Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Effects of Remote Ischemic Conditioning on Cerebral Hemodynamics in Ischemic Stroke
Authors Qin C , Yan X , Jin H, Zhang R, He Y , Sun X, Zhang Y, Guo ZN, Yang Y
Received 21 September 2019
Accepted for publication 16 December 2019
Published 23 January 2020 Volume 2020:16 Pages 283—299
DOI https://doi.org/10.2147/NDT.S231944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Chen Qin,1,* Xiuli Yan,1,* Hang Jin,1 Ruyi Zhang,2 Yaode He,1 Xin Sun,1 Yihe Zhang,1 Zhen-Ni Guo,1,3 Yi Yang1,3
1Department of Neurology, The First Hospital of Jilin University, Changchun 130021, People’s Republic of China; 2Department of Cardiovascular Center, The First Hospital of Jilin University, Changchun 130021, People’s Republic of China; 3Clinical Trial and Research Center for Stroke, Department of Neurology, The First Hospital of Jilin University, Changchun 130021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Yang; Zhen-Ni Guo
Department of Neurology, The First Hospital of Jilin University, Xinmin Street 1#, Changchun 130021, People’s Republic of China
Tel +86-13756661217; +86-18186872986
Fax +86-431-88782378; +86-431-88782378
Email [email protected]; [email protected]
Abstract: Ischemic stroke is one of the most common cerebrovascular diseases and is the leading cause of disability all over the world. It is well known that cerebral blood flow (CBF) is disturbed or even disrupted when ischemic stroke happens. The imbalance between demand and shortage of blood supply makes ischemic stroke take place or worsen. The search for treatments that can preserve CBF, especially during the acute phase of ischemic stroke, has become a research hotspot. Animal and clinical experiments have proven that remote ischemic conditioning (RIC) is a beneficial therapeutic strategy for the treatment of ischemic stroke. However, the mechanism by which RIC affects CBF has not been fully understood. This review aims to discuss several possible mechanisms of RIC on the cerebral hemodynamics in ischemic stroke, such as the improvement of cardiac function and collateral circulation of cerebral vessels, the protection of neurovascular units, the formation of gas molecules, the effect on the function of vascular endothelial cells and the nervous system. RIC has the potential to become a therapeutic treatment to improve CBF in ischemic stroke. Future studies are needed to highlight our understanding of RIC as well as accelerate its clinical translation.
Keywords: remote ischemic conditioning, cerebral hemodynamics, ischemic stroke, cerebral blood flow
Introduction
Ischemic stroke is a common kind of cerebrovascular disease with high morbidity, mortality, and disability rates. More than 10 million people worldwide suffer from ischemic stroke each year.1 It becomes a socio-economic problem when it is more prevalent among a younger age group, with resultant permanent disability, cognitive and motor disorders, and dementia. This brings ischemic stroke to the attention of scientists who are endeavoring to search for advanced clinical treatments to improve the prognosis of patients with ischemic stroke. Remote ischemic conditioning (RIC) offers practical value: it is effective, noninvasive, economical, and convenient. It has thus been researched intensively by cardiovascular disease specialists for many years; it has also begun to be an object of study in cerebrovascular disease.
The normal function of the brain is based on the stable CBF and cerebral autoregulation. Several studies reveal the fact that RIC can affect cerebral hemodynamics in ischemic stroke.2,3 The mechanism by which RIC influences cerebral hemodynamics is beyond full comprehension and needs our further exploration. Table 1 shows experimental and clinical studies available at present of RIC in ischemic stroke. In September 2019, a literature search in PubMed was performed based on the combination of the following terms: “remote ischemic conditioning”, “ischemic stroke”, “ischemic preconditioning”, “ischemic perconditioning”, “ischemic postconditioning”, “cerebral hemodynamics” and “cerebral blood flow”. The reference list of relevant papers was also screened. The search was limited to publications in English, and the final references included were chosen based on the relevance to the scope of this review.
 |  |  |  |
Table 1 Experimental and Clinical Studies Available at Present of Remote Ischemic Conditioning in Ischemic Stroke |
Findings of Animal Experiments and Clinical Studies
RIC mainly exerts endogenous protective effects on important organs of the body through nerve, humoral, and immune-inflammatory pathways. Its safety and efficacy in ischemic stroke have been reported.40,41 Recent studies have shown that RIC can alleviate ischemia-reperfusion (I/R) injury.42–44 Cerebral ischemia leads to cerebral hypoperfusion. After reperfusion, hyperperfusion is the first to be observed in almost all experimental animals, followed by hypoperfusion.45 Neither hyperperfusion nor hypoperfusion is harmless to the recovery from cerebral ischemia. Wang et al found that after ischemia reperfusion, the hyperperfusion time in the control group lasted for 30 mins, followed by several hours of hypoperfusion. In the RIC group, in contrast, the hyperperfusion time was shortened to 20 mins, and the decreasing of hyperperfusion values was observed while the hypoperfusion values increased. These findings suggest that RIC can reduce I/R injury and improve disturbed CBF by reducing the duration and degree of hyperperfusion.42 Zhao et al demonstrated for the first time that RIC had remarkable effects on CBF in focal cerebral ischemic rat models. They found that bilateral common carotid artery occlusion reduced CBF to approximately 30% of the baseline, and additional middle cerebral artery occlusion further decreased CBF to 20% approximately. After the common carotid artery was released, a transient hyperemic response was observed, which could be broken off by three cycles of reperfusion and occlusion of the bilateral common carotid artery. The results indicated that RIC could protect against I/R injury and be conducive to CBF.43 Clinical trials also show this positive result. Meng et al evaluated the protective effects of RIC in patients with symptomatic atherosclerotic intracranial arterial stenosis. They found RIC could prevent recurrent stroke and shorten the average time to recovery. The degree of the abnormally elevated peak systolic velocities decline was more pronounced in the RIC group vs the control group.44 In addition, the middle cerebral artery peak systolic blood flow velocity and pulsatility index did not change significantly before, during, or after RIC in AIS patients treated with thrombectomy.37 And it has been demonstrated that RIC may reduce infarction tissue risk as an adjunct therapy to thrombolysis in patients with acute ischemic stroke.40
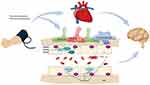
Research on cerebral hemodynamics has shifted from purely vascular concepts to a complex interaction of biochemical and molecular mechanisms. Factors that can affect the cardiac function, the coupling of nerves and blood vessels, the content of blood gas, the blood viscosity, the body temperature, and the automatic regulation of cerebral blood vessels may affect cerebral hemodynamics.46 The influence of RIC on the cerebral hemodynamics of ischemic stroke may occur through the following means: it may improve cardiac function, improve collateral circulation of cerebral vessels, protect neurovascular units (NVU), induce the formation of gas molecules, affect the function of vascular endothelial cells, and affect the nervous system [Figure 1].
Mechanisms
RIC Can Improve Cardiac Function
The cerebrovascular system and the cardiovascular system are highly correlated in anatomy and physiological functions. Left ventricular ejection enters the cerebrovascular system through the aortic arch to supply blood and oxygen to the brain tissue. The brain receives one fifth of the cardiac output. It has been reported that moderate and regular physical exercise can improve cardiac function, increase CBF, and improve microcirculation.47–49 Lieshout et al found that there was a linear correlation between CBF and cardiac output in healthy subjects, that is, when the muscles of the lower limbs were tense, cardiac output increased and the mean blood flow velocity of the middle cerebral artery (MCA) increased simultaneously.50 Evidence also showed that left ventricle ejection fraction was one of the strongest predictors of the poststroke improvement. Preserved ejection fraction is associated with early favorable outcome in ischemic stroke.51 All the evidence supports that improving cardiac function could be a nice way to affect cerebral hemodynamics.
Recently, several studies have shown that RIC may have an exercise equivalent.2,52 For example, a prospective study showed that dialysates prepared from plasma of human subjects undergoing high-intensity exercise or RIC were both protective in reducing the infarct size of an isolated rabbit heart after I/R injury.52 Kono et al studied 10 heart failure patients with left ventricular ejection fraction reduced and 10 healthy subjects. All subjects received RIC treatment for 1 week. The results showed that RIC increased coronary microcirculation in both patients and healthy subjects.53 Previous experimental studies showed that although RIC failed to improve the left ventricular ejection fraction, RIC could reduce the level of plasma N-terminal pro brain-type natriuretic peptide, improve the myocardial systolic function of most patients with severe compensatory heart failure, reduce systolic blood pressure, and reduce the cardiac afterload, so as to have a positive impact on hemodynamics.54 In a randomized controlled study involving 50 subjects with mild ischemic heart failure, RIC treatment of the upper limb twice a day for 6 weeks could improve the cardiac function of patients, improve the left ventricular ejection fraction, extent the 6 mins walk distance, and reduce the level of B-type natriuretic peptide, thereby affecting the New York Heart Function Assessment score.55 RIC may play the role of myocardial protection through the survival activating factor enhancement (SAFE) pathway and the reperfusion injury salvage kinases (RISK) pathway:
The SAFE Pathway
The SAFE pathway is a novel protective pathway against reperfusion injuries, which consists of the Janus kinase 2 (JAK2) signal transducer and activator of transcription 3 (STAT-3) signaling cascade.56 Previous research has indicated the cardioprotection by RIC was associated with activation of the SAFE signaling pathway.57 Tamareille et al reported that local ischemic postconditioning +RIC increased phospho-STAT-3 levels when compared to local ischemic postconditioning alone, which highlighted the key role of the SAFE pathway.58 Additional study has revealed that remote ischemic postconditioning protects myocardial cells by the recruitment of the RISK and SAFE pathways. However, remote ischemic preconditioning may not.59 The activation of SAFE pathway may be different in different RIC protocols.
The RISK Pathway
The RISK pathway is another universal signaling cascade composed of two parallel cascades, the phosphoinositide-3 kinase/Akt and MEK1-ERK1/2. Activation of the RISK pathway by RIC has been confirmed to be associated with cardioprotection in many experimental models.60 Recent studies also observe that interaction exists between the RISK and SAFE signaling pathways in mediating RIC.58 Further studies are required to determine the relationship between the SAFE and RISK pathway and their function of myocardial protection.
Based on the researches cited above, RIC may exert their myocardial protection through the SAFE pathway and RISK pathway, thereby improving cerebral hemodynamics. Long-term RIC treatment may be beneficial to patients either with heart failure or with ischemic stroke. The protective effects of exercise and RIC on cardiac function and cerebral hemodynamics deserve further study.
RIC Can Improve Cerebral Collateral Circulation
The primary collaterals consist of circulatory anastomoses that constitute the circle of Willis, and the secondary collaterals consist of the pial or leptomeningeal collaterals. The collateral circulation plays an important role in maintaining tissue viability in the first hours of ischemic stroke. It has been reported that collateral circulation is a key factor in predicting the prognosis of ischemic stroke.61 Thus, promoting collateral circulation formation and strengthening collateral circulation function are important strategies for the treatment or prevention of ischemic diseases.62 Evidence showed that the higher the ischemic edge microvascular density, the better the clinical outcome in stroke patients.63 RIC has been proven that it could significantly develop leptomeningeal anastomoses and enhance the vessel diameter of anterior cerebral artery-middle cerebral artery anastomoses. RIC may play a neuroprotective role by enhancing the leptomeningeal collateral circulation.64 In addition, it has been reported that RIC significantly induced angiogenesis and collaterals formation, which was manifested as an increase in the number and volume of blood vessels. And these changes had an effect on improving CBF.3 RIC may improve the collateral circulation of cerebral vessels through the following ways:
Notch Signaling Pathway
The Notch signaling pathway is a biologically ancient intercellular signaling pathway. The Notch receptor is a highly conserved membrane-bound receptor, which directly transfers signals from the cell surface to the nucleus by regulating intramembrane proteolysis. Notch1 and Notch4 are the only two kinds of Notch receptors expressed in vascular endothelial cells, which have an important effect on the vascular development and physiological process of vertebrates. These effects include the regulation of the arterial/venous differentiation of endothelial cells and vascular smooth muscle cells, the regulation of the germination and branching of blood vessels during normal development and tumor angiogenesis, and the differentiation and physiological responses of vascular smooth muscle cells.65 Lawson et al first confirmed the effect of the Notch signaling pathway on arteriovenous differentiation in zebrafish experiments. They found that loss of the Notch signaling pathway lead to defective arteriovenous differentiation. Activation of the Notch signaling pathway, by contrast, resulted in repression of venous cell development, and promoted the differentiation of cells into arteries. The Notch signaling pathway is essential for the normal development of arteries and veins.66 It has been demonstrated that the promoting of the Notch signaling pathway contributed to the proliferation of endothelial progenitor cells and angiogenesis of the brain in cerebral ischemic stroke mice.67 And blockade of Notch signaling decreased vascular smooth muscle cells’ investment of developing arteries.68 In the mouse model of hind limb ischemia, the arteriogenesis of the ischemic hindlimb of mice with Notch1 heterozygous deletion was also impaired.69 In conclusion, the Notch signaling pathway plays an important role in the normal function of angiogenesis.
Ren et al utilized the middle cerebral artery occlusion (MCAO) model in rats and divided the rats into a sham operation group, an MCAO control group, and an MCAO + RIC group. The study showed that compared with the MCAO control group, Notch1 and the intracellular domain expression of Notch1 in the MCAO +RIC group was increased, the artery diameter was enlarged, the leptomeningeal anastomoses branch was significantly increased, and local CBF was also significantly elevated.2 Therefore, RIC may stimulate the Notch1 signaling pathway to promote collateral circulation generation and improve CBF in ischemic regions, thereby affecting cerebral hemodynamics. The Notch signaling pathway may be a new therapeutic target for ischemic stroke.
eNOS/NO Pathway
The production of nitric oxide (NO) in vivo is catalyzed by three different enzymes, neuronal nitric oxide synthase (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS). NO production by vascular endothelial cells is mainly dependent on eNOS.70 The eNOS catalyzes the conversion of L-arginine to L-citrulline to generate NO. Nitrite is the storage pool of NO generated by endogenous eNOS, which is reduced to NO in the hypoxic area, mediating vascular dilatation and increasing CBF. Murohara et al research showed that vascular remodeling was impaired in an eNOS knockout murine model of operatively induced hindlimb ischemia.71 Thus, the eNOS/NO pathway is considered one of the major regulators of angiogenesis after ischemia.
An experiment divided 30 adult rats into a sham operation group, a bilateral carotid artery occlusion group (2VO), and a 2VO+RIC group. The experiment found that compared with 2VO group, in the 2VO+RIC group, the expression of phosphorylated endothelial nitric oxide synthase (p-eNOS) increased, microvascular density and collateral vessels increased, and the cerebral perfusion significantly increased as well. Intraperitoneal injection of NOS inhibitors can reverse this phenomenon.72 Moreover, RIC treatment could up-regulate the content of nitrite in the plasma of mice, and the up-regulation of nitrite was related to the increase of CBF.73 Hoda et al reported that RIC up-regulated the expression of eNOS mRNA in blood vessels of the regulatory site by about 10 times, and increased the plasma concentration of NO.74 These results suggest that RIC can improve cerebral perfusion in ischemic regions by promoting angiogenesis, and this effect is mediated by eNOS/NO pathway. Nitrite levels are easily measured in the blood and can therefore be used as a promising circulating biomarker for ischemic treatment.
VEGF Pathway
Vascular endothelial growth factor (VEGF) is now considered one of the most effective highly specific cell factors promoting vascular endothelial growth, participating directly in the angiogenesis of ischemic or hypoxic tissues or organs. In the human genome, VEGF A, B, C, D, E and placental growth factor are included. They need to bind to the corresponding receptors and activate different downstream signaling pathways to play their respective roles. VEGF-A was the first discovered among them, with the richest content and the strongest function in tissues and cells. Therefore, VEGF refers to VEGF-A in most literature. Accumulating evidence supports the protective role of VEGF in inducing angiogenesis and increasing vascular permeability, thus affecting hemodynamics.75–77 Zhang et al used male Wistar rats to construct the MCAO model, and they discovered that VEGF expression began to increase 2 hrs after cerebral infarction, and lasted for at least 28 days. The increase in the number of new capillaries in the ischemic area was correlated with the up-regulation of VEGF, indicating that VEGF mediated the angiogenesis in the ischemic area and increased cerebral blood perfusion.78 In addition, VEGF-A is also a potent vasodilator and has been reported to not only induce neuroprotection directly in ischemic disorders, but also to improve cerebral autoregulation through hypoxia-inducible transcription factor-1-regulated pathways.79
Evidence suggests that RIC is able to elevate the circulating VEGF significantly, even at the mRNA and protein levels,80 which is considered to be a key mediator of protective RIC effects.81 Ueno et al investigated the relationship between RIC and VEGF by clamping abdominal aortas in mice. The results also showed that RIC could increase the level of VEGF in plasma, thus producing a neuroprotective effect.82 All the studies above have confirmed that RIC can effectively promote the up-regulation of VEGF expression, so as to play its physiological role in promoting the neovascularization in the ischemic area and affecting cerebral hemodynamics. This is probably a main molecular biological mechanism through which RIC mediates brain protection.
RIC Can Prevent the Collapse of Pial Collaterals
The cerebral collaterals are pivotal auxiliary vascular pathways. They can maintain blood flow to ischemic tissue up to a point when the primary vascular routes to the brain are obstructed.83
An experiment showed constriction of pial collaterals and distal MCA segments at all time points after MCAO was apparent in controls, but this did not happen in RIC-treated animals. This result demonstrated that RIC could prevent the collapse or constriction of cerebral collaterals after ischemia took place. And it could also improve CBF through its influence on cerebral collaterals. But no significant effect or interaction was observed in blood flow velocity.10
Above all, it is proved that RIC has a positive influence on cerebral collateral circulation. On the one hand, RIC can promote the establishment of collateral circulation in several ways. On the other hand, RIC can prevent the collapse of pial collaterals after ischemia. That is, RIC is beneficial to the status of collateral circulation and collateral blood flow. Therefore, RIC is a potential therapeutic method for ischemic stroke.
RIC Can Protect the Neurovascular Unit
The concept of “neurovascular unit (NVU)” was first introduced by Lo et al in 2003.84 The NVU is a special structural and functional unit of the mammalian nervous system. It includes vascular cells (endothelium, pericytes, and vascular smooth muscle cells), glial cells (astrocytes, microglia, and oligodendroglia), and neurons, which are tightly connected to modulate regional blood flow in response to local metabolic demand.85,86 This modulation enables rapid increase in CBF in response to neuronal activation, despite the relatively stable global blood flow.86 Many physiological studies have confirmed that there is communication between cerebral blood vessels and adjacent nerve cells in NVU. The NVU plays a crucial role in modifying cerebrovascular function, controlling CBF and permeability in health and in specific diseases.87 Therefore, the regulation of regional and local cerebral hemodynamics depends on the structural and functional integrity of the NVU.88
Several diseases can lead to impaired cellular communication between neurovascular unit components, and thus result in brain dysfunction. Microvessel responses, astrocyte injury, and neuron injury occur almost simultaneously when the ischemia/reperfusion (I/R) happens.89 Damage to any part of the NVU could lead to dysfunction in cerebral hemodynamics. Thus, we should make efforts to protect the functional and structural integrity of the NVU in patients with ischemic stroke. Han et al first demonstrated that ischemic treatment could reduce the I/R injury to the cellular structures of neurons, astrocytes, and microvessels.90 Researches then advanced to RIC. Astrocytes are important part of NVU, they undergo rapid hypertrophy and hyperplasia in response to injury of brain tissue. Cheng et al reported that RIC could protect NVU by adjusting the proportion of astrocyte subtypes and weakening the activation of astrocytes in the brains of ischemic mice, thereby improving neurological function as well as reducing mortality, infarct area, and hemispheric swelling after ischemic stroke.5
The evidences above give us reason to think that RIC can be effective in improving CBF by protecting NVU during ischemic stroke. The understanding of NVU highlights that attention should be shifted from neurons or blood vessels, respectively, to the whole NVU. It also provides a platform for potential therapies for ischemic stroke. RIC may be an effective and promising treatment for ischemic stroke due to its convenience of operation and positive impact on NVU. However, there is still much to learn about the mechanism and function of the neurovascular unit and RIC.
RIC Can Induce the Formation of Gas Molecules
Numerous studies have demonstrated that hypoxia can induce vascular endothelial cells to produce gaseous messenger molecules, such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S).91–93 These gasotransmitters share several common properties among their physiological and pathological functions.
NO
As mentioned above, NO is considered one of the main endothelium-derived vasodilation factors. NO plays a potential role in cerebral autoregulation, the expansion of cerebral blood vessels, and the improvement of CBF. A study discovered that a decrease in transmural pressure from 60 mmHg resulted in the increasing release of vascular NO by intraparenchymal arterioles isolated from rats. The result supports that NO contributes to the autoregulatory vasodilation intrinsic to the vessel during hypotension.94 White et al compared the effect of the NOS inhibitor N(G)-monomethyl-L-arginine on dynamic autoregulation with that of noradrenaline in healthy humans. They found reduced NO secretion was associated with impaired cerebral autoregulation, suggesting that nitric oxide mediates cerebral autoregulation in humans.95 Recently, a number of studies have focused on the function of NO on cerebral autoregulation.96–98 It is demonstrated that inhaled nitric oxide had the function of preventing impairment of cerebral autoregulation, reducing hippocampal necrosis and blocking the reductions in CBF.96–98 Thus, NO is an important medium of the regulating of cerebral hemodynamics and contributes to the adequate blood supply to the brain. However, there are still dissenting voices.99,100 The effect of NO on cerebral hemodynamics needs further study, including the effective dose and its possible toxic effect.
CO
CO is a gaseous second messenger endogenously generated from heme by heme oxygenase (HO). Three isoforms of HO (e.g., HO-1, HO-2, and HO-3) have been discovered.101 Endogenous CO production occurring at low concentrations is thought to be protective.101 In animal models, 250 ppm CO in the central nervous system have been demonstrated to have protective effects.102,103 CO can generate cyclic guanosine phosphate by activating guanosine cyclase. The increase of intracellular cyclic guanosine phosphate can expand blood vessels and inhibit platelet aggregation. An increasing number of studies have shown that endogenous CO may be one of the factors involved in regulating vascular tension during hypoxia.104 The vascular tone of the resisting arteries and arterioles determines the resistance of the surrounding vessels, which helps to regulate blood pressure and blood flow, thus affecting the hemodynamics inside tissues and organs.105 Several experiments confirm that CO also can influence NVU by its impact on neurogenesis, angiogenesis, and synaptic plasticity.102,106,107 HO-1 knockout mice (HO-1(-/-)) had fewer positive cells for axon migrating markers after MCAO.108 Mice exposed to 250 ppm CO for 2 hrs each day have significant endothelial progenitor cells mobilization.106 And, relatively low concentration of endogenous CO production is thought to play an important role at the synapse leading to long-term potentiation.109 Therefore, the potential efficacy of CO therapy in ischemic stroke may be its influence on neurogenesis, angiogenesis, and synaptic plasticity, which can improve the CBF by affecting NVU.
H2S
H2S can also be produced by cells under ischemic conditions, and it has been considered the third important gaseous signaling molecule following NO and CO. It used to be regarded as a poisonous gas. However, as research progresses, an increasing number of experiments have revealed its role as a bioactive molecule in biological systems. There are conflicting results concerning the role of H2S in ischemic stroke.110–114 Chen et al showed that increased production of H2S in the brain was significantly correlated with either poor clinical outcome or early deterioration in clinical stroke.110 There is also strong information supporting the important role that H2S plays in the induction of angiogenesis,111 regulation of neuronal activity,113 vascular relaxation,114 and protection against I/R injury in important organs. It is confirmed that H2S could protect neurons against hypoxic injury via the K(ATP)/PKC/ERK1/2/Hsp90 pathway.115 Wang et al proved that H2S protected blood-brain barrier (BBB) integrity following middle cerebral artery occlusion (MCAO).116 Jang et al reported that treatment with H2S augmented angiogenesis in the peri-infarct area, and it improved functional outcomes after 2 weeks significantly through PI3K/AKT signaling in a rat MCAO model.117 Their findings manifest that H2S has potential therapeutic value in regenerative and even hemodynamic recovery after stroke. Similar to NO, H2S can relax smooth muscle cells and expand blood vessels. H2S synthase has been found in mammals.93 So H2S may have the potential of becoming the mediator of RIC affecting cerebral hemodynamics.
It is reasonable to infer that RIC may induce the generation of endogenous gas molecules through the I/R of distant limbs.93,118 Gas molecules enter the central nervous system through blood circulation, thereby regulating cerebral vascular tone and affecting cerebral hemodynamics. Natural products that are induced by RIC can provide an innovative tool for improving treatments for stroke recovery. More investigations should be done to gain a clearer understanding of these gas molecules. And considerable attention should be paid to RIC as a target for novel therapeutic treatment against for ischemic stroke.
RIC Can Affect the Function of Vascular Endothelial Cells
Blood vessels are composed of the tunica adventitia, tunica media and the tunica intima. The contractile force of blood vessels is mainly regulated by the contractile force of tunica media smooth muscle cells, while the latter are affected by vascular endothelial cells. Vascular endothelial cells can generate NO, prostacyclin, hydrogen peroxide, and hydrogen sulfide, and activate potassium ion channels. Vascular endothelial cells change vascular reactivity through the production and release of vasoactive factors, which can regulate local vascular perfusion and are important substances for regulating vascular tone.119
Nakamura et al studied 15 smokers and 15 non-smokers, who were given upper extremity RIC six times a day for 1 month. The results showed that the RIC stimulation could significantly increase the level of circulating progenitor cells in the non-smoking group, enhance the response of the forearm blood flow to acetylcholine, and enhance the endothelium-dependent vasodilatory function. No such changes were observed in the smoking group. RIC may be a simple and safe treatment for peripheral vascular endothelial protection.120 A recent study also confirmed that RIC can improve the endothelium-dependent vasodilatory function of brachial arteries in the forearm.121
The effector of RIC may act on the whole body through blood circulation. Therefore, it is inferred that the effector produced by RIC may have the same effect on cerebrovascular endothelial cells, thereby affecting cerebrovascular reactivity and improving cerebral hemodynamics.
RIC Can Affect the Nervous System
The nervous system also plays an important part in the effects of RIC on cerebral hemodynamics. Primarily, the proper function of RIC is reliant on the presence of intact neuronal pathways. There is a trend toward attenuation of RIC protection when femoral and sciatic nerves are sectioned in a rabbit model.122 Local muscle ischemia induced through RIC may lead to the release of adenosine bradykinin or opioid, which may activate the nervous system. A recent study confirmed that RIC could reduce the I/R injury of endothelial cells and enhance the endothelium-dependent vasodilatory function by mediating the production of glucagon-like peptide-1, thus affecting the circulatory system. Glucagon-like peptide-1 is an endocrine hormone released by L cells of the small intestine under the regulation of the efferent activity of the vagus nerve.121 It has been discovered that selective severing of the posterior gastric branch of the vagus nerve can eliminate the protective effect of RIC, and stimulation of this branch can induce the protective effect of RIC. Scientists thus believe that the circulating factors of RIC are generated and released into the systemic circulation by the internal organs innervated by the posterior gastric branch of the vagus nerve.112 Activation of the vagus nerve by RIC may also inhibit inflammatory processes mediated by the liver and spleen via the cholinergic anti-inflammatory pathway.123 A study from Azevedo et al showed that neurovascular coupling was impaired in individuals with autonomic dysfunction, and so was cerebrovascular regulation.124 It also has been reported that activation of other parasympathetic nerves by RIC, such as the sphenopalatine ganglion, may increase CBF.125
Therefore, RIC can affect the nervous system, especially the vagus nerve, and exert an influence on cerebral hemodynamics. The vagus nerve may become a potential therapeutic target for ischemic stroke. However, the interaction between the sympathetic and parasympathetic nerves is extremely mazy. It is difficult to affect only one without affecting the other. Further studies are needed to confirm the interaction between the nervous system and its relationship with RIC.
Conclusions
In conclusion, during the initial stage of ischemic stroke, the CBF decreases and the autoregulation of the cerebral vascular system is damaged, leading to cerebral ischemia and hypoxia, and this may lead to a poor prognosis. Multiple mechanisms are involved in the process of regulation of CBF. Several animal experiments and clinical studies have shown that RIC can trigger the endogenous protection mechanisms through a variety of ways, thus having a positive impact on cerebral hemodynamics. RIC has the potential to become a therapeutic treatment to improve CBF during the initial phase of ischemic stroke with the advantages of being simple, safe, non-invasive, and inexpensive. Renewed efforts are needed to improve our understanding of RIC and to provide important insights into developing more effective therapies for ischemic stroke.
Acknowledgments
We thank Liyang Bao for assistance with figure preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. 2015;45(3):161–176. doi:10.1159/000441085
2. Ren C, Li S, Wang B, et al. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav Brain Res. 2018;340:87–93. doi:10.1016/j.bbr.2016.10.036
3. Khan MB, Hafez S, Hoda MN, et al. Chronic remote ischemic conditioning is cerebroprotective and induces vascular remodeling in a VCID model. Transl Stroke Res. 2018;9(1):51–63. doi:10.1007/s12975-017-0555-1
4. Chen GZ, Shan XY, Li XS, Tao HM. Remote ischemic postconditioning protects the brain from focal ischemia/reperfusion injury by inhibiting autophagy through the mTOR/p70S6K pathway. Neurol Res. 2018;40(3):182–188. doi:10.1080/01616412.2018.1424696
5. Cheng X, Zhao H, Yan F, et al. Limb remote ischemic post-conditioning mitigates brain recovery in a mouse model of ischemic stroke by regulating reactive astrocytic plasticity. Brain Res. 2018;1686:94–100. doi:10.1016/j.brainres.2018.02.019
6. Kitagawa K, Saitoh M, Ishizuka K, Shimizu S. Remote limb ischemic conditioning during cerebral ischemia reduces infarct size through enhanced collateral circulation in murine focal cerebral ischemia. J Stroke Cerebrovasc Dis. 2018;27(4):831–838. doi:10.1016/j.jstrokecerebrovasdis.2017.09.068
7. Gao X, Liu Y, Xie Y, Wang Y, Qi S. Remote ischemic postconditioning confers neuroprotective effects via inhibition of the BID-mediated mitochondrial apoptotic pathway. Mol Med Rep. 2017;16(1):515–522. doi:10.3892/mmr.2017.6652
8. Huang D, Liu H, Qu Y, Wang P. Non-invasive remote ischemic postconditioning stimulates neurogenesis during the recovery phase after cerebral ischemia. Metab Brain Dis. 2017;32(6):1805–1818. doi:10.1007/s11011-017-0068-3
9. Khaksari M, Mehrjerdi FZ, Rezvani ME, Safari F, Mirgalili A, Niknazar S. The role of erythropoietin in remote renal preconditioning on hippocampus ischemia/reperfusion injury. J Physiol Sci. 2017;67(1):163–171. doi:10.1007/s12576-016-0451-6
10. Ma J, Ma Y, Dong B, Bandet MV, Shuaib A, Winship IR. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J Cereb Blood Flow Metab. 2017;37(8):3001–3014. doi:10.1177/0271678X16680636
11. Ramagiri S, Taliyan R. Protective effect of remote limb post conditioning via upregulation of heme oxygenase-1/BDNF pathway in rat model of cerebral ischemic reperfusion injury. Brain Res. 2017;1669:44–54. doi:10.1016/j.brainres.2017.05.016
12. Xu W, Jin W, Zhang X, Chen J, Ren C. Remote limb preconditioning generates a neuroprotective effect by modulating the extrinsic apoptotic pathway and TRAIL-receptors expression. Cell Mol Neurobiol. 2017;37(1):169–182. doi:10.1007/s10571-016-0360-5
13. Zhang W, Wang Y, Bi G. Limb remote ischaemic postconditioning-induced elevation of fibulin-5 confers neuroprotection to rats with cerebral ischaemia/reperfusion injury: activation of the AKT pathway. Clin Exp Pharmacol Physiol. 2017;44(6):656–663. doi:10.1111/cep.2017.44.issue-6
14. Chen G, Ye X, Zhang J, et al. Limb remote ischemic postconditioning reduces ischemia-reperfusion injury by inhibiting NADPH oxidase activation and MyD88-TRAF6-P38MAP-kinase pathway of neutrophils. Int J Mol Sci. 2016;17(12):1971. doi:10.3390/ijms17121971
15. Liu ZJ, Chen C, Li XR, et al. Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci Ther. 2016;22(1):43–52. doi:10.1111/cns.12448
16. Wang J, Han D, Sun M, Feng J. A combination of remote ischemic perconditioning and cerebral ischemic postconditioning inhibits autophagy to attenuate plasma HMGB1 and induce neuroprotection against stroke in rat. J Mol Neurosci. 2016;58(4):424–431. doi:10.1007/s12031-016-0724-9
17. Li P, Su L, Li X, et al. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion injury via upregulating expression of Nrf2, HO-1 and NQO-1 in mice. Int J Neurosci. 2016;126(6):552–559. doi:10.3109/00207454.2015.1042973
18. Li S, Hu X, Zhang M, et al. Remote ischemic post-conditioning improves neurological function by AQP4 down-regulation in astrocytes. Behav Brain Res. 2015;289:1–8. doi:10.1016/j.bbr.2015.04.024
19. Li H, Zhou S, Wu L, et al. The role of p38MAPK signal pathway in the neuroprotective mechanism of limb postconditioning against rat cerebral ischemia/reperfusion injury. J Neurol Sci. 2015;357(1–2):270–275. doi:10.1016/j.jns.2015.08.004
20. Qi Z, Dong W, Shi W, et al. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl Stroke Res. 2015;6(3):198–206. doi:10.1007/s12975-015-0393-y
21. Xiao Y, Hafeez A, Zhang Y, et al. Neuroprotection by peripheral nerve electrical stimulation and remote postconditioning against acute experimental ischaemic stroke. Neurol Res. 2015;37(5):447–453. doi:10.1179/1743132815Y.0000000032
22. Zong Y, Jiang L, Zhang M, et al. Limb remote ischemic postconditioning protects cerebral ischemia from injury associated with expression of HIF-1alpha in rats. BMC Neurosci. 2015;16:97. doi:10.1186/s12868-015-0235-6
23. Chen G, Yang J, Lu G, Guo J, Dou Y. Limb remote ischemic post-conditioning reduces brain reperfusion injury by reversing eNOS uncoupling. Indian J Exp Biol. 2014;52(6):597–605.
24. Cheng Z, Li L, Mo X, et al. Non-invasive remote limb ischemic postconditioning protects rats against focal cerebral ischemia by upregulating STAT3 and reducing apoptosis. Int J Mol Med. 2014;34(4):957–966. doi:10.3892/ijmm.2014.1873
25. Hoda MN, Bhatia K, Hafez SS, et al. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl Stroke Res. 2014;5(4):484–490. doi:10.1007/s12975-013-0318-6
26. Hoda MN, Siddiqui S, Herberg S, et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke. 2012;43(10):2794–2799. doi:10.1161/STROKEAHA.112.660373
27. Hu S, Dong H, Zhang H, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012;1459:81–90. doi:10.1016/j.brainres.2012.04.017
28. Peng B, Guo QL, He ZJ, et al. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res. 2012;1445:92–102. doi:10.1016/j.brainres.2012.01.033
29. Qi ZF, Luo YM, Liu XR, et al. AKT/GSK3beta-dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neurosci Ther. 2012;18(12):965–973. doi:10.1111/cns.12016
30. Sun J, Tong L, Luan Q, et al. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J Cereb Blood Flow Metab. 2012;32(5):851–859. doi:10.1038/jcbfm.2011.199
31. Wei D, Ren C, Chen X, Zhao H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012;7(2):e30892. doi:10.1371/journal.pone.0030892
32. Yuan HJ, Zhu XH, Luo Q, et al. Noninvasive delayed limb ischemic preconditioning in rats increases antioxidant activities in cerebral tissue during severe ischemia-reperfusion injury. J Surg Res. 2012;174(1):176–183. doi:10.1016/j.jss.2010.11.001
33. Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2011;42(10):2960–2962. doi:10.1161/STROKEAHA.111.622340
34. Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94. doi:10.1016/j.brainres.2009.07.029
35. Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151(4):1099–1103. doi:10.1016/j.neuroscience.2007.11.056
36. Che R, Zhao W, Ma Q, et al. rt-PA with remote ischemic postconditioning for acute ischemic stroke. Ann Clin Transl Neurol. 2019;6(2):364–372. doi:10.1002/acn3.2019.6.issue-2
37. Zhao W, Che R, Li S, et al. Remote ischemic conditioning for acute stroke patients treated with thrombectomy. Ann Clin Transl Neurol. 2018;5(7):850–856. doi:10.1002/acn3.2018.5.issue-7
38. Li Y, Liang K, Zhang L, Hu Y, Ge Y, Zhao J. Upper limb ischemic postconditioning as adjunct therapy in acute stroke patients: a randomized pilot. J Stroke Cerebrovasc Dis. 2018;27(11):3328–3335. doi:10.1016/j.jstrokecerebrovasdis.2018.07.039
39. England TJ, Hedstrom A, O’Sullivan S, et al. RECAST (remote ischemic conditioning after stroke trial): a pilot randomized placebo controlled Phase II trial in acute ischemic stroke. Stroke. 2017;48(5):1412–1415. doi:10.1161/STROKEAHA.116.016429
40. Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45(1):159–167. doi:10.1161/STROKEAHA.113.001346
41. Zhao W, Meng R, Ma C, et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, randomized controlled trial. Circulation. 2017;135(14):1325–1335. doi:10.1161/CIRCULATIONAHA.116.024807
42. Wang JY, Shen J, Gao Q, et al. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39(3):983–990. doi:10.1161/STROKEAHA.107.499079
43. Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26(9):1114–1121. doi:10.1038/sj.jcbfm.9600348
44. Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853–1861. doi:10.1212/WNL.0b013e318271f76a
45. Karapanayiotides T, Meuli R, Devuyst G, et al. Postcarotid endarterectomy hyperperfusion or reperfusion syndrome. Stroke. 2005;36(1):21–26. doi:10.1161/01.STR.0000149946.86087.e5
46. Bor-Seng-Shu K, Kita WS, Figueiredo EG, et al. Cerebral hemodynamics: concepts of clinical importance. Arq Neuropsiquiatr. 2012;70:357–365. doi:10.1590/S0004-282X2012000500010
47. Liu Z, Liu HY, Zhou H, et al. Moderate-intensity exercise affects gut microbiome composition and influences cardiac function in myocardial infarction mice. Front Microbiol. 2017;8:1687. doi:10.3389/fmicb.2017.01687
48. Chapman SB, Aslan S, Spence JS, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5:75. doi:10.3389/fnagi.2013.00075
49. Leardini-Tristao M, Borges JP, Freitas F, et al. The impact of early aerobic exercise on brain microvascular alterations induced by cerebral hypoperfusion. Brain Res. 2017;1657:43–51. doi:10.1016/j.brainres.2016.11.030
50. van Lieshout JJ, Pott F, Madsen PL, van Goudoever J, Secher NH. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke. 2001;32(7):1546–1551. doi:10.1161/01.STR.32.7.1546
51. Rojek A, Gasecki D, Fijalkowski M, et al. Left ventricular ejection fraction and aortic stiffness are independent predictors of neurological outcome in acute ischemic stroke. J Hypertens. 2016;34(12):2441–2448. doi:10.1097/HJH.0000000000001095
52. Michelsen MM, Stottrup NB, Schmidt MR, et al. Exercise-induced cardioprotection is mediated by a bloodborne, transferable factor. Basic Res Cardiol. 2012;107(3):260. doi:10.1007/s00395-012-0260-x
53. Kono Y, Fukuda S, Hanatani A, et al. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Devel Ther. 2014;8:1175–1181. doi:10.2147/DDDT.S68715
54. Pryds K, Nielsen RR, Jorsal A, et al. Effect of long-term remote ischemic conditioning in patients with chronic ischemic heart failure. Basic Res Cardiol. 2017;112(6):67. doi:10.1007/s00395-017-0658-6
55. Chen L, Zhou Q, Jin H, et al. Effects of remote ischaemic conditioning on heart rate variability and cardiac function in patients with mild ischaemic heart failure. Heart Lung Circ. 2018;27(4):477–483. doi:10.1016/j.hlc.2017.03.164
56. Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47(1):32–40. doi:10.1016/j.yjmcc.2009.03.019
57. Youn YJ, Yoo BS, Son JW, et al. Remote ischemic conditioning by effluent collected from a novel isolated hindlimb model reduces infarct size in an isolated heart model. Korean Circ J. 2017;47(5):714–726. doi:10.4070/kcj.2017.0092
58. Tamareille S, Mateus V, Ghaboura N, et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011;106(6):1329–1339. doi:10.1007/s00395-011-0210-z
59. Huang J, Xu D, Guo Q, et al. Remote ischemic postconditioning improves myocardial dysfunction via the risk and safe pathways in a rat model of severe hemorrhagic shock. Shock (Augusta, Ga). 2018;49(4):460–465. doi:10.1097/SHK.0000000000000940
60. Skyschally A, Kleinbongard P, Lieder H, et al. Humoral transfer and intramyocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice. Am J Physiol Heart Circ Physiol. 2018;315(1):H159–h172. doi:10.1152/ajpheart.00152.2018
61. Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26(7):1789–1797.
62. Liu J, Wang Y, Akamatsu Y, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi:10.1016/j.pneurobio.2013.11.004
63. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25(9):1794–1798. doi:10.1161/01.STR.25.9.1794
64. Zhang Y, Ma L, Ren C, et al. Immediate remote ischemic postconditioning reduces cerebral damage in ischemic stroke mice by enhancing leptomeningeal collateral circulation. J Cell Physiol. 2018.
65. Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134(15):2709–2718. doi:10.1242/dev.004184
66. Lawson ND, Scheer N, Pham VN et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;1128(19):3675–83.
67. Liu XL, Wang G, Song W, Yang WX, Hua J, Lyu L. microRNA-137 promotes endothelial progenitor cell proliferation and angiogenesis in cerebral ischemic stroke mice by targeting NR4A2 through the Notch pathway. J Cell Physiol. 2018;233(7):5255–5266. doi:10.1002/jcp.26312
68. Chang L, Noseda M, Higginson M, et al. Differentiation of vascular smooth muscle cells from local precursors during embryonic and adult arteriogenesis requires Notch signaling. Proc Natl Acad Sci U S A. 2012;109(18):6993–6998. doi:10.1073/pnas.1118512109
69. Kikuchi R, Takeshita K, Uchida Y, et al. Pitavastatin-induced angiogenesis and arteriogenesis is mediated by Notch1 in a murine hindlimb ischemia model without induction of VEGF. Lab Invest. 2011;91(5):691–703. doi:10.1038/labinvest.2011.5
70. Jeffrey Man HS, Tsui AK, Marsden PA. Nitric oxide and hypoxia signaling. Vitam Horm. 2014;96:161–192.
71. Murohara T, Asahara T, Silver M, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101(11):2567–2578. doi:10.1172/JCI1560
72. Ren C, Li N, Li S, et al. Limb ischemic conditioning improved cognitive deficits via eNOS-dependent augmentation of angiogenesis after chronic cerebral hypoperfusion in rats. Aging Dis. 2018;9(5):869–879. doi:10.14336/AD.2017.1106
73. Hess DC, Hoda MN, Khan MB. Humoral mediators of remote ischemic conditioning: important role of eNOS/NO/Nitrite. Acta Neurochir Suppl. 2016;121:45–48.
74. Hoda MN, Hess DC, Ergul A, Fagan SC. Response to letter regarding article, “Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type”. Stroke. 2013;44(4):e37. doi:10.1161/STROKEAHA.111.000541
75. Wang X, Freire Valls A, Schermann G, et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev Cell. 2017;42(5):462–478.e467. doi:10.1016/j.devcel.2017.08.002
76. Chakrabarti S, Rizvi M, Morin K, Garg R, Freedman JE. The role of CD40L and VEGF in the modulation of angiogenesis and inflammation. Vascul Pharmacol. 2010;53(3–4):130–137. doi:10.1016/j.vph.2010.05.003
77. Ashina K, Tsubosaka Y, Kobayashi K, Omori K, Murata T. VEGF-induced blood flow increase causes vascular hyper-permeability in vivo. Biochem Biophys Res Commun. 2015;464(2):590–595. doi:10.1016/j.bbrc.2015.07.014
78. Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi:10.1097/00004647-200204000-00002
79. Sorond FA, Tan CO, LaRose S, et al. Deferoxamine, cerebrovascular hemodynamics, and vascular aging: potential role for hypoxia-inducible transcription factor-1-regulated pathways. Stroke. 2015;46(9):2576–2583. doi:10.1161/STROKEAHA.115.009906
80. Zhou M, Lu S, Lu G, et al. Effects of remote ischemic postconditioning on fracture healing in rats. Mol Med Rep. 2017;15(5):3186–3192. doi:10.3892/mmr.2017.6348
81. Limani P, Linecker M, Oberkofler CE, et al. Remote ischemic preconditioning: a novel strategy in rescuing older livers from ischemia-reperfusion injury in a rodent model. Ann Surg. 2016;264(5):797–803. doi:10.1097/SLA.0000000000001765
82. Ueno K, Samura M, Nakamura T, et al. Increased plasma VEGF levels following ischemic preconditioning are associated with downregulation of miRNA-762 and miR-3072-5p. Sci Rep. 2016;6:36758. doi:10.1038/srep36758
83. Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10(10):909–921. doi:10.1016/S1474-4422(11)70195-8
84. Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi:10.1038/nrn1106
85. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi:10.1016/j.neuron.2008.01.003
86. Tan CO, Taylor JA. Integrative physiological and computational approaches to understand autonomic control of cerebral autoregulation. Exp Physiol. 2014;99(1):3–15. doi:10.1113/expphysiol.2013.072355
87. McConnell HL, Kersch CN, Woltjer RL, Neuwelt EA. The translational significance of the neurovascular unit. J Biol Chem. 2017;292(3):762–770. doi:10.1074/jbc.R116.760215
88. Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6(1):43–50. doi:10.1038/nn980
89. Del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158(3):972–982. doi:10.1016/j.neuroscience.2008.08.028
90. Han D, Zhang S, Fan B, et al. Ischemic postconditioning protects the neurovascular unit after focal cerebral ischemia/reperfusion injury. J Mol Neurosci. 2014;53(1):50–58. doi:10.1007/s12031-013-0196-0
91. Rassaf T, Heiss C, Hendgen-Cotta U, et al. Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic Biol Med. 2006;41(2):295–301. doi:10.1016/j.freeradbiomed.2006.04.006
92. Zhou JL, Zhu XG, Ling T, Zhang JQ, Chang JY. Effect of endogenous carbon monoxide on oxidant-mediated multiple organ injury following limb ischemia-reperfusion in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2002;16(4):273–276.
93. Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2015;172(6):1587–1606. doi:10.1111/bph.12811
94. Kajita Y, Takayasu M, Dietrich HH, Dacey RG
95. White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clin Sci. 2000;99(6):555–560. doi:10.1042/cs0990555
96. Pastor P, Curvello V, Hekierski H, Armstead WM. Inhaled nitric oxide protects cerebral autoregulation through prevention of impairment of ATP and calcium sensitive K channel mediated cerebrovasodilation after traumatic brain injury. Brain Res. 2019;1711:1–6. doi:10.1016/j.brainres.2019.01.008
97. Curvello V, Pastor P, Hekierski H, Armstead WM. Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury through inhibition of ET-1, ERK MAPK and IL-6 upregulation in pigs. Neurocrit Care. 2019;30(2):467–477. doi:10.1007/s12028-018-0638-1
98. Hekierski H, Pastor P, Curvello V, Armstead WM. Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal neuronal cell necrosis after traumatic brain injury in newborn and juvenile pigs. J Neurotrauma. 2019;36(4):630–638. doi:10.1089/neu.2018.5824
99. Thompson BG, Pluta RM, Girton ME, Oldfield EH. Nitric oxide mediation of chemoregulation but not autoregulation of cerebral blood flow in primates. J Neurosurg. 1996;84(1):71–78. doi:10.3171/jns.1996.84.1.0071
100. Zhang R, Wilson TE, Witkowski S, Cui J, Crandall GG, Levine BD. Inhibition of nitric oxide synthase does not alter dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol. 2004;286(3):H863–H869.
101. Choi YK. Role of carbon monoxide in neurovascular repair processing. Biomol Ther (Seoul). 2018;26(2):93–100. doi:10.4062/biomolther.2017.144
102. Choi YK, Maki T, Mandeville ET, et al. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med. 2016;22(11):1335–1341. doi:10.1038/nm.4188
103. Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42(9):2605–2610. doi:10.1161/STROKEAHA.110.607101
104. Chodorowski Z, Sein Anand J, Nowak-Banasik L, Szydlowska M, Klimek J, Kaletha K. Carbon monoxide–a regulator of vascular tone in hypoxia? Przegl Lek. 2005;62(6):438–440.
105. Tykocki NR, Boerman EM, Jackson WF. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr Physiol. 2017;7(2):485–581.
106. Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost. 2009;7(8):1401–1408. doi:10.1111/j.1538-7836.2009.03478.x
107. Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science (New York, NY). 1993;260(5116):1946–1950. doi:10.1126/science.8100368
108. Nada SE, Tulsulkar J, Shah ZA. Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761(R)) after permanent ischemic stroke in mice. Mol Neurobiol. 2014;49(2):945–956. doi:10.1007/s12035-013-8572-x
109. Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science (New York, NY). 1993;259(5093):381–384. doi:10.1126/science.7678352
110. Wong PT, Qu K, Chimon GN, et al. High plasma cyst(e)ine level may indicate poor clinical outcome in patients with acute stroke: possible involvement of hydrogen sulfide. J Neuropathol Exp Neurol. 2006;65(2):109–115. doi:10.1097/01.jnen.0000199571.96472.c7
111. Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106(51):21972–21977. doi:10.1073/pnas.0908047106
112. Mastitskaya S, Basalay M, Hosford PS, Ramage AG, Gourine A, Gourine AV. Identifying the source of a humoral factor of remote (Pre)conditioning cardioprotection. PLoS One. 2016;11(2):e0150108. doi:10.1371/journal.pone.0150108
113. Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide Biol Chem. 2014;41:4–10. doi:10.1016/j.niox.2014.01.002
114. Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science (New York, NY). 2008;322(5901):587–590. doi:10.1126/science.1162667
115. Tay AS, Hu LF, Lu M, Wong PT, Bian JS. Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience. 2010;167(2):277–286. doi:10.1016/j.neuroscience.2010.02.006
116. Wang Y, Jia J, Ao G, et al. Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J Neurochem. 2014;129(5):827–838. doi:10.1111/jnc.12695
117. Jang H, Oh MY, Kim YJ, et al. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res. 2014;92(11):1520–1528. doi:10.1002/jnr.23427
118. Shahid M, Tauseef M, Sharma KK, Fahim M. Brief femoral artery ischaemia provides protection against myocardial ischaemia-reperfusion injury in rats: the possible mechanisms. Exp Physiol. 2008;93(8):954–968. doi:10.1113/expphysiol.2007.041442
119. Zaborska KE, Wareing M, Austin C. Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone. Br J Pharmacol. 2017;174(20):3388–3397. doi:10.1111/bph.v174.20
120. Nakamura S, Kimura M, Goto C, et al. Cigarette smoking abolishes ischemic preconditioning-induced augmentation of endothelium-dependent vasodilation. Hypertension. 2009;53(4):674–681. doi:10.1161/HYPERTENSIONAHA.108.126078
121. Verouhis D, Saleh N, Settergren M, Sorensson P, Gourine A, Pernow J. Remote ischemic conditioning protects against endothelial ischemia-reperfusion injury via a glucagon-like peptide-1 receptor-mediated mechanism in humans. Int J Cardiol. 2019;274:40–44. doi:10.1016/j.ijcard.2018.09.061
122. Donato M, Buchholz B, Rodriguez M, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol. 2013;98(2):425–434. doi:10.1113/expphysiol.2012.066217
123. Hess DC, Hoda MN, Bhatia K. Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke. 2013;44(4):1191–1197. doi:10.1161/STROKEAHA.112.678482
124. Azevedo E, Castro P, Santos R, et al. Autonomic dysfunction affects cerebral neurovascular coupling. Clin Auton Res. 2011;21(6):395–403. doi:10.1007/s10286-011-0129-3
125. Weber C. Far from the heart: receptor cross-talk in remote conditioning. Nat Med. 2010;16(7):760–762. doi:10.1038/nm0710-760
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.