Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Efficacy and Patient Tolerability of Split-Dose Sodium Picosulfate/Magnesium Citrate (SPMC) Oral Solution Compared to the Polyethylene Glycol (PEG) Solution for Bowel Preparation in Outpatient Colonoscopy: An Evidence-Based Review
Authors de Miranda Neto AA, de Moura DTH, Hathorn KE, Tustumi F, de Moura EGH , Ribeiro IB
Received 31 March 2020
Accepted for publication 14 September 2020
Published 7 October 2020 Volume 2020:13 Pages 449—457
DOI https://doi.org/10.2147/CEG.S237649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Antonio Afonso de Miranda Neto,1 Diogo Turiani Hourneaux de Moura,1,2 Kelly E Hathorn,2 Francisco Tustumi,3 Eduardo Guimarães Hourneaux de Moura,1 Igor Braga Ribeiro1
1Gastrointestinal Endoscopy Unit, Hospital Das Clínicas, University of São Paulo School of Medicine, São Paulo, Brazil; 2Division of Gasteoenterology, Hepatology and Endoscopy – Brigham and Women´s Hospital – Harvard Medical School, Boston, MA, USA; 3Gastrointestinal Surgery Unit, Hospital Das Clínicas, University of São Paulo School of Medicine, São Paulo, Brazil
Correspondence: Igor Braga Ribeiro
Gastrointestinal Endoscopy Unit, Hospital Das Clínicas, University of São Paulo School of Medicine, São Paulo, Brazil
Tel +5592981377788
Email [email protected]
Background: Colonoscopy is the gold standard exam for evaluation of colonic abnormalities and for screening and surveillance for colorectal cancer. However, the efficacy of colonoscopy is dependent on the quality of the pre-colonoscopy bowel preparation. Polyethylene glycol (PEG) and sodium picosulfate/magnesium citrate (SPMC) have emerged as two of the most commonly used bowel preparation agents. We conducted an evidence-based review of current evidence to further investigate the efficacy and patient tolerability of split-dose SPMC oral solution compared to PEG solution for colonoscopy bowel preparation.
Methods: A systematic search was performed using Pubmed (MEDLINE), Web of Science, EMBASE, and Cochran Central Register of Controlled Trials databases. All studies on split-dose bowel preparation with SPMC and PEG were reviewed. Relevant studies regarding colonoscopy and bowel preparations were also included. Randomized controlled trials were prioritized due to the high quality of evidence.
Results: Eight randomized controlled trials were included. Split-dose SPMC and PEG were associated with similar results for adequacy of bowel preparation. Split-dose SPMC was associated with increased patient tolerability and compliance.
Conclusion: Split-dose SPMC and PEG are both adequate and safe for bowel preparation for outpatient colonoscopy, with split-dose SPMC being more tolerable for patients. Additional RCTs comparing these and other bowel preparation solutions are necessary to further investigate quality of bowel preparation, patient preference, and cost-effectiveness of the various options.
Keywords: colonoscopy, bowel, polyethylene, glycol, sodium, picosulfate, magnesium, citrate, PEG, SPMC, tolerability, adenoma
Introduction
Colonoscopy remains the gold standard exam for the investigation of colonic mucosal abnormalities and is an integral part of colorectal cancer screening and surveillance programs. However, the efficacy of colonoscopy in the detection of high-risk lesions is greatly dependent on the quality of the pre-colonoscopy bowel preparation1,5 and, even in emergency procedures, adequate and thorough bowel preparation can improve patient safety and outcomes.6–14 There are various factors, such as patient medical comorbidities, tolerance and compliance, and cost burden of preparations which are important considerations in selecting an agent. Polyethylene glycol (PEG) and sodium picosulfate/magnesium citrate (SPMC) have emerged as two of the most commonly used agents worldwide.
PEG is a non-absorbable, large polymer that remains in the gut lumen resulting in an osmotic lavage effect and can be osmotically balanced with non-fermentable electrolyte solutions.15 While PEG has been the most commonly used bowel preparation agent, it requires the consumption of a large volume of liquid, resulting in poor patient tolerance and compliance. Thus, in recent years, studies have investigated other preparation agents in attempts to improve patient adherence and thus quality of preparation and adenoma detection rates.
SPMC is one such alternative preparation, which serves as a purgative laxative and is generally made up of two components: Sodium Picosulfate, a prodrug metabolized by the colonic flora into its active metabolite which stimulates peristalsis and increases bowel movement frequency, and magnesium oxide and citric acid, which react to create magnesium citrate, which induces catharsis and leads to increased fluid retention within the colon via its osmotic effect.15,16 The SPMC bowel preparation reduces effective volume of the colon-cleansing solutions to 2-liters (2-L) from the standard 4-liters (4-L) of the PEG solution,16 which is thought to improve patient satisfaction and adherence, while still achieving a similar cleansing effect. Additionally, there is evidence that administration of the preparation agent in a split dose, ie, giving preparation in separate doses on the day prior or day of the procedure, is superior to administration in a single dose in terms of patient convenience, tolerance and palatability, improved quality of bowel preparation, and increased adenoma detection rate.17,18
This review was designed to summarize present evidence about the efficacy and tolerability of split-dose SPMC oral solution compared to PEG solution for colonoscopy.
Methods
Individualized systematic searches of PubMed (Medline), EMBASE, Web of Science, and Cochrane Library were acquired of available literature from inception through February 2020. The combinations of keywords used were:
((Cathartic) OR (Bowel Evacuants) OR (Purgatives) OR (Bowel Preparation) OR (Colon cleans*) OR (Bowel cleans*)) AND ((PEG-based) OR (PEG) OR (Polyethylene Glycol) OR (Macrogol) OR (Polyethylene Oxide) OR (Polyethyleneoxide) OR (Polyoxyethylene) OR (Polyglycol) OR (Carbowax)) AND ((SPMC) OR (picosulfate-magnesium) OR (picosulfate/magnesium) OR (picosulfate AND magnesium) OR (picosulfate sodium) OR (sodium picosulfate) OR (picoprep) OR (Picolax) OR (Picosulfol) OR (Laxoberal))
All relevant full-text articles in English, regardless of the year of publication, were included. From the initial search results, duplicates were extracted, and then the titles and abstracts of all potentially relevant studies were screened for eligibility. Two reviewers (AAMN, IBR) independently screened the titles and abstracts of all the articles according to the below-predefined eligibility and exclusion criteria, extracting relevant information, for ensuring relevance to the selected topic. Any differences were resolved by mutual agreement and in consultation with a third reviewer (DTHM). Additionally, we had scanned the reference lists of included studies and gray literature was searched.
Studies which didn't fulfil the eligibility criteria were excluded. All studies evaluating the quality of split-dose preparations of SPMC and PEG, patient tolerability, and patient compliance were included. We included only randomized controlled trials (RCTs) with full texts published, due to the better quality of evidence, that were published or presented as original research articles in the English language. Studies were excluded from this review according to the following criteria: use of alternative bowel preparation solutions (not SPMC or PEG solutions); use for an indication other than outpatient colonoscopy; not adults patients; patients with dietary restrictions; and/or not using split doses for bowel preparation. The detailed process of study selection is shown in Figure 1.
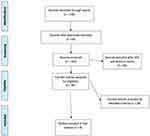 |
Figure 1 Flow chart for study selection. |
Literature Results
We retrieved a total of 132 records from the electronic literature search. A total of eight randomized controlled trials were included in the final analysis and were reviewed. The study characteristics and patient demographics of the included studies are summarized in Table 1. The quality of the bowel preparation was evaluated on one of three previously validated scoring systems: Four of the articles used the Ottawa Bowel Preparation Scale (OBPS), three of the articles used the Boston Bowel Prep Score (BBPS), and three of the articles used the Aronchick Score. The bowel preparation validated scoring systems used are summarized in Table 2.
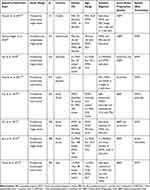 |
Table 1 Characteristics of the Studies Included in the Review |
 |
Table 2 Characteristics of Bowel Validated Scoring Systems |
Rostom et al19 conducted a single-center randomized controlled trial in which 171 patients whom were scheduled for outpatient colonoscopy were randomized to either same-day or 2-day split-dose SPMC or PEG lavage. Bowel preparation quality was recorded in a blinded manner by the endoscopist using the OBPS prior to washing or suctioning. This group found that SPMC was inferior to PEG (P=0.019, mean OBPS: 4.14±2.64 vs 5.11±3.44). Additionally, they demonstrated that a 1-day split dose was inferior compared to a 2-day split-dose regimen (P<0.001, mean OBPS: 3.68±2.82 vs 5.69±3.06). Two-day split dosing also resulted in a better right colon cleanliness score compared to 1-day split dosing (right bowel, P<0.001, OBPS 1.27±0.11 vs 2.10±0.12).
Kojecky et al20 conducted a randomized, endoscopist-blinded, multicenter study in which they evaluated the quality of bowel preparation, in a single or a split-dose preparation for 973 outpatients who received PEG, SPMC, or ethylene glycol/ascorbic acid (PEGA). Satisfactory bowel cleansing (Aronchick score 1+2) was observed more frequently when a split dose was used, irrespective of the solution type (P<0.006, PEG 90.1 vs 68.8%, PEGA 86.0 vs 71.6%, SPMC 84.3 vs 60.2%). In terms of patient tolerance, PEG was the worst tolerated (P<0.001), and SPMC was the best tolerated, followed by PEGA (P<0.006). It was also observed that tolerability did not correlate with the regimen or amount of the solution used.
Mathus-Viegen et al21 recently conducted a non-inferiority randomized trial comparing PEG-electrolyte solution plus bisacodyl (PEG-sc + B) and SPMC with 2 liters ascorbic-acid-enriched. A total of 341 patients underwent colonoscopy, and those patients reported significantly fewer physical complaints and a higher completion rate with SPMC compared to PEG-Asc+B; in particular, patients receiving SPMC reported increased ease of consumption and improved taste compared to the PEG-Asc+B preparation. In the event of a repeat colonoscopy, 59.7% of patients in the PEG-Asc+B compared to 93.6% of patients in the SPMC group confirmed that they would opt for the same preparation again. Additionally, in this study, researchers reported that the observed changes in hemodilution and changes in electrolytes, including bicarbonate and magnesium, were largely attributable to the preparation used but were not clinically significant. Thus, they concluded that SPMC was non-inferior to PEG-Asc+B in terms of quality of bowel preparation and showed in this study that the effects on blood electrolyte concentrations were clinically insignificant.
Seo et al22 conducted a randomized, endoscopist-blinded, single center controlled trial comparing SPMC vs 2L-PEG/Asc in 223 outpatients undergoing colonoscopy. There was no significant difference in overall quality of bowel preparation on the OBPS between the two groups; however, when broken down by each individual segment of the bowel, there was a trend towards improved quality of preparation in the right colon in the SPMC group compared to the PEG/Asc group (OBPS scores; P=0.08, 1.55±0.66 vs 1.74±0.88). SPMC was also better tolerated than PEG/Asc based on ease of consumption and preference to receive the agents again in the future. The authors observed that total adverse events like nausea, abdominal pain, and abdominal bloating were significantly lower in SPMC group compared with the PEG/Asc group (P=0.031, 47.4 vs 62.4%).
Yoo et al,23 in another randomized, single-center, observer-blinded study evaluated 200 prospectively enrolled outpatients who received a split-dose preparation of either SPMC or PEG-Asc low-volume bowel preparations for colonoscopy. This group demonstrated that PEG-Asc was similar to SPMC in terms of quality of bowel preparation (P=0.718, ≥6 BBPS: 80% vs 82%; adequate Aronchick grade: P=0.352, 93% vs 96%). He also observed that SPMC caused fewer gastrointestinal symptoms (ie, abdominal fullness and general abdominal discomfort). Patients in the SPMC group reported significantly better palatability than PEG–Asc (mean±SD, score 1/excellent–5/bad: 2.39±0.73 vs 3.06±0.93, P<0.001).
Jeon et al24 conducted a endoscopist-blinded randomized, single-center, controlled trial comparing 2-L PEG-Asc vs SPMC on both intention-to-treat (ITT) analysis (total of 388 patients) and per protocol (PP) analysis (total of 356 patients.) No significant differences in preparation adequacy were observed in ITT and PP analyses when assessed with the BBPS (P>0.05). The polyp and adenoma detection rate (PDR and ADR) were greater than 60 and 40% in both groups, respectively (P>0.05). While patient compliance levels were higher in the 2-L PEG/Asc group compared to the SPMC group (P<0.001), patient satisfaction (ITT, P=0.014; PP, P=0.032) and palatability (ITT and PP, P<0.001) levels were higher in the SPMC group than in the 2-L PEG/Asc group. Despite this, ease of consumption and future intention to reuse if necessary were similar in both groups (P>0.05, ITT and PP).
Manes et al25 conducted an endoscopist-blinded, multicenter randomized study assessing 285 outpatients undergoing colonoscopy. Patients were randomized to receive either SPMC or PEG-Asc. Then, depending on the time of their scheduled colonoscopy, they were divided into either same-day or split-dose preparation regimens. Patients with a procedure earlier than 12:00 pm were instructed to complete the preparation in one night, starting at 5:00 pm the day before the procedure. Patients with a procedure later than 12:00 pm were instructed to consume the preparation in a split-dose regimen; they started the first half at 5:00 pm and consumed the second half the morning of their scheduled procedure. It was shown that the mean BBPS score for both the entire colon (6.8±1.76 for SPMC group vs 6.6±1.7 for PEG-Asc group) and for the right colon (1.95±0.73 for SPMC group vs 1.96±0.71 for PEG-Asc group) were comparable between groups. In addition, 97.1% patients in the SPMC group and 84.8% in the PEG-Asc group reported no or mild discomfort (P<0.0003) and 97.883.4% expressed their willingness to repeat the preparation (P<0.0001). The palatability was better in the SPMC cohort and related symptoms occurred more frequently in the PEG-ASC cohort. Regardless of which preparation was used, the split regimen was associated with better cleansing compared with the same-day method (OR=3.39; 95% CI=1.1–10.4; P=0.03). Predictors of poor cleansing were comorbid medical conditions, discomfort during preparation, and incomplete consumption (<75%) of preparation.
Kim et al26 conducted a randomized, multicenter, single-blinded, non-inferiority study comparing split-dose Conventional 4-L PEG versus split-dose SPMC/bisacodyl. A total of 365 patients were analyzed on intention-to-treat analysis; 18 in the PEG group and 28 patients in the SPMC did not complete the entire preparation, and thus 319 patients were evaluated in a per protocol (PP) analysis (166 in the PEG cohort vs 153 in the SPMC cohort). This group observed that the total mean BBPS score was similar between the two groups in both the ITT (SPMC/bisacodyl: 7.3±1.6 vs Conventional 4-L PEG: 7.2±1.7; P=0.329) and PP (SPMC/bisacodyl: 7.3±1.6 vs Conventional 4-L PEG: 7.2±1.6; P=0.680) analysis. The mean visual analog scale (VAS), used to analyze compliance and satisfaction level, and Likert scale (LS) score, used to analyze ease of use, were higher in the SPMC group in both ITT (P<0.001, 7.58±1.94 vs 5.79±2.43) and PP analyses (P<0.001, 7.62±1.95 vs 5.92±2.35). The adverse event rate was lower in the SPMC group than in the PEG group (P<0.05). Thus, it was concluded that SPMC preparation was comparable to conventional PEG with respect to bowel preparation adequacy and superior with respect to compliance, satisfaction, and safety.
Discussion
This review assessed the efficacy and patient tolerability of split-dose SPMC vs PEG for outpatient colonoscopy. The use of SPMC split-dose preparations correlated with improved tolerability, fewer physical complaints from patients, and a higher adherence and completion rate in most cases. However, in terms of adequacy of bowel preparation, the split-dose SPMC and PEG preparations were similar in the majority of studies. Adequate bowel preparation is an integral part of adequate screening and surveillance in patients referred to colonoscopy. Poor bowel preparation may cause incomplete visualization of the colon, and may lead to missed lesions, procedure failure, prolonged procedure time (increasing both cecal intubation and withdrawal time), and an increased risk of complications.27,28 The traditional PEG 3- or-4-L regimens are still widely used and are associated with excellent efficacy when well tolerated. However, some studies have demonstrated poor compliance because of the large volumes of these solutions.29
In a recent update from 2019, ESGE guidelines for bowel preparation for colonoscopy recommend the use of split-dose bowel preparation for elective colonoscopy (strong recommendation, and high-quality evidence).30 Typically, the standard dose of a bowel preparation is split between the day before and the morning of the procedure. The second dose should be administered between 3–8 hours before the planned start of the colonoscopy procedure,31 as patients must have completed the preparation a minimum of 2 hours before sedation to avoid potential aspiration.32 The guidelines reference a meta-analysis involving 47 RCTs, including four different bowel preparation regimens (polyethylene glycol, sodium phosphate, picosulfate, or oral sulfate solutions) with a total of 13,478 patients, where it was shown that split-dose regimens, regardless of the type and dose of the cleansing agent, provided excellent or good colon cleansing more frequently than day-before bowel preparation (OR=2.51, 95% CI=1.86–3.39). This result was confirmed in sub-analyses restricted to PEG (OR=2.60, 95% CI=1.46–4.63), sodium phosphate (OR=9.34, 95% CI=2.12–41.11), and picosulfate (OR=3.54, 95% CI=1.95–6.45). Split dosing was also associated with a higher proportion of patients willing to repeat the preparation (OR=1.90, 95% CI=1.05–3.46).33
Similarly, ASGE guidelines for bowel preparation for colonoscopy34 recommend split-dose regimens for all patients and/or same day preparations for afternoon colonoscopies with a portion of the preparation taken within 3–8 hours of the procedure, both to enhance colonic cleansing as well as improve patient tolerance (moderate quality of evidence). In these guidelines, experts recommend that bowel preparations be individualized by the prescribing provider for each patient based on efficacy, cost, safety, and tolerability considerations balanced with the patient's overall health, comorbid conditions, and preferences (high quality of evidence). Both PEG and SPMC are good options for bowel preparations. However, these guidelines recommend that the SPMC preparation should be used cautiously in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency as ascorbic acid may provoke hemolysis in these patients. In a systematic review and meta-analysis comparing different regimens, including split-dose regimens of SPMC and PEG for colonoscopy preparation, Jin et al35 analyzed a total of 25 RCTs and observed that no difference was found in polyp detection rate (RR=0.94; 95% CI=0.82–1.08, P=0.37; I2=46%) nor adenoma detection rate (RR=0.88; 95% CI=0.74–1.05, P=0.16; I2=37%). However, adverse events, such as nausea, vomiting, and bloating, were less frequent in the SPMC group (RR=0.78, 95% CI=0.66–0.93, P=0.004; I2=88%). Additionally, a higher proportion of patients were likely to complete the SPMC regimen (RR=1.08; 95% CI=1.04–1.13, P<0.001; I2=95%) and the percentage of patients who were willing to repeat an identical bowel preparation in the future was significantly higher in the SPMC group compared to the PEG group (RR=1.44; 95% CI=1.25–1.67, P<0.001; I2=95%). In terms of colon cleansing, there was no significant difference between the two agents, although there was a trend in favor of the PEG solution (RR=0.93, 95% CI=0.86–1.01, P=0.07; I2=87%).
Similarly, in a recent systematic review and meta-analysis, Rocha et al7 analyzed 16 RCTs and compared SPMC and PEG before elective outpatient colonoscopy. The authors concluded that SPMC and PEG can be used for split preparations as there are no difference in bowel cleaning success, tolerability, and adverse events, but SPMC should be the preferred choice for day-before preparations because of its improved tolerability. There was no difference observed between the two preparations when comparing polyp or adenoma detection rates.
However, while these aforementioned studies saw no differences in adverse events, it should be noted that because of hyperosmolarity and magnesium content, solutions containing SPMC are contraindicated in patients with congestive heart disease, hypermagnesemia, rhabdomyolysis, gastrointestinal ulcerations, and severe impairment of renal function, which can lead to magnesium accumulation. In a retrospective study36 using administrative data to research adults >65 years old, SPMC was associated with an increased risk of hospital admission due to hyponatremia when compared with PEG solution. Although occasionally not well tolerated given lower palatability and increased volume, PEG is considered generally safe for patients with pre-existing electrolyte imbalances and for patients who cannot tolerate a significant sodium load (for example those with renal failure, congestive heart failure, or advanced liver disease with ascites).37
Conclusion
Studies comparing split-dose SPMC and PEG for bowel preparation for outpatient colonoscopy demonstrate that both are effective in terms of satisfactory bowel cleansing when evaluated with the OBPS, BBPS, and/or the Aronchick scoring system. Furthermore, split-dose SPMC may be associated with improved patient tolerance, adherence rates, and less side-effects.
Ethical Statement
The study was approved by the Research Ethics Committee of the University of São Paulo School of Medicine Hospital das Clínicas.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Moura reports personal fees from Boston Scientific, personal fees from Olympus, outside the submitted work. The authors report no other conflicts of interest.
References
1. Guo X, Shi X, Kang X, et al. Risk factors associated with inadequate bowel preparation in patients with functional constipation. Dig Dis Sci. 2020;65(4):1082–1091.
2. Guo B, Zuo X, Li Z, et al. Improving the quality of bowel preparation through an app for inpatients undergoing colonoscopy: A randomized controlled trial. J Adv Nurs. 2020;76(4):1037–1045.
3. Butterly LF, Nadel MR, Anderson JC, et al. Impact of colonoscopy bowel preparation quality on follow-up interval recommendations for average-risk patients with normal screening colonoscopies. J Clin Gastroenterol. 2020;54(4):356–364.
4. Hopkins RL, Parsons D, Hoyo L, Jacobson BC. Evaluating the practice of canceling colonoscopies for presumed inadequate bowel preparation. Gastrointest Endosc. 2020;92:382–386. doi:10.1016/j.gie.2020.03.3750
5. Fuccio L, Frazzoni L, Spada C, et al. Factors that affect adequacy of colon cleansing for colonoscopy in hospitalized patients. Clin Gastroenterol Hepatol []. 2020; doi:10.1016/j.cgh.2020.02.055
6. Ribeiro IB, Bernardo WM, Martins BDC, et al. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open [Internet]. 2018;(5):E558–67.
7. Rocha RSDP, Ribeiro IB, de Moura DTH, et al. Sodium picosulphate or polyethylene glycol before elective colonoscopy in outpatients? A systematic review and meta-analysis. World J Gastrointest Endosc. 2018;10(12):422–441.
8. Ribeiro IB, Moura DTHD, Thompson CC, Moura EGHD. Acute abdominal obstruction: colon stent or emergency surgery? An evidence-based review. World J Gastrointestinal Endoscopy. 2019;11(3):193–208.
9. Ribeiro IB, Bernardo WM, Martins BDC, et al. Erratum: colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6(5):C1.
10. DTH DM, BFBH DM, Manfredi MA, et al. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc. 2019;11(5):329–344.
11. Delgado AADA, Moura DTHD, Ribeiro IB, et al. Propofol vs traditional sedatives for sedation in endoscopy: A systematic review and meta-analysis. World J Gastrointest Endosc. 2019;11(11):573–588.
12. Ribeiro IB, DTH DM, Sachdev AH. Stent as a bridge to surgery for colonic obstruction: do we really need more systematic reviews with meta-analysis of the same articles? Gastrointest Endosc. 2019;90(4):704–705.
13. Guacho J, Ribeiro I, de Moura D, et al. Propofol versus midazolam sedation for elective endoscopy in patients with cirrhosis. a systematic review and meta-analysis of randomized controlled trials. 2020. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0040-1704586.
14. Proença I, Ribeiro I, de Moura D, et al. Fecal microbiota transplantation for metabolic syndrome and obesity: a systematic review and meta-analysis based on randomized clinical trials. 2020. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0040-1704624.
15. Sarre R. Bowel preparation. Aust Prescr. 2005;28(1):16–17.
16. Frommer D. Cleansing ability and tolerance of three bowel preparations for colonoscopy. Dis Colon Rectum. 1997;40(1):100–104.
17. Radaelli F, Paggi S, Hassan C, et al. Split-dose preparation for colonoscopy increases adenoma detection rate: a randomised controlled trial in an organised screening programme. Gut. 2017;66(2):270–277.
18. Mohamed R, Hilsden RJ, Dube C, Rostom A. Split-dose polyethylene glycol is superior to single dose for colonoscopy preparation: results of a randomized controlled trial. Can J Gastroenterol Hepatol. 2016;2016:1–6. doi:10.1155/2016/3181459
19. Rostom A, Dube C, Bishay K, Antonova L, Heitman SJ, Hilsden R A randomized clinical prospective trial comparing split-dose picosulfate/magnesium citrate and polyethylene glycol for colonoscopy preparation. Green J, editor. PLoS One []. 2019;14(3):e0211136.doi:10.1371/journal.pone.0211136
20. Kojecky V, Matous J, Keil R, et al. A head-to-head comparison of 4-L polyethylene glycol and low-volume solutions before colonoscopy: which is the best? A multicentre, randomized trial. Int J Colorectal Dis. 2017;32(12):1763–1766.
21. Mathus-Vliegen EMH, van der Vliet K, Wignand-van der Storm IJ. Split-dose bowel cleansing with picosulphate is safe and better tolerated than 2-l polyethylene glycol solution. Eur J Gastroenterol Hepatol. 2018;30(7):709–717.
22. Seo SI, Kang JG, Kim HS, Jang MK, Kim HY, Shin WG. Efficacy and tolerability of 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate: a randomized controlled trial. Int J Colorectal Dis. 2018;33(5):541–548.
23. Yoo IK, Lee JS, Chun HJ, et al. A randomized, prospective trial on efficacy and tolerability of low-volume bowel preparation methods for colonoscopy. Dig Liver Dis. 2015;47(2):131–137. doi:10.1016/j.dld.2014.10.019
24. Jeon SR, Kim HG, Lee JSJSJS, et al. Randomized controlled trial of low-volume bowel preparation agents for colonic bowel preparation: 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate. Int J Colorectal Dis. 2015;30(2):251–258. doi:10.1007/s00384-014-2066-9
25. Manes G, Amato A, Arena M, Pallotta S, Radaelli F, Masci E. Efficacy and acceptability of sodium picosulphate/magnesium citrate vs low-volume polyethylene glycol plus ascorbic acid for colon cleansing: A randomized controlled trial. Colorectal Dis. 2013;15(9):1145–1153.
26. Kim HG, Huh KC, Koo HS, et al. Sodium picosulfate with magnesium citrate (SPMC) plus laxative is a good alternative to conventional large volume polyethylene glycol in bowel preparation: A multicenter randomized single-blinded trial. Gut Liver. 2015;9(4):494–501. doi:10.5009/gnl14010
27. Hoy SM, Scott LJ, Wagstaff AJ. Sodium Picosulfate/Magnesium Citrate. Drugs. 2009;69(1):123–136.
28. Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207–1214.
29. Kilgore TW, Abdinoor AA, Szary NM, et al. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011;73(6):1240–1245.
30. Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: european Society of Gastrointestinal Endoscopy (ESGE) Guideline – update 2019. Endoscopy. 2019;51(08):775–794.
31. Bryant RV, Schoeman SN, Schoeman MN. Shorter preparation to procedure interval for colonoscopy improves quality of bowel cleansing. Intern Med J. 2013;43(2):162–168.
32. American Society of Anesthesiologists. &NA; practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 2011;114(3):495–511.doi:10.1097/ALN.0b013e3181fcbfd9
33. Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split-dose preparations are superior to day-before bowel cleansing regimens: a meta-analysis. Gastroenterology. 2015;149(1):79–88.
34. Saltzman JR, Cash BD, Pasha SF, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781–794.
35. Jin Z, Lu Y, Zhou Y, Gong B. Systematic review and meta-analysis: sodium picosulfate/magnesium citrate vs. polyethylene glycol for colonoscopy preparation. Eur J Clin Pharmacol. 2016;72:523–532.
36. Weir MA, Fleet JL, Vinden C, et al. Hyponatremia and sodium picosulfate bowel preparations in older adults. Am J Gastroenterol. 2014;109(5):686–694.
37. Marschall H-U, Bartels F. Life-threatening complications of nasogastric administration of polyethylene glycol-electrolyte solutions (Golytely) for bowel cleansing. Gastrointest Endosc. 1998;47(5):408–410.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
