynuclear and lymphocyte migration, and inhibited fibroblast activity.2
The efficacy of cyclosporine from 15 days of use is mainly associated with inhibition of Th2 lymphocyte proliferation and IL-2 production, and a reduction in the number of cells and inflammatory mediators on the ocular surface. This study shows comparable efficacy for dosages of 2% and 0.1%.22 Cyclosporine takes the form of a cationic emulsion attracted by the negatively charged particles of cell membranes, increasing its retention on the ocular surface. This could explain the similar efficacy despite a dose that is 20 times lower.23 In addition, the number of drops administered is also lower with cyclosporine 0.1% (approx. 2 drops per day on inclusion then 1 drop per day by end of follow-up) that with cyclosporine 2% (approx. 3 drops per day on inclusion then 2 drops per day by end of follow-up). The efficacy of a lower dose of cyclosporine could be useful for limiting the doses received by patients, but also as this enables the prescription of a drug that is more easily available thanks to an officinal formula which is easier to store after opening, for example during school hours.17,24,25
It should be noted that clinical progression is identical regardless of the clinical form of VKC. In addition, visual acuity is maintained in both groups. Maintenance of visual acuity is data that is rarely compiled in studies of cyclosporine. This progression profile is reassuring and tends to show that complications are also of iatrogenic origin, in particular secondary to corticosteroid treatment.10
The reduction in corticosteroid use thus remains the key reason for introducing cyclosporine in severe VKC. This study has shown extensive use of corticosteroids with 19 drops per month of dexamethasone in the cyclosporine 0.1% group on inclusion and 18 in the cyclosporine 2% group. The number of drops per month fell to 4 drops per month in both groups after 12 months of follow-up. The stable or slightly higher use of dexamethasone at three months should be noted. A large number of patients continued to use a high posology of dexamethasone drops during the first month after inclusion, during the initial treatment of the inflammation caused by VKC (for two to six weeks, in order to treat the keratitis) and before the onset of action of cyclosporine. Cyclosporine at 0.1% or 2% can limit the use of topical corticosteroids during recurrence of inflammation, and is an important addition to the range of treatments available for this allergic pathology, in particular in severe cases of VKC.2,19
The safety of the treatment was also evaluated using questionnaires. The most common adverse effects were a burning sensation on administration, tearing, redness, and transient blurred vision. Discomfort was experienced by 41% of children treated with cyclosporine 2% and 37% of those treated with cyclosporine 0.1% at three months. This rate of discomfort fell to 8% in both groups after one year of follow-up. Cyclosporine is thus relatively well tolerated. The reduction in concentration does not correlate with a reduction in discomfort.
Conclusions
In children with severe VKC, a significant reduction in symptomatic and clinical scores is observed with cyclosporine 2% and 0.1%. Both doses enabled a substantial reduction in the use of topical corticosteroids. The tolerance of both treatments is good and improves over the course of follow-up.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2:73–88. doi:10.1007/s40123-013-0019-y
2. Singhal D, Sahay P, Maharana PK, Raj N, Sharma N, Titiyal JS. Vernal keratoconjunctivitis. Surv Ophthalmol. 2019;64:289–311. doi:10.1016/j.survophthal.2018.12.001
3. Sacchetti M, Abicca I, Bruscolini A, Cavaliere C, Nebbioso M, Lambiase A. Allergic conjunctivitis: current concepts on pathogenesis and management. J Biol Regul Homeost Agents. 2018;32:49–60.
4. Fauquert JL. Diagnosing and managing allergic conjunctivitis in childhood: the allergist’s perspective. Pediatr Allergy Immunol. 2019;30:405–414. doi:10.1111/pai.13035
5. Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478–485. doi:10.1097/ACI.0b013e32833e16e4
6. De Smedt S, Wildner G, Kestelyn P. Vernal keratoconjunctivitis: an update. Br J Ophthalmol. 2013;97:9–14. doi:10.1136/bjophthalmol-2011-301376
7. Ben-Eli H, Erdinest N, Solomon A. Pathogenesis and complications of chronic eye rubbing in ocular allergy. Curr Opin Allergy Clin Immunol. 2019;19:526–534. doi:10.1097/ACI.0000000000000571
8. Esposito S, Fior G, Mori A, Osnaghi S, Ghiglioni D. An update on the therapeutic approach to vernal keratoconjunctivitis. Paediatr Drugs. 2016;18:347–355. doi:10.1007/s40272-016-0185-1
9. Pacharn P, Vichyanond P. Immunomodulators for conjunctivitis. Curr Opin Allergy Clin Immunol. 2013;13:550–557. doi:10.1097/ACI.0b013e328364d86a
10. Mandal R, Maiti P, Sasmal NK, et al. Ocular effects of long term use of topical steroids among children and adolescents with vernal keratoconjunctivitis: a prospective observational study. J Indian Med Assoc. 2011;109:
11. Daniell M, Constantinou M, Vu HT, Taylor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006;90:461–464. doi:10.1136/bjo.2005.082461
12. Spadavecchia L, Fanelli P, Tesse R, et al. Efficacy of 1.25% and 1% topical cyclosporine in the treatment of severe vernal keratoconjunctivitis in childhood. Pediatr Allergy Immunol. 2006;17:527–532. doi:10.1111/j.1399-3038.2006.00427.x
13. Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013;13:308–314. doi:10.1007/s11882-013-0345-0
14. Wan KH, Chen LJ, Rong SS, Pang CP, Young AL. Topical cyclosporine in the treatment of allergic conjunctivitis: a meta-analysis. Ophthalmology. 2013;120:2197–2203. doi:10.1016/j.ophtha.2013.03.044
15. Ebihara N, Ohashi Y, Uchio E, et al. A large prospective observational study of novel cyclosporine 0.1% aqueous ophthalmic solution in the treatment of severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2009;25:365–372. doi:10.1089/jop.2008.0103
16. Bremond-Gignac D, Doan S, Amrane M, et al. Twelve-month results of cyclosporine A cationic emulsion in a randomized study in patients with pediatric vernal keratoconjunctivitis. Am J Ophthalmol. 2020;212:116–126. doi:10.1016/j.ajo.2019.11.020
17. Leonardi A, Doan S, Amrane M, et al. A randomized, controlled trial of cyclosporine A cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126:671–681. doi:10.1016/j.ophtha.2018.12.027
18. Yücel OE, Ulus ND. Efficacy and safety of topical cyclosporine A 0.05% in vernal keratoconjunctivitis. Singapore Med J. 2016;57:507–510. doi:10.11622/smedj.2015161
19. Chatterjee A, Bandyopadhyay S, Bandyopadhyay SK. Efficacy, safety and steroid-sparing effect of topical cyclosporine A 0.05% for vernal keratoconjunctivitis in Indian children. J Ophthalmic Vis Res. 2019;14:412–418. doi:10.18502/jovr.v14i4.5439
20. Pucci N, Novembre E, Cianferoni A, et al. Efficacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol. 2002;89:298–303. doi:10.1016/S1081-1206(10)61958-8
21. Erdinest N, Solomon A. Topical immunomodulators in the management of allergic eye diseases. Curr Opin Allergy Clin Immunol. 2014;14:457–463. doi:10.1097/ACI.0000000000000089
22. Wan XC, Dimov V. Pharmacokinetic evaluation of topical calcineurin inhibitors for treatment of allergic conjunctivitis. Expert Opin Drug Metab Toxicol. 2014;10:543–549. doi:10.1517/17425255.2014.884070
23. Baudouin C, de la Maza MS, Amrane M, et al. One-year efficacy and safety of 0.1% cyclosporine a cationic emulsion in the treatment of severe dry eye disease. Eur J Ophthalmol. 2017;27:678–685. doi:10.5301/ejo.5001002
24. De Smedt SK, Nkurikiye J, Fonteyne YS, Tuft SJ, Gilbert CE, Kestelyn P. Vernal keratoconjunctivitis in school children in Rwanda: clinical presentation, impact on school attendance, and access to medical care. Ophthalmology. 2012;119(9):1766–1772. doi:10.1016/j.ophtha.2012.03.041
25. Sacchetti M, Baiardini I, Lambiase A, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. Am J Ophthalmol. 2007;144:557–563. doi:10.1016/j.ajo.2007.06.028
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
