Back to Journals » Clinical Ophthalmology » Volume 14
Effects of VEGF Inhibitor Conbercept on Corneal Neovascularization Following Penetrating Keratoplasty in Rabbit Model
Authors Liu H, Zhang XR , Xu HC, Ma Y, Huang LY , Zhai LY, Zhao Y
Received 7 May 2020
Accepted for publication 9 July 2020
Published 31 July 2020 Volume 2020:14 Pages 2185—2193
DOI https://doi.org/10.2147/OPTH.S260302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Huan Liu,1,2 Xiao-Rong Zhang,1,2 Hong-Chang Xu,1 Yue Ma,1 Li-Ying Huang,1 Li-Ying Zhai,1 Ying Zhao2
1Division of Ocular Injuries, Department of Ophthalmology, The Third Affiliated Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 2Hebei OPO Eye Bank, The Third Affiliated Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Xiao-Rong Zhang Email [email protected]
Purpose: To evaluate the effects of the vascular endothelial growth factor inhibitor conbercept (KH902) on corneal neovascularization and wound healing following penetrating keratoplasty in rabbits.
Methods: Conbercept was administered to New Zealand white rabbits through topical and subconjunctival routes. Corneal neovascularization and wound healing were examined by slit-lamp photography and histological analyses. The expressions of vascular endothelial growth factor inhibitor, α-smooth muscle actin, and keratocan in the corneal grafts were measured by real-time quantitative polymerase chain reaction (RT-qPCR).
Results: The anterior segment photographs demonstrated that corneal neovascularization started in the 2nd week. In the 4th week, histologically, the superficial corneal stroma layer showed disordered arrangement, and there were large numbers of dense inflammatory cells and blood vessels in the stroma layer. Vascular endothelial growth factor in the experimental groups was significantly decreased at all time points compared with the control group (both P = 0.001). Expression of α-smooth muscle actin in corneal grafts demonstrated an increase in time even it was lower in experimental groups, but the difference was not statistically significant (P equaled to 0.507 and 0.723, respectively). There were no significant differences with the expression of keratocan in all groups except that it significantly declined at the 4th week as to the second week in all groups and P values were 0.022, 0.020 and 0.014 in control (C), topical (E1), and subconjunctival (E2) group, respectively.
Conclusion: The study found that conbercept inhibited the formation of corneal neovascularization without affecting keratocan-mediated corneal wound healing and there were no significant differences between topical administration of different doses of conbercept on the rabbit corneal neovascularization after penetrating keratoplasty in this study.
Keywords: α-smooth muscle actin, conbercept, corneal neovascularization, keratocan, penetrating keratoplasty, vascular endothelial growth factor, VEGF
Introduction
Corneal disorders are a major cause of blindness worldwide. Keratoplasty is the most common surgical intervention used for the treatment of corneal blindness.1 However, despite the use of conventional immunosuppressants, graft rejection is a major risk factor associated with keratoplasty, particularly in high-risk patients with contraindications to corticosteroids.1–3 Previous reports have shown that the rate of graft rejection may be as high as 60–90%, depending on the criteria used to classify “high-risk”.4 One of the major factors causing graft rejection is corneal neovascularization (CNV). CNV is the formation of a new blood vessel outgrowth from the limbus that leads to the loss of transparency of the cornea, thus leading to graft failure.3,5 The development of CNV is mainly induced by chronic hypoxia and inflammation, which may be associated with the prolonged usage of contact lens, ocular trauma, uveitis, glaucoma, and various immunological disorders.6 The mechanism underlying CNV may be through the molecular pathways associated with angiogenesis.7 To date, due to the lack of specific treatments, selected immunosuppressive agents and topical steroids remain the widely used therapeutic interventions for the clinical management of CNV, despite their known adverse effects associated with long-term use.
Novel therapies targeting angiogenic (fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF)) and anti-angiogenic (angiostatin, endostatin, pigment epithelial-derived factor) factors have been previously employed in CNV, age-related macular degeneration (AMD) and certain other conditions.2,6,8 Currently, anti-VEGF agents are widely utilized in antiangiogenic therapy.2,8
A novel anti-VEGF agent, conbercept (KH902), a fusion receptor protein consisting of a human immunoglobulin Fc region and VEGF receptor extracellular domains, was developed by Chengdu Kanghong Biotechnology Co. Ltd., Chengdu, China, and approved for the treatment of wet AMD by the State Food and Drug Administration of China in December 2013. The chemical structure of conbercept is similar to that of aflibercept, which is an anti-VEGF agent that binds to all the isoforms of VEGF-A, VEGF-B, and placental growth factor (PIGF). However, conbercept has a higher affinity to VEGF compared to aflibercept because of an additional fourth Ig-like domain of VEGFR-2 in the Fab fragment, which improves the three-dimensional structure and increases the dimerization efficiency of conbercept.9,10
Thus, this study aims at evaluating the effects of conbercept on rabbit CNV following penetrating keratoplasty (PKP) by measuring the expression levels of VEGF. We also analyzed the expression of α-smooth muscle actin (α-SMA), a marker associated with myofibroblasts. Furthermore, we identified the corneal wound healing by analyzing the keratocan expression levels and the general histological changes in the rabbit eye. Many factors attributes to the wound healing. Keratocan, one of 3 principal keratan sulfate proteoglycans in the extracellular matrix with long keratan sulfate chains, is characteristically expressed in the cornea and it preserves corneal transparency and intensity.11 The core protein of keratocan is encoded by the KERA gene, which is a member of class II small leucine-rich repeat proteoglycan (SLRP) gene family.12 In the process of evolution, the keratocan expression has gradually reduced in most tissues except the cornea, and it is more regulated than the other members of the keratan sulfate proteoglycans family. Although keratocan in the cornea comprises only a few portions of the SLRPs, the absence of keratocan may cause.12 Wound healing in the cornea is a complicated process, and its mechanism including the underlying genetic factors are not fully understood yet.
Materials and Methods
Animals
All the animal study protocols for the experiments conducted were reviewed and approved by the Institutional Animal Care and Use Committee of Hebei Medical University, Hebei, China. The protocols adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. 108 New Zealand white adult rabbits, weighing 2.2–2.8 kg, were obtained from Animal Research Center of Hebei Medical University (Shijiazhuang, Hebei, China).
Experimental CNV Model
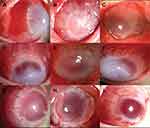
All the procedures were carried out using rabbits under general anesthesia by intravenous injection of pentobarbital sodium 2% (1.5 mL/kg). In total, 36 rabbits were sacrificed and both the eyes were enucleated, and these 72 corneas were harvested as donors for further corneal transplantation. Topical proparacaine (0.5%) was applied to the corneal surface and topical pilocarpine 1% (Wuhan Wujing Pharmaceutical Co. Ltd., Wuhan, China) was used to contract the pupil before conducting the surgical procedure. PKP was performed on the right eye of 72 rabbits. The sutures were placed and the knots tied, but not buried, to stimulate the formation of CNV (Figure 1).
After PKP, these 72 rabbits were randomly divided into 3 groups (n = 24), namely, control (C), topical (E1), and subconjunctival injection (E2) groups. All the rabbits were administered routine topical tobramycin eye drops (Alcon, Novartis, USA) every 2 hours/day for 2 weeks postoperatively including control group. In order to allow neovascularization to occur, no corticosteroid eye drops were administered postoperatively as is routinely done following surgery. While in the E1 group, topical conbercept (5%) were administered 4 times a day, in the E2 group, a subconjunctival injection (0.1 mL) of 10% conbercept was administered only once a week during the 8 weeks of this study.
Slit-Lamp Photographic Data and General Histology
All the rabbits were examined by slit lamp every other day for the first 2 weeks to observe the corneal wound healing and the formation of neovascularization, followed by examination once a week in this study. Corneal graft samples obtained at the 2nd, 4th, 6th, and 8th weeks were processed for light microscopy. These samples were embedded in paraffin blocks to obtain 5 μm cross-sections for hematoxylin and eosin (H & E) staining.
RNA Isolation and RT-qPCR of Corneal Grafts
6 rabbits from each group were sacrificed at the end of the 2nd, 4th, 6th, and 8th weeks postoperatively. The corneal grafts were extracted and stored at −80 °C until they were analyzed. Total RNA was isolated from the pooled rabbit corneal grafts in each group using an RNA extraction kit (Beijing Zhuangmeng International Bio-Gene Technology Co. Ltd., Beijing, China). After confirming the purity and structural integrity of the total RNA samples using NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and 1.5% agarose gel electrophoresis, respectively, 5 µg of RNA from each group was reverse transcribed using Reverse Transcriptase Kit (M-MLV) (Beijing Zhuangmeng International Bio-Gene Technology Co. Ltd., Beijing, China), according to the manufacturer’s protocol. The obtained cDNA was stored at −20 °C for further real-time quantitative polymerase chain reaction (RT-qPCR). qPCR was then performed using 0.5 µL cDNA as a template and the primers listed in Table 1 (Bosheng Chemical Limited Co., Shijiazhuang, China). Subsequent amplification was performed using the fast RT-PCR system (ABI 7500, Blue Lion Biotech, Carnation, WA, USA). The amplification protocol consisted of 94 °C for 3 minutes; followed by 40 cycles of 94 °C for 15 seconds of denaturation, 60 °C (30 seconds) of annealing, and 72 °C (30 seconds) of extension.
 |
Table 1 Specific PCR Primers Used for the Amplification of the VEGF, α-SMA and Keratocan |
Assessment of Corneal VEGF, α-SMA and Keratocan Levels
The expressions of VEGF, α-SMA, and keratocan in the cornea were measured by RT-qPCR using commercial primers (Table 1). GAPDH was used as an internal reference. The values for VEGF, α-SMA, and keratocan expression were analyzed in comparison to the values for GAPDH expression. The gene expression of the samples was expressed by the cycle threshold (Ct). ΔCt was calculated by the difference between the Ct of the target gene for each sample and the Ct of the internal reference, ΔΔCt was the difference between ΔCt between the different groups of the sample, which was expressed as 2−ΔΔCt, relative to the expression of the gene.
Statistical Analysis
Statistical analyses were performed on the computer (SPSS, ver. 6.1; SPSS Science, Chicago, IL, USA). The results were expressed as mean ± standard deviation (M ± SD). The experimental groups were compared with the control group using the one-way analysis of variance (ANOVA) test. The values with P < 0.05 were considered to be statistical significance.
Results
Morphologic Evaluation
Throughout the 8 weeks duration of this study, we recorded the morphologic changes in the corneal grafts obtained from groups C, E1, and E2 by slit-lamp examination (Figure 1). The images of the anterior segment show that CNV started in the 2nd week, postoperatively. Slit-lamp examination showed that the corneal grafts in the control group developed numerous blood vessels (Figure 1A-C) as compared to the grafts in groups E1 (Figure 1D-F) and E2 (Figure 1G-I) at the 2nd, 4th, and 8th week, respectively. Neovascularization seemed to be most prominent in the 4th week in all the experimental groups (Figure 1B-H), and it decreased by the 8th week postoperatively (Figure 1C-I). The histological examination of the control group revealed that the collagen fibers were arranged neatly, the corneal stroma had inflammatory cells infiltration and neovascularization had developed especially in the superficial stroma layer at 2nd week (Figure 2A). In the 4th week, the superficial corneal stroma layer showed disordered arrangement, and there was a large number of dense inflammatory cells and blood vessels in the stroma layer (Figure 2B). In the 6th week, derangement of collagen fibers, a monolayer of endothelial cells near the neovascular lumen, and the accumulation of inflammatory cells around the neovascularization were observed (Figure 2C). In the 8th week, in addition to the derangement of collagen fibers, significant fibroblast proliferation, and scar formation were seen. The number of inflammatory cells and blood vessels decreased (Figure 2D). As compared to the control group, fewer inflammatory cells and blood vessels were observed in the corneal stroma layer in E1 (Figure 2E-H) and E2 (Figure 2I-L) groups at the same time points.
Expression of VEGF mRNA in the Corneal Grafts
The expression of corneal VEGF in the control and experimental groups increased at first and then declined with time, with the highest level of VEGF was recorded in the 4th week during the 8 weeks study (Figure 3). The expression of VEGF was then declined at all checked time points, as compared to the control group, the E1 and E2 groups both showed significant differences (both P = 0.001, ANOVA) (Table 2). There were no significant differences in the expression of VEGF between the experimental E1 and E2 groups (P = 0.805).
 |
Table 2 The Expression of VEGF mRNA in the Cornea Grafts of Rabbits |
 |
Figure 3 Changes of corneal VEGF mRNA expression in different groups. |
Expression of α-SMA in the Corneal Grafts
A time-dependent increase in the levels of α-SMA was observed in all the groups (Figure 4). In general, the expression levels of α-SMA in all the experimental groups tended to be somewhat lower than that in the control group at all the 4 time points of 2nd, 4th, 6th and 8th week, however, the differences were not statistically significant, control vs E1 group (P = 0.507), control vs E2 group (P = 0.723) (Table 3). The data for the expression of α-SMA in the corneal grafts are summarized in Table 3.
 |
Table 3 The mRNA Expression of α-SMA in the Cornea of Rabbits |
 |
Figure 4 The changes of expression of corneal α-SMA in different groups after PKP. |
Expression of Keratocan in the Corneal Grafts
The details of keratocan expression in the corneal grafts are outlined in Table 4. The keratocan expression levels declined with time in the control, E1, and E2 groups (Figure 5). As compared to the control group, both the E1 and E2 groups did not show significant differences (P= 0.832 and 0.740, respectively) (Table 4). Keratocan expression significantly dropped in the 4th week as compared with the 2nd week in all groups (P = 0.022, 0.020, and 0.014 in groups C, E1, and E2 respectively). Then it reduced from the 6th week onwards.
 |
Table 4 The mRNA Expression of Keratocan in the Cornea of Rabbits |
 |
Figure 5 Changes of corneal keratocan mRNA expression in different groups. |
Discussion
Avascularity is crucial for corneal transparency and maintenance of visual acuity.2–4,13 Numerous antiangiogenic systems regulating corneal avascularity and thus facilitating clear vision and angiogenic prerogative, have been previously identified.14 CNV causes various ocular surface disorders13,15,16 that may be clinically challenging and lead to a significant loss of visual function.2,17 VEGF is one of the most important factors involved in the pathogenesis of CNV. Various members of the human VEGF family, such as VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF), have been previously identified.18 Receptor tyrosine kinases (RTKs) of the VEGF receptors, VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4), communicate with the respective VEGFs.18 The most significant member of the VEGF family, VEGF-A, plays an important role in angiogenesis by binding to VEGFR-2, and thus stimulates the process of migration and proliferation of the vascular endothelial cells, as well as increasing the dilation and permeability of blood vessels.19–21 VEGFs mediate the development of lymphatic and blood vessels by regulating angiogenesis. Furthermore, they are upregulated in inflamed and vascularized corneas in both, animals and human beings.22 Similarly, the binding of VEGF-C or VEGF–D to VEGFR-2 or VEGFR −3 accelerates lymphangiogenesis.23,24 VEGFs also act as chemo-attractants for inflammatory cells like macrophages that generate additional pro-angiogenic factors.25
Current clinical strategies for the management of CNV mainly involve the use of non-steroidal anti-inflammatory agents and corticosteroids. However, serious adverse effects, such as corneal melting and elevated intraocular pressure, have been associated with the prolonged use of these drugs.2,17,26 In this study, to better observe the effects of conbercept (KH902) on CNV in the rabbit model, corticosteroid eye drops were not administered postoperatively, as routinely advised. The alternative treatments for CNV involve fine-needle diathermy, laser photocoagulation, and photodynamic therapy;27–29 however, corneal stromal injuries and regrowth of blood vessels are often associated with these procedures. Surgical interventions for recovering the ocular surface, such as amniotic membrane transplantation, have also been investigated and have variable degrees of success in CNV treatment.30
Recent studies have suggested that drugs targeting VEGF may have therapeutic potential in CNV.18,31-33 Anti-VEGF agents, ranibizumab and bevacizumab, are humanized monoclonal antibodies that selectively bind to the isoforms of VEGF-A and thus inhibit it efficiently.34 Bevacizumab was initially approved for the treatment of metastatic colorectal cancer. For use in CNV, the preferred route of administration is generally topical. Several studies have examined the dose-response relationship of bevacizumab administered topically for the management of CNV.35,36 However, Kim et al37 have the use of topical bevacizumab (1.25%) for 2 months may lead to the loss of integrity of the corneal epithelium and stromal thinning. Thus, it is important to be cautious while using topical bevacizumab in patients with a deficiency of corneal epithelial for a prolonged period. In addition, inhibiting new blood vessel development by neutralizing VEGF-A has been shown to enhance corneal graft survival in various animal models.38 Specifically, ranibizumab has been previously shown to significantly lower CNV in rats.39 Owing to its lower molecular weight (48 kD) and size (1/3rd) compared to bevacizumab, ranibizumab can penetrate the cornea more efficiently and thus manage CNV more effectively than bevacizumab.17 Topical ranibizumab also diminishes the severity of CNV by decreasing the caliber of blood vessels.15 It has been reported that conbercept significantly improves visual acuity and enhances anatomical outcomes in patients with neovascular AMD, polypoidal choroidal vasculopathy (PCV), and other ocular neovascular disorders.8–10,40,41 But the efficacy of conbercept in the treatment of CNV has not been widely reported yet.42,43
A previous study on conbercept demonstrated that it significantly inhibits the formation of new blood vessels and promotes the recovery of existing vessels in a mouse model of alkali burn-induced CNV, and the cornea tissue shows a reduction in the levels of PIGF and VEGF.42 In this study, we found that VEGF expression in corneal grafts in all the groups increased with time, and the peak levels were seen in the 4th week postoperatively, and then dropped gradually. This trend may be related to the severity of corneal inflammation associated with surgical wound healing. Wu et al43 reported that topical conbercept is an effective therapeutic intervention in the alkali burn-induced rabbit model of CNV. The authors also reported that the inhibition of VEGF expression gradually enhances with an increase in the concentrations of conbercept. But there were no significant differences between the groups of different concentrations.
In this study, we found that corneal repairing and neovascularization were very prominent before the 4th week. After the 4th week, it got into a renovating period where the concentration of VEGF and other inflammatory factors in corneal tissue started decreasing gradually, thus leading to the regression of immature neovascularization but had no significant effects on mature neovascularization, and the severity of CNV declined. The changes of CNV observed by slit lamp were consistent with the changes of VEGF measured in the experiment.
Activation of myofibroblasts starts following various injuries to repair damaged extracellular matrix (ECM) in cornea. The basic processes regulating myofibroblast formation and its function are quite similar in all tissues.44 Fibroblasts differentiate into myofibroblasts in granulation tissue, which are characterized by α-SMA expression. The induction of α-SMA in corneal stromal cells promotes their contraction for wound closure and extracellular matrix remodeling.45 Our study revealed that the expression of α-SMA in all of the experimental groups tended to be somewhat lower than that in the control group during the 8 weeks experiment, however, the differences were not statistically significant as comparing to the control group.
We found that the keratocan expression declined with time in all the experimental groups including the control group following PKP. There were no other significant differences between groups except the significant decline in keratocan expression in the 4th week as compared to the 2nd week. This indicated that surgical injury to the cornea led to a decrease in the corneal stromal cells following PKP and a simultaneous increase in keratocan expression. After the initial keratocyte apoptosis, increasing numbers of cells experience the pro‐inflammatory process of necrosis. Migration and proliferation of the existing keratocytes proceeded from 12 to 24 hours, which aroused activated keratocytes, fibroblasts, and myofibroblasts that were responsible for the regrowth of the exhausted stromal cells.46 This study did not find the different concentrations of topical conbercept affecting the corneal transparency by regulating keratocan mediated corneal wound healing.
Conclusion
The serious condition of CNV has long been identified but effective therapies are still lacking. In this preliminary study, we demonstrated that conbercept inhibited the formation of CNV following PKP without affecting the wound healing process in the rabbit model of CNV. There were no significant differences in the efficacy of different concentrations of topical conbercept used postoperatively. There is still a need for quantifying the histology and microscopy data in the future. The efficacy and safety of topical conbercept against CNV in humans need further investigation.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. George AJ, Larkin DF. Corneal transplantation: the forgotten graft. Am J Transplant. 2004;4(5):678–685. doi:10.1111/j.1600-6143.2004.00417.x
2. Roshandel D, Eslani M, Baradaran-Rafii A, et al. Current and emerging therapies for corneal neovascularization. Ocul Surf. 2018;16(4):398–414. doi:10.1016/j.jtos.2018.06.004
3. Zhai LY, Zhang XR, Liu H, Ma Y, Xu HC. Observation of topical tacrolimus on high-risk penetrating keratoplasty patients: a randomized clinical trial study. Eye. 2019. doi:10.1038/s41433-019-0717-3
4. Joseph A, Raj D, Shanmuganathan V, Powell RJ, Dua HS. Tacrolimus immunosuppression in high-risk corneal grafts. Br J Ophthalmol. 2007;91(1):51–55. doi:10.1136/bjo.2006.097428
5. Dana MR, Schaumberg DA, Kowal VO, et al. Corneal neovascularization after penetrating keratoplasty. Cornea. 1995;14(6):604–609.
6. Ahmed A, Berati H, Nalan A, Aylin S. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Exp Ophthalmol. 2009;37(7):730–736. doi:10.1111/j.1442-9071.2009.02112.x
7. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med. 1995;1(1):27–31. doi:10.1038/nm0195-27
8. Liu K, Song Y, Xu G, et al. Conbercept for treatment of neovascular age-related macular degeneration: results of the randomized Phase 3 PHOENIX study. Am J Ophthalmol. 2019;197(1):156–167. doi:10.1016/j.ajo.2018.08.026
9. Peng Y, Zhang X, Mi L, et al. Efficacy and safety of conbercept as a primary treatment for choroidal neovascularization secondary to punctate inner choroidopathy. BMC Ophthalmol. 2017;17(1):87. doi:10.1186/s12886-017-0481-8
10. Zhang M, Zhang J, Yan M, et al. A Phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2011;118(4):672–678. doi:10.1016/j.ophtha.2010.08.008
11. Tasheva ES, Funderburgh JL, Corpuz LM, Conrad GW. Cloning, characterization and tissue-specific expression of the gene encoding bovine keratocan, a corneal keratan sulfate proteoglycan. Gene. 1998;218(1–2):63–68. doi:10.1016/S0378-1119(98)00390-4
12. Chakravarti S. Focus on molecules: keratocan (KERA). Exp Eye Res. 2006;82(2):183–184. doi:10.1016/j.exer.2005.09.009
13. Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19(2):125–133. doi:10.1016/j.semcdb.2007.08.014
14. Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing. Trans Am Ophthalmol Soc. 2006;104:264–302.
15. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43(3):245–269. doi:10.1016/S0039-6257(98)00035-6
16. Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother. 2007;41(4):614–625. doi:10.1345/aph.1H316
17. Ferrari G, Dastjerdi MH, Okanobo A, et al. Topical ranibizumab as a treatment of corneal neovascularization. Cornea. 2013;32(7):992–997. doi:10.1097/ICO.0b013e3182775f8d
18. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. doi:10.1096/fasebj.13.1.9
19. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi:10.1038/nm0603-669
20. Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200(6):581–597. doi:10.1046/j.1469-7580.2002.00066.x
21. Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274(4):H1054–1058. doi:10.1152/ajpheart.1998.274.3.H1054
22. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41(9):2514–2522.
23. Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15(7):290–298. doi:10.1002/j.1460-2075.1996.tb00359.x
24. Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA. 1998;95(2):548–553. doi:10.1073/pnas.95.2.548
25. Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–1050. doi:10.1172/JCI20465
26. Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative corneal transplantation studies research group. Ophthalmology. 1994;101(9):1536–1547.
27. Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41(8):2148–2153.
28. Sheppard JD, Epstein RJ, Lattanzio FA, Marcantonio D, Williams PB. Argon laser photodynamic therapy of human corneal neovascularization after intravenous administration of dihematoporphrin ether. Am J Ophthalmol. 2006;141(3):524–529. doi:10.1016/j.ajo.2005.11.003
29. Yoon KC, Im SK, Park HY. Recurrent herpes simplex keratitis after Verteporfin photodynamic therapy for corneal neovascularization. Cornea. 2010;29(4):465–467. doi:10.1097/ICO.0b013e3181b53310
30. Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12(2):242–249. doi:10.1097/00055735-200108000-00002
31. Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57(5):415–429. doi:10.1016/j.survophthal.2012.01.007
32. Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39(1):18–22.
33. Doctor PP, Bhat PV, Foster CS. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008;27(9):992–995. doi:10.1097/ICO.0b013e31817786ad
34. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi:10.1038/nrd1381
35. Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127(4):381–389. doi:10.1001/archophthalmol.2009.18
36. Koenig Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin®) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1375–1382. doi:10.1007/s00417-009-1099-1
37. Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115(6):e33–38. doi:10.1016/j.ophtha.2008.02.013
38. Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45(8):2666–2673. doi:10.1167/iovs.03-1380
39. Sener E, Yuksel Y, Yildiz DK, et al. The impact of subconjunctivally injected EGF and VEGF inhibitors on experimental corneal neovascularization in rat model. Curr Eye Res. 2011;36(11):1005–1013. doi:10.3109/02713683.2011.601840
40. Qu J, Cheng Y, Li X, Yu L, Ke X, AURORA Study Group. Efficacy of intravitreal injection of conbercept in polypoidal choroidal vasculopathy: subgroup analysis of the Aurora study. Retina. 2016;36(5):926–937. doi:10.1097/IAE.0000000000000875
41. Li X, Xu G, Wang Y, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized Phase 2 study: AURORA study. Ophthalmology. 2014;121(9):1740–1747. doi:10.1016/j.ophtha.2014.03.026
42. Zhou AY, Bai YJ, Zhao M, Yu WZ, Li XX. KH902, a recombinant human VEGF receptor fusion protein, reduced the level of placental growth factor in alkali burn induced-corneal neovascularization. Ophthalmic Res. 2013;50(3):180–186. doi:10.1159/000353437
43. Wu Y, Xue C, Lu Y, Huang Z. The inhibitory effect of different concentrations of KH902 eye drops on corneal neovascularization induced by alkali burn. Indian J Ophthalmol. 2017;65(11):1127–1132. doi:10.4103/ijo.IJO_339_17
44. Hinz B. Myofibroblasts. Exp Eye Res. 2016;142(1):56–70. doi:10.1016/j.exer.2015.07.009
45. Chen J, Li H, SundarRaj N, Wang JH. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil Cytoskeleton. 2007;64(4):248–257. doi:10.1002/cm.20178
46. Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18(4):529–551. doi:10.1016/S1350-9462(98)00033-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.