Back to Journals » Patient Preference and Adherence » Volume 17
Effects of Telephone-Based Brief Motivational Interviewing on Self-Management, Medication Adherence, and Glycemic Control in Patients with Uncontrolled Type 2 Diabetes Mellitus in a Rural Community in Thailand
Authors Sawaengsri N, Maneesriwongul W , Schorr EN, Wangpitipanit S
Received 18 May 2023
Accepted for publication 10 August 2023
Published 24 August 2023 Volume 2023:17 Pages 2085—2096
DOI https://doi.org/10.2147/PPA.S418514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Naruemon Sawaengsri,1 Wantana Maneesriwongul,1 Erica N Schorr,2 Supichaya Wangpitipanit1
1Ramathibodi School of Nursing, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2School of Nursing, University of Minnesota, Minneapolis, MN, USA
Correspondence: Wantana Maneesriwongul, Ramathibodi School of Nursing, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Thung Phaya Thai, Ratchathewi, Bangkok, 10400, Thailand, Tel +662 2011600, Fax +662 2012858, Email [email protected]
Introduction: Owing to the increased prevalence of type 2 diabetes mellitus (T2DM) and the high proportion of patients with uncontrolled T2DM, effective interventions for disease management are needed.
Objective: The study aim was to test the effects of brief motivational interviewing (MI) on patients’ self-management, medication adherence, and glycemic control.
Methods: A single-group repeated measures trial was used to examine the effects of usual care only and usual care plus telephone-based brief MI. Participants were 29 patients with uncontrolled T2DM recruited from a rural primary care setting in Nakhon Sawan, Thailand. Participants received usual care during the first 4 weeks, followed by usual care plus brief MI during weeks 4– 8. Outcomes of self-management, medication adherence, fasting blood sugar (FBS) levels, and hemoglobin A1c (HbA1c) levels were assessed at baseline, 4 weeks, and 8 weeks. Data were analyzed using descriptive statistics, one-way repeated measures analysis of variance, and Friedman test.
Results: Significant changes in self-management (p < 0.001), medication adherence (p < 0.001), and FBS (p < 0.05) were observed over the 8-week study. In multiple comparisons, self-management was the only parameter significantly different across baseline, 4, and 8 weeks (p < 0.05, < 0.001, and < 0.001, respectively); medication adherence was significantly different between 4 and 8 weeks (p < 0.05), and between baseline and 8 weeks (p < 0.001); and FBS was significantly different between 4 and 8 weeks (p < 0.05). HbA1c declined over the 8-week study, but not significantly.
Conclusion: An intervention combining telephone-based brief MI with usual care significantly increased self-management, medication adherence, and glycemic control (ie, FBS) after 4 weeks, whereas usual care only significantly increased self-management. Phone-based brief MI may be an effective way for healthcare providers to remotely enhance patients’ self-management and glycemic control, thus reducing barriers related to time and geographic location.
Keywords: motivational interviewing, tele-intervention, stage of change, medication adherence, glycemic control, diabetes, T2DM
Introduction
Diabetes mellitus (DM) is now the ninth-leading cause of death worldwide and causes more than 1 million deaths per year.1 The global prevalence of type 2 DM (T2DM) is estimated to increase from 6,059 cases per 100,000 in 2017 to 7,079 cases per 100,000 by 2030.2 This substantial increase in the burden of T2DM constitutes a major global public health problem,2 because poor glycemic control can lead to target organ damage, including to the heart, blood vessels, eyes, kidneys, and peripheral nerves;3 increase the risk of serious complications;4 and overwhelm healthcare systems.5 The increased risk of all-cause mortality associated with DM is higher among people living in rural areas,6 probably owing to undiagnosed and inadequate DM management leading to poor glycemic control.6–8
Suboptimal or poor glycemic control has been linked to poor self-management9 and partly attributed to a lack of awareness.10 It is important that patients with DM are self-aware11 and self-motivated12 so that they feel responsible for developing a feasible and practical self-care plan to achieve glycemic control.11 Patients with better self-management practices are more likely to achieve glycemic control13 and thereby reduce the risk of serious DM complications.14 Several systematic reviews have reported that DM self-management substantially reduces hemoglobin A1c (HbA1c) level and improves glycemic control.15–17 Patient self-management comprises a range of behaviors and activities to control disease.18–20 It requires intrinsic motivation21 and self-confidence, which can be developed through motivational interviewing (MI).21–23
MI is “a collaborative, person-centered form of guiding to elicit and strengthen motivation for change” (p.137).24 It uses supportive and empathic counseling styles that are based on the approach of Rogers (1972).25 MI also draws on Bem’s self-perception theory (1972)26 and Janis and Mann’s decisional balance theory (1977).27 MI interventions focus on enhancing an individual’s intrinsic motivation to engage in healthy behaviors by developing their self-efficacy and confidence in their ability to change.23 MI conceptualizes the readiness to change and the stages of change using the framework of the transtheoretical model of behavioral change.28 MI interventions can be used to help individuals to overcome ambivalence and accomplish the various tasks required to move from the precontemplation stage to the maintenance stage of change. Recently, interest in MI in the DM behavioral field has increased substantially.29 Various studies have used MI to increase individuals’ intrinsic motivation to enhance DM self-management and glycemic control.30,31 Several systematic reviews have reported empirical evidence that MI is an effective intervention to improve DM management behaviors such as physical activities,31 diet and weight management,31,32 DM self-management,33,34 medication adherence,31 and glycemic control.33–35
A brief MI (Brief MI) (ie, 30-min sessions) method has been developed to facilitate behavioral change in brief consultations. This can be used in consultations when time is limited. Brief MI has been reported to have good outcomes36 and can be as effective as full-length MI in enhancing patient motivation to achieve glycemic control.21 Brief MI sessions have also been shown to improve DM self-management and glycemic control.37–41
In Thailand, the prevalence of DM increased from 8.9% in 2014 to 9.5% in 2020.42 According to the Thai Health Data Center, the proportion of patients with poorly controlled T2DM and the mortality rate in a remote community of Than Thahan subdistrict, in Nakhon Sawan province (76.92%; 3.24%, respectively), are substantially higher than those reported in the whole district (66.38%; 2.55%), the whole province (66.22%; 2.11%), and the whole country (74.52%; 1.27%).43 The high rate of suboptimal glycemic control in remote communities may increase the risk of all-cause mortality owing to serious DM complications44 and overwhelming rural healthcare systems.45 Therefore, effective interventions that are appropriate for primary care settings (such as health promoting hospitals (HPH), which are primary care service facilities available at the subdistrict level in Thailand) are needed to prevent suboptimal glycemic control in people with DM in Than Thahan subdistrict.
To our knowledge, there is only one study from Thailand that used usual care plus a brief MI intervention in patients with uncontrolled T2DM in a secondary care hospital. The results indicated that the intervention substantially improved knowledge, medication adherence, and fasting blood sugar (FBS) level.41 For people living in rural communities, distance and lack of transportation are major barriers to healthcare access and delivery. Thus, in this study, we examined the effects of an intervention using usual care plus telephone-based brief MI among patients with uncontrolled T2DM living in a rural community. We hypothesized that usual care plus telephone-based brief MI would have significant effects on self-management, medication adherence, and glycemic control.
Materials and Methods
A single-group repeated measures trial was conducted to examine the effects of usual care only and usual care plus telephone-based brief MI.
Participants
The target population was patients with uncontrolled T2DM in Nakhon Sawan province, in central Thailand. Potential participants were recruited from the HPH in Than Thahan subdistrict, which is in a rural setting.
Eligible patients with uncontrolled T2DM were recruited according to the following inclusion criteria: (1) aged >35 years; (2) a diagnosis of T2DM for at least 12 months; (3) poor glycemic control, defined as HbA1c ≥7%; (4) receiving oral hypoglycemic medications for at least 6 months; (5) no severe medical comorbidity; (6) owns a mobile phone for telephone consultations; (7) able to communicate in Thai; and (8) willing to participate in the study and sign a consent form. Patients were excluded if they had severe complications such as myocardial infarction, had received insulin injections, or were not able to complete the study.
The sample size was calculated based on the findings from a previous study.46 Based on a power analysis using a power of 0.80 and a significance level of 0.05, the estimated minimum sample size needed for this study was 25 patients.47 Of 32 eligible patients, 30 patients voluntarily agreed to participate and signed a participant consent form. After 4 weeks following enrollment, one participant dropped out because of work commitments (see Figure 1).
 |
Figure 1 Study flow chart. |
Study Intervention
Participants received usual care only during the first 4 weeks, followed by usual care plus telephone-based brief MI during weeks 4 to 8 (Table 1).
 |
Table 1 Intervention Protocol and Assessments |
For the usual care, participants received a regular follow-up visit at a DM clinic. The visit included an assessment of blood pressure and blood sugar; brief health advice on diet, physical activity, medications, and adherence to medication and follow-up appointments; a prescription refill; and a scheduled appointment for the next follow-up visit.
For the usual care plus telephone-based brief MI, the structure of the telephone-based brief MI was adapted from the protocol developed by Arunsangsod et al48 and Pratuangtham and Jerawatana (2018),49 and was based on the MI principles of Miller and Rollnick23 and the stages of change of the transtheoretical model of behavioral change.28 This model describes five different stages of change that individuals move through: 1) precontemplation (unaware of change), 2) contemplation (considering change), 3) preparation (determining and preparing to take action within 30 days), 4) action (adopting healthy behaviors or ceasing unhealthy behaviors and maintaining the new behavior over the last 6 months), and 5) maintenance (maintaining the new behavior for over 6 months).28
In this study, the telephone-based brief MI comprised four sessions provided by the first author (N.S.), who received MI training from an advanced practice nurse at a DM clinic and from online training courses. The first session was conducted at week 4. Each participant received an individual face-to-face session during an in-person visit to the HPH. During the initial session, each individual’s stage of change was assessed and the MI intervention was targeted according to the participant’s current behavioral stage. Participants then received two follow-up sessions via telephone during weeks 5 and 7. At the completion of 8 weeks, participants received their last session in person at the HPH (Table 1).
Outcomes and Measurement
Primary study outcomes included patient-reported outcomes (ie, self-management and medication adherence) and laboratory parameters of glycemic control (ie, FBS and HbA1c). In this study, self-management and medication adherence were measured using self-administered questionnaires.
Self-management was assessed using the Thai version of the revised Summary of Diabetes Self-Care Activities scale (Thai-revised SDSCA) translated using back-translation19 from the original version of Toobert et al.18 The SDSCA in Thai comprises 19 items on five subscales: diet (7), physical activity (2), self-monitoring (3), foot care (5), and medication taking (2). Respondents rate their performance of these DM self-care activities during the last 7 days using a scale that ranges from 0 to 7. There are 15 positive and 4 negative items.20 Negative items are scored reversely so that higher sum scores indicate a higher level of DM self-management. The Thai-revised SDSCA has a Cronbach’s alpha reliability of 0.76 to 0.77.50,51
Medication adherence was assessed using the 30-day medication adherence visual analogue scale (VAS). The VAS is an inexpensive and valid tool that measures medication adherence among patients with chronic conditions and has been used in both research and primary care settings.52,53 Scores on this scale strongly correlate with clinical outcomes.52,54 Participants were asked to place a mark on a horizontal line of 100 mm to indicate their best estimation of the proportion of medication doses taken over the last 30 days. The scale ranges from 0% (no medication adherence) to 100% (perfect medication adherence).
FBS and HbA1c levels were used to evaluate participants’ glycemic control. These were measured at the Nong Bua Hospital laboratory, which is regularly accredited by the Thailand Medical Laboratory Accreditation System. FBS level was measured using the Horiba ABX Pentra 400 analyzer (norm 70–115 mg/dl). HbA1c was measured using a Labotron LD 620 analyzer (norm 4.0–6.2%).
Ethical Considerations
The study was approved by the ethics committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Thailand (COA.MURA2022/182). The study was conducted according to the Declaration of Helsinki. All participants provided informed consent prior to participation.
Data Collection
Following ethical approval and the permission to conduct this study from the Nakhon Sawan Provincial Health Office, the lead researcher contacted the Director of Nong Bua District Hospital and the HPH of Than Thahan subdistrict to inform them of the study. Patients with T2DM registered in the Than Thahan registry were screened by a nurse working at the HPH. Potential participants were recruited and provided their informed consent prior to participation. A total of 30 patients with uncontrolled T2DM met the inclusion criteria and agreed to participate in the study. Data were collected at three time points during the study: baseline, 4 weeks, and 8 weeks. A flow chart of the study is shown in Figure 1.
Statistical Analysis
Data analysis was conducted using SPSS 18.0. One participant dropped out because of a work transfer, so 29 participants were included in the data analysis. To test whether the data were normally distributed, the skewness and kurtosis were analyzed. Standardized values of skewness and kurtosis within −1.96 and 1.96 indicate a normal distribution.55,56 Background characteristics and health outcomes were analyzed using descriptive statistics (ie, frequency, percentage, range, mean, and standard deviation (SD)). If assumptions for normality were met, a one-way repeated measures analysis of variance was used to test changes in each hypothesized outcome over the three study time points (baseline, 4 weeks, and 8 weeks). Friedman test was used for non-normally distributed data.
Results
Demographic and Clinical Characteristics
Of the 221 patients with T2DM who were screened, 30 patients with uncontrolled T2DM met the inclusion criteria and agreed to participate in this study. One participant dropped out 4 weeks after enrollment. There were 29 participants at the completion of the 8-week study. Participants were aged between 40 and 72 years (mean = 60.45, SD = 7.70 years). Most participants were women and were married (72.4%). Most of the participants (86.3%) had universal healthcare insurance and the average duration of T2DM was 6.95 years (SD = 4.68 years). Nearly all participants had one or more comorbidities (96.6%) and one-fifth had DM complications (20.7%). Table 2 provides details of the participant background characteristics.
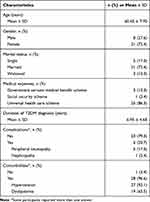 |
Table 2 Background Characteristics of the Participants (N = 29) |
Stages of Change
At the baseline assessment (pre-intervention), participants were in various stages of behavioral change: precontemplation stage (n = 2), contemplation stage (n = 7), preparation stage (n = 12), and action stage (n = 8). After receiving one telephone-based brief MI session, all participants moved to the action stage and remained in the action stage throughout the duration of the study.
Intervention Effects
Table 3 shows the effects of the intervention, which comprised patient-reported outcomes (self-management and medication adherence) and laboratory parameters of glycemic control (FBS and HbA1c).
 |
Table 3 Means and Mean Differences at Baseline, 4-Weeks, and 8-Weeks After Enrollment (N = 29) |
Effects on Patient-Reported Outcomes
Self-Management
The findings indicated a significant improvement over 8 weeks in overall self-management scores (F = 54.598, p < 0.001) and self-management subscale scores: dieting (χ2 = 34.579, p < 0.001), physical activity (F = 14.711, p < 0.001), self-monitoring (χ2 = 18.075, p < 0.001), foot care (χ2 = 21.664, p < 0.001), and medication taking (χ2 = 7.969, p < 0.05).
Multiple comparisons showed that individuals improved significantly in overall self-management scores at baseline, 4 weeks, and 8 weeks (p < 0.05, p < 0.001, and p < 0.001, respectively). For each of the self-management subscales, the multiple comparisons for dieting, physical activity, self-monitoring, and foot care showed no statistically significant differences between baseline and 4 weeks (p > 0.05), but significant differences (ie, improvements) were found between 4 and 8 weeks (p < 0.001, p < 0.001, p < 0.05, and p < 0.05, respectively), and between baseline and 8 weeks (p < 0.001, p < 0.05, p < 0.001, and p < 0.001, respectively). Multiple comparisons for scores on the medication-taking subscale of self-management showed significant improvements between 4 and 8 weeks (p < 0.05), whereas the differences between baseline and 4 weeks, and between baseline and 8 weeks, were not significant (all p > 0.05).
Medication Adherence
The findings also indicated significant improvement over 8 weeks in medication adherence (assessed by VAS scores) (χ2 = 17.354, p < 0.001). Multiple comparisons for medication adherence showed no significant improvement between baseline and 4 weeks (p > 0.05), but the differences between 4 and 8 weeks (p < 0.05), and between baseline and 8 weeks (p < 0.001), were significant. Table 3 shows details of the multiple comparisons of the patient-reported outcomes at different time points.
Effects on Laboratory Parameters
The glycemic control laboratory parameters assessed were FBS and HbA1c levels. Significant changes over 8 weeks in FBS were observed (χ2 = 6.591, p < 0.05). Although HbA1c level tended to decline over 8 weeks, the change across the time points was not significant (F = 2.706, p > 0.05). However, this is not unexpected, as lowering HbA1c levels is a gradual process that requires a minimum of 8 to 12 weeks to observe significant changes.
Multiple comparisons of FBS level showed no statistically significant change between baseline and 4 weeks (p > 0.05), but there was a significant change in FBS level between 4 and 8 weeks (p < 0.05). Although FBS level tended to decline between baseline and 8 weeks, the difference was not significant (p = 0.057) (see Table 3).
Figure 2 shows changes in the mean scores on self-management, medication adherence (assessed by VAS scores), FBS, and HbA1c at baseline, 4 weeks, and 8 weeks.
Discussion
In this study, we sought to examine the effects of usual care only and usual care plus telephone-based brief MI on self-management, medication adherence, and glycemic control in patients with T2DM. Our findings demonstrated that the usual care for patients with uncontrolled T2DM, which comprised a routine DM service, led to positive improvements in overall self-management, whereas usual care plus telephone-based brief MI led to positive improvements in overall self-management, medication adherence, and FBS level over 8 weeks. These results suggest that compared with individuals who received usual care only, those who received telephone-based brief MI experienced greater motivation to improve their DM self-management, medication adherence, and glycemic control (ie, FBS).
These findings that usual care plus telephone-based brief MI has a positive effect on overall self-management, medication adherence, and FBS in patients with uncontrolled T2DM are similar to those of previous studies on the effects of brief MI or MI among patients with DM in Thailand,39,41,57,58 China,38 Iran,59 Turkey,60 England,61 and the USA.62 These previous studies demonstrated that MI had positive effects on self-management, in terms of dieting and physical activity in patients with uncontrolled T2DM,60 on medication adherence in patients with DM,59,62 and on FBS level.58 The present findings are also consistent with previous reports that brief MI positively affects physical activity levels in pregnant women with gestational DM,61 medication adherence,41 overall self-management,38,39,57 and FBS level in patients with T2DM.41
Our results suggest that brief MI can be as effective as full-length MI sessions21 in increasing patient motivation to achieve glycemic control, and that 20–30-min sessions can have large effects on patient-reported outcomes (observed range of d: 0.662–1.396). A plausible possible explanation is that the telephone-based brief MI intervention enhanced individuals’ motivation and/or goals to be healthy; thus, they improved their level of self-management in dieting, physical activity, self-monitoring, medication taking, and foot care. Patients who show improved DM self-management are also more likely to show improved glycemic control.13,63 However, our findings regarding HbA1c levels differed from those of previous studies.37–40,57,64 Although we found no statistically significant effect of time on changes in HbA1c, we observed a trend of declining HbA1c over the three time points at which it was assessed. This may be because the follow-up period (8 weeks) was limited by the COVID-19 pandemic in Thailand, which lasted until the end of September 2022.
Strengths
This study had several strengths. First, we focused on patients with uncontrolled T2DM living in a rural community. The use of telephone-based brief MI interventions could help to reduce the effect of healthcare service barriers such as limited time and geographic location, and a telephone-based brief MI intervention may be a more cost-effective intervention for patients with uncontrolled T2DM. Second, we focused on patients who had not developed severe DM complications. The brief MI intervention could promote secondary and tertiary T2DM prevention by improving self-management, medication adherence, and glycemic control among patients with suboptimal glycemic control and thus preventing adverse DM complications.
Limitations
There were two major limitations, both related to study methodology. First, the significant improvement observed in patient outcomes should be interpreted with caution because individuals who agreed to participate in the study may have been more likely to change or to have had a greater readiness to change compared with the general population. A related point is that the use of a small and self-selected sample (ie, non-probability sampling) may have led to sampling bias, which could have distorted the study findings. Second, follow-up at 4-week intervals (baseline, 4 weeks, and 8 weeks) was too short to observe significant changes in HbA1c and to reflect the long-term sustainability of the effects of the intervention on HbA1c level. However, the follow-up period was limited by the fact that the study was conducted during the COVID-19 pandemic.
Recommendations
We make the following recommendations for future research on this topic: 1) use a randomized controlled trial design to monitor the effects of telephone-based brief MI on self-management, medication adherence, and glycemic control, 2) extend the follow-up period to a minimum of 12 weeks to increase the likelihood of observing significant changes in HbA1c, and 3) increase the study duration beyond 6 months to determine the long-term sustainability of the effects of brief MI on patient outcomes.
Conclusion
The results of this study indicate that telephone-based brief MI with usual care can significantly improve self-management, medication adherence, and glycemic control (ie, FBS) in patients with uncontrolled T2DM after 4 weeks. Phone-based brief MI can be considered an effective tool for healthcare providers to remotely enhance patients’ self-management and glycemic control.
Data Sharing Statement
Data supporting this study are available from the corresponding author.
Acknowledgments
We thank all participants for their time and effort at various stages of this study. We also thank Dr.Nipaporn Butsing for statistical consulting.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Noncommunicable Diseases Progress Monitor 2022. Geneva: World Health Organization; 2022.
2. Khan MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi:10.2991/jegh.k.191028.001
3. World Health Organization. Global report on diabetes. Geneva: World Health Organization; 2016. Available from: https://www.who.int/publications/i/item/9789241565257.
4. Papatheodorou K, Papanas N, Banach M, et al. Complications of Diabetes 2016. J Diabetes Res. 2016;2016:1–3. doi:10.1155/2016/6989453
5. International Diabetes Federation. Diabetes atlas-eight edition; 2017. Available from: https://diabetesatlas.org/atlas/eighth-edition/.
6. Bragg F, Holmes MV, Iona A, et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA. 2017;317(3):280–289. doi:10.1001/jama.2016.19720
7. Almigbal TH. Driving distance and glycemic control in patients with insulin-treated diabetes mellitus: results from the diabetes and driving study. J Nat Sci Med. 2021;4(3):244–249. doi:10.4103/jnsm.jnsm_147_20
8. Zgibor JC, Gieraltowski LB, Talbott EO, et al. The association between driving distance and glycemic control in rural areas. J Diabetes Sci Technol. 2011;5(3):494–500. doi:10.1177/193229681100500304
9. Tan MY, Magarey J. Self-care practices of Malaysian adults with diabetes and sub-optimal glycaemic control. Patient Educ Couns. 2008;72(2):252–267. doi:10.1016/j.pec.2008.03.017
10. Hashim MJ, Mustafa H, Ali H. Knowledge of diabetes among patients in the United Arab Emirates and trends since 2001: a study using the Michigan diabetes knowledge test. East Mediterr Health J. 2016;22(10):742–748. doi:10.26719/2016.22.10.742
11. Steinberg MP, Miller WR. Motivational Interviewing in Diabetes Care. New York, NY: Guilford Publications; 2015.
12. Shigaki C, Kruse RL, Mehr D, et al. Motivation and diabetes self-management. Chronic Illn. 2010;6(3):202–214. doi:10.1177/1742395310375630
13. Ngetich E, Pateekhum C, Hashmi A, et al. Illness perceptions, self-care practices, and glycemic control among type 2 diabetes patients in Chiang Mai, Thailand. Arch Public Health. 2022;80(134):1–10. doi:10.1186/s13690-022-00888-1
14. Adu MD, Malabu UH, Malau-Aduli AEO, et al. Enablers and barriers to effective diabetes self-management: a multi-national investigation. PLoS One. 2019;14(6):e0217771. doi:10.1371/journal.pone.0217771
15. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926–943. doi:10.1016/j.pec.2015.11.003
16. Hildebrand JA, Billimek J, Lee JA, et al. Effect of diabetes self-management education on glycemic control in Latino adults with type 2 diabetes: a systematic review and meta-analysis. Patient Educ Couns. 2020;103(2):266–275. doi:10.1016/j.pec.2019.09.009
17. Vas A, Devi ES, Vidyasagar S, et al. Effectiveness of self-management programmes in diabetes management: a systematic review. Int J Nurs Pract. 2017;23(5):
18. Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi:10.2337/diacare.23.7.943
19. Keeratiyutawong P, Hanucharurnkl S, Melkus G, et al. Effectiveness of a self-management program for Thais with type 2 diabetes: an integrative review. Thai J Nurs Res. 2006;10(2):85–97.
20. Kanan P, Piaseu N, Malathum P, Belza B . Predictors of diabetes self-management in older adults with poorly controlled type 2 diabetes mellitus. PRIJNR. 2015;23(4):357–367. doi:10.2147/PPA.S335363
21. Swanson V, Maltinsky W. Motivational and behaviour change approaches for improving diabetes management. Pract Diab. 2019;36(4):121–125. doi:10.1002/pdi.2229
22. Douaihy A, Kelly TM, Gold MA. Motivational Interviewing: A Guide for Medical Trainees. United States of America: Oxford University Press; 2014.
23. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. London: Guildford; 2002.
24. Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother. 2009;37(2):129–140. doi:10.1017/S1352465809005128
25. Rogers CR. On Becoming a Person. Boston, Mass: Houghton Mifflin; 1972.
26. Bem DJ. Self-perception theory. In: Advances in Experimental Social Psychology. New York: Academic Press; 1972.
27. Janis IL, Mann L. Decision-Making: A Psychological Analysis of Conflict, Choice, and Commitment. New York: Free Press; 1977.
28. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–1114. doi:10.1037//0003-066x.47.9.1102
29. Welch GW, Rose GS, Ernst DB. Motivational Interviewing and diabetes: what is it, how is it used, and does it work? Diabet Spect. 2006;19:5–11. doi:10.2337/diaspect.19.1.5
30. Carpenter R, DiChiacchio T, Barker K. Interventions for self-management of type 2 diabetes: an integrative review. Int J Nurs Sci. 2018;6(1):70–91. doi:10.1016/j.ijnss.2018.12.002
31. Salimi C, Momtazi S, Zenuzian S. A review on effectiveness of motivational interviewing in the management of diabetes mellitus. J Psychol Clin Psychiatry. 2016;5(4):00294. doi:10.15406/jpcpy.2016.05.00294
32. Ekong G, Kavookjian J. Motivational interviewing and outcomes in adults with type 2 diabetes: a systematic review. Patient Educ Couns. 2016;99(6):944–952. doi:10.1016/j.pec.2015.11.022
33. Song D, Xu T, Sun Q. Effects of motivational interviewing on self-management in patients with diabetes mellitus: a meta-analysis. Inter J Nurs Sci. 2014;1:291–297. doi:10.1016/j.ijnss.2014.06.002
34. Lestari SP, Wihastuti TA, Ismail DD. Effectiveness of motivational interviewing on self-management of type 2 diabetes mellitus patients: a systematic review. J Kedokteran Brawijaya. 2021;31(4):253–259. doi:10.21776/ub.jkb.2021.031.04.11
35. Berhe KK, Gebru HB, Kahsay HB. Effect of motivational interviewing intervention on HgbA1C and depression in people with type 2 diabetes mellitus (systematic review and meta-analysis). PLoS One. 2020;15(10):e0240839. doi:10.1371/journal.pone.0240839
36. Rollnick S, Heather N, Bella A. Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Mental Health. 1992;1:25–37. doi:10.3109/09638239209034509
37. Calhoun D, Brod R, Kirlin K, et al. Effectiveness of motivational interviewing for improving self-care among northern plains Indians with type 2 diabetes. Diabetes Spectr. 2010;23(2):107–114. doi:10.2337/diaspect.23.2.107
38. Chen SM, Creedy D, Lin HS, et al. Effects of motivational interviewing intervention on self-management, psychological and glycemic outcomes in type 2 diabetes: a randomized controlled trial. Int J Nurs Stud. 2012;49(6):637–644. doi:10.1016/j.ijnurstu.2011.11.011
39. Jerawatana R, Reutrakul S, Siripitayakunkit A. The effect of advanced practice nurse-led intervention program on outcomes in diabetes patients with complex problems. Rama Nurs J. 2018;24(1):51–68.
40. Kıral MA, Cansu GB. Glycemic regulation in patients with type 2 diabetes mellitus: effects of motivational interviewing. Ankara Med J. 2022;22(3):336–346. doi:10.5505/amj.2022.87854
41. Pokpirom P, Rookkapan K. Effect of brief motivational Interviewing with education and telephone follow up by pharmacists among type 2 Diabetic patients: a randomized controlled Trial. Thai J Pharm Pract. 2020;12(4):985–996.
42. Aekplakorn W. The Sixth Thai National Health Examination Survey (NHES IV&VI). Bangkok: Aksorn Graphic and Design; 2019.
43. Ministry of Public Health. Health data center. Available from: https://hdcservice.moph.go.th/hdc/main/search.php?search=%E0%B9%80%E0%B8%9A%E0%B8%B2%E0%B8%AB%E0%B8%A7%E0%B8%B2%E0%B8%99.
44. Gill G, Gebrekidan A, English P, et al. Diabetic complications and glycaemic control in remote North Africa. An Inter J Med. 2008;101(10):793–798.
45. Birabwa C, Bwambale MF, Waiswa P, et al. Quality and barriers of outpatient diabetes care in rural health facilities in Uganda - A mixed methods study. BMC Health Serv Res. 2019;19(1):706. doi:10.1186/s12913-019-4535-x
46. Likhitluecha N, Atthachaiwat A, Wongsuvansiri S, et al. Development of care management model for patients with uncontrolled diabetes. Journal of Nursing Division. 2017;44(2):141–158.
47. Stevens JP. Applied Multivariate Statistics for the Social Sciences.
48. Arunsangsod K, Maneesriwongkul W, Panpakdee O. Effect of brief motivational interviewing on knowledge, motivation and medication adherence among patients with hypertension. J Pub Health Nurse. 2016;28(3):129–144.
49. Pratuangtham SR, Jerawatana R. Effectiveness of diabetes self – management education in Thais with type 2 diabetes. PRIJNR. 2018;23(1):74–86.
50. Navicharern R. Diabetes self-management, fasting blood sugar and quality of life among type 2 diabetic patients with foot ulcers. J Med Assoc Thai. 2012;95(2):156–162.
51. Klungthumnium K, Wirojratana V, Jitramontree N, et al. The relationships between illness representations, emotional representation and self-care behaviors in older persons with uncontrolled type 2 diabetes. J Royal Thai Army Nurs. 2016;17(2):135–144.
52. Finitsis DJ, Pellowski JA, Huedo-Medina TB, et al. Visual analogue scale (VAS) measurement of antiretroviral adherence in people living with HIV (PLWH): a meta-analysis. J Behav Med. 2016;39(6):1043–1055. doi:10.1007/s10865-016-9770-6
53. Rickles NM, Mulrooney M, Sobieraj D, et al. A systematic review of primary care-focused, self-reported medication adherence tools. J Am Pharm Assoc. 2023;63(2):477–490.e1. doi:10.1016/j.japh.2022.09.007
54. Goruntla N, Mallela V, Nayakanti D. Impact of pharmacist-directed counseling and message reminder services on medication adherence and clinical outcomes in type 2 diabetes mellitus. J Pharm Bioallied Sci. 2019;11(1):69–76. doi:10.4103/jpbs.JPBS_211_18
55. Curran-Everett D, Taylor S, Kafadar K. Fundamental concepts in statistics: elucidation and illustration. J Appl Physiol. 1998;85(3):775–786. doi:10.1152/jappl.1998.85.3.775
56. Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486–489. doi:10.5812/ijem.3505
57. Hinkhaw C, Hanprasitkam K, Jianvitayakij S. Effects of a combination of self-management and motivational interviewing program for delayed progression of diabetic nephropathy on self-management behaviors and clinical outcomes among type 2 diabetic patients with the third-stage diabetic nephropathy. JPNC. 2019;30(2):185–202.
58. Wattanakorn K, Deenan A, Puapan S, et al. Effects of an eating behaviour modification program on Thai people with diabetes and obesity: a randomised clinical trial. PRIJNR. 2013;17(4):356–370.
59. Miri Z, Rezaee N, Faghihi H, et al. Effect of cognitive-behavioral training combined with motivational interviewing on treatment adherence and hemoglobin A1c in patients with diabetes and depressive symptoms. Med Surg Nurs J. 2021;10(3):e120496. doi:10.5812/msnj.120496
60. Oz HS, Buyulsoy GD. The effects of motivational interview on healthy behaviour and quality of life in the uncontrolled type 2 diabetes patients. Inter J Car Sci. 2022;15(2):1194–1201.
61. Smith R, Ridout A, Livingstone A, et al. Motivational interviewing to increase physical activity in women with gestational diabetes. British J Midwifery. 2021;29(10):550–556. doi:10.12968/bjom.2021.29.10.550
62. Ekong G, Chou C, Lakin J, et al. Pharmacist-led motivational interviewing for diabetes medication adherence in a worksite wellness program. J Am Pharm Assoc. 2020;60(6):e224–e229. doi:10.1016/j.japh.2020.07.025
63. Al-Khawaldeh OA, Al-Hassan MA, Froelicher ES. Self-efficacy, self-management, and glycemic control in adults with type 2 diabetes mellitus. J Diabetes Complications. 2012;26(1):10–16. doi:10.1016/j.jdiacomp.2011.11.002
64. Rahmani S, Mansoobifar M, Sirifi MR, et al. Effectiveness of motivational interviewing based on the ability, information and motivation model on adherence to treatment and glycosylated hemoglobin in females with type 2 diabetes mellitus. IJDO. 2022;14(1):44–50. doi:10.21776/ub.jkb.2021.031.04.11
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

