Back to Journals » Drug Design, Development and Therapy » Volume 18
Effects of Remifentanil Gradual Withdrawal Combined with Postoperative Infusion on Postoperative Hyperalgesia in Patients Undergoing Laparoscopic hysterectomy: A Factorial Design, Double-Blind, Randomized Controlled Trial
Authors Luo M, Han X , Li H, Zhou G, Chen H, Gao F
Received 27 November 2023
Accepted for publication 20 February 2024
Published 27 February 2024 Volume 2024:18 Pages 583—595
DOI https://doi.org/10.2147/DDDT.S451913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Meng Luo,1,2,* Xue Han,1,2,* Huan Li,1,2 Guangyue Zhou,1,2 Haoxuan Chen,1,2 Fang Gao1,2
1Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang Gao, Department of Anesthesiology, the Affiliated Hospital of Xuzhou Medical University, No. 99 Huaihai West Road, Xuzhou, Jiangsu, 221002, People’s Republic of China, Tel +86-18052268331, Email [email protected]
Background: Remifentanil-induced hyperalgesia (RIH) increases the risk of persistent postoperative pain, making early postoperative analgesic therapy ineffective and affecting postoperative patient satisfaction. This study aimed to verify the effects of gradual withdrawal of remifentanil combined with postoperative pump infusion of remifentanil on postoperative hyperalgesia and pain in patients undergoing laparoscopic hysterectomy.
Methods: This trial was a factorial design, double-blind, randomized controlled trial. Patients undergoing laparoscopic hysterectomy were randomly allocated to the control group, postoperative pump infusion of remifentanil group, gradual withdrawal of remifentanil group, or gradual withdrawal plus postoperative pump infusion of remifentanil group (n = 35 each). The primary outcome was postoperative mechanical pain thresholds in the medial forearm. The secondary outcomes included postoperative mechanical pain thresholds around the incision, pain numeric rating scale scores, analgesic utilization, awakening agitation or sedation scores, a 15-item quality of recovery survey, and postoperative complications.
Results: Gradual withdrawal of remifentanil significantly increased postoperative pain thresholds versus abrupt discontinuation (P < 0.05), whereas postoperative infusion did not show significant differences compared to the absence of infusion (P > 0.05). The combined gradual withdrawal and postoperative infusion group exhibited the highest thresholds and had the lowest postoperative pain scores and analgesic requirements as well as the highest quality of recovery scores (P < 0.05). No significant differences were observed for agitation scores, sedation scores, or complication rates (P > 0.05).
Conclusion: The novel combined gradual withdrawal and postoperative infusion of remifentanil uniquely attenuates postoperative hyperalgesia, pain severity, analgesic necessity, and improves recovery quality after laparoscopic hysterectomy.
Keywords: opioid-induced hyperalgesia, remifentanil-induced hyperalgesia, laparoscopic hysterectomy, mechanical pain thresholds, postoperative recovery
Introduction
Opioid-induced hyperalgesia (OIH) represents a paradoxical reduction in pain thresholds following opioid administration, manifesting primarily as hypersensitivity to painful stimuli.1 Proposed OIH mechanisms include increased activation of downstream pain pathways by opioids,2 the induction of long-term potentiation (LTP) at synapses,3 inflammatory responses with spinal glial cell activation,4 the role of endogenous neuropeptides,5 alterations in opioid receptor function and number6 and reduced function of inhibitory neurotransmitter receptors7 among others. Studies have shown that chronic pain occurs in about 5–32% of hysterectomy patients.8 Inadequate perioperative analgesia and unchecked hyperalgesia engender significant risk for the development of intractable postoperative pain syndromes.9 Remifentanil possesses unique pharmacokinetic properties, including rapid onset, fleeting half-life, and independence of hepatic and renal clearance, allowing for sustained infusion without bioaccumulation. This pharmacologic profile has enabled the widespread utilization of remifentanil for both acute and chronic analgesia as well as the maintenance of general anesthesia.10 However, investigations have revealed that among opioid analgesics, remifentanil elicits the highest rates of OIH.11
In recent years, diverse pharmacological interventions such as ketamine, colistin, non-steroidal anti-inflammatory drugs (NSAIDs), nitric oxide, and magnesium sulfate have demonstrated efficacy in attenuating remifentanil-induced hyperalgesia (RIH) to varying extents in animal models and clinical trials.12–16 However, supplemental dosing regimens introduce the potential for adverse effects. Comelon proposed that gradual withdrawal of remifentanil may confer a non-pharmacological approach to preventing OIH.17 One study found that approximately 36% of patients undergoing gradual remifentanil cessation required rescue analgesia in the postanesthesia care unit (PACU), but demonstrated that the addition of a postoperative remifentanil infusion after tapering significantly reduced rescue analgesia requirements and pain numeric rating scale (NRS) scores without increasing side effects compared to tapering alone.18 The efficacy of combining gradual withdrawal with postoperative pump infusion of remifentanil in mitigating postoperative hyperalgesia and acute pain has not been prospectively evaluated. Therefore, this study aimed to determine the efficacy of this novel regimen in attenuating postoperative hyperalgesia and acute pain. We hypothesized that both gradual withdrawal of remifentanil and postoperative pump infusion of remifentanil would reduce the incidence and severity of postoperative hyperalgesia in patients undergoing laparoscopic hysterectomy. Additionally, we anticipated these methods would provide superior analgesia, improve quality of recovery without increasing adverse effects, and that the combination of the two methods would be superior to the use of a single agent.
Materials and Methods
Participants
The study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University on April 13, 2023 (no. XYFY2023-KL049-02). The trial was registered before patient enrollment with the Chinese Clinical Trial Registry (ChiCTR2300074524). Written informed consent was obtained from all patients.
Patients who underwent laparoscopic hysterectomy under general anesthesia at the Affiliated Hospital of Xuzhou Medical University between April 2023 and October 2023 were selected for the study. Inclusion criteria: (1) patients who underwent elective laparoscopic hysterectomy; (2) age 18–65 years; (3) ASA I–II; (4) BMI 18–30 kg/m2; (5) voluntary provision of informed consent. Exclusion criteria: (1) contraindications to surgery or anesthesia; (2) allergy or contraindication to remifentanil; (3) neuropsychiatric disorders, drug and alcohol abuse; (4) significant cardiovascular, cerebrovascular, gastrointestinal, pulmonary, hematologic, or neurological comorbidity; (5) opioid or analgesic use within 48 hours preoperatively; (6) symptoms or history suggestive of peripheral or central neuropathy or chronic pain syndromes; (7) the surgical procedure has changed; (8) patients refused to participate in the trial.
Randomization of the Study Participants
A randomized number table was used to divide the patients into the control group (C group), postoperative pump infusion of remifentanil group (P group), gradual withdrawal of remifentanil group (G group), and gradual withdrawal plus postoperative pump infusion of remifentanil group (GP group). The details of each patient’s remifentanil administration method were stored in an opaque, sealed envelope and were opened only by researchers before anesthesia induction. All participants, preoperative and postoperative follow-up assessors, and statisticians were blinded to group allocation.
Intraoperative Anesthesia Management
Basal pain thresholds were obtained preoperatively by designated staff not involved in anesthesia or surgery.
After fasting, patients were monitored for peripheral oxygen saturation (SpO2), heart rate (HR), non-invasive blood pressure (NIBP), electrocardiogram and partial pressure of end-tidal carbon dioxide (PETCO2), and the range of carbon dioxide pneumoperitoneal pressure was maintained at 12–14 mmHg. All patients were given intravenous midazolam (0.04 mg/kg), etomidate (0.3 mg/kg), sufentanil (0.4 μg/kg), and rocuronium (1 mg/kg) for induction of anesthesia. Following the induction, tracheal intubation was performed using a video laryngoscopy technique. This was achieved under careful monitoring, confirmed by bilateral chest auscultation to verify effective mechanical ventilation. Subsequently, patients were connected to an anesthesia machine after tracheal intubation and mechanically ventilated. The tidal volume was set at 6–8 mL/ kg and the respiratory rate at 12–16 bpm, and the PETCO2 was maintained between 35–45 mmHg. Sevoflurane combined with remifentanil was used to maintain anesthesia, the initial end-expiratory concentration of sevoflurane was 2.0%, and the depth of anesthesia was adjusted to the bispectral index (BIS) value of 40–60. Sevoflurane concentration was adjusted according to the BIS, the mean arterial pressure (MAP) and the heart rate, and inotropes were given intermittently during the operation. The patients were continuously infused with crystalloid intraoperatively, when the BP was lower than 20% of the basal value, rapid infusion of fluids was used to expand the volume. Intravenous phenylephrine (40 μg/dose) or ephedrine (3 mg/dose) was given if necessary, and when the heart rate was lower than 45 beats/min, intravenous atropine (0.3–0.5 mg/dose) was given. At the time of skin suturing, sevoflurane was stopped and intravenous tropisetron (2 mg) was given. Remifentanil was stopped at the end of skin suturing. Patients were admitted to PACU after surgery.
In the PACU, any residual neuromuscular blockade was reversed with neostigmine (0.04 mg/kg) and atropine (0.01 mg/kg) once the tidal volume of spontaneous ventilation surpassed 200 mL. Patients were continuously monitored for the return of consciousness, evidenced by appropriate responses to verbal cueing. Tracheal extubation was performed when patients exhibited spontaneous eye opening and a respiratory rate exceeding 10 breaths per minute voluntarily. Pain scores were evaluated every 10 minutes post-extubation. Fentanyl (50 μg) was given for pain scores greater than 3 or intolerable pain. The patients were reassessed in 10 minutes, and the dose was repeated if needed. Flurbiprofen (50 mg) was added if 2 fentanyl doses were ineffective. Patients were discharged to the ward after acceptable pain scores and no nausea or vomiting. Flurbiprofen was given for pain scores greater than 4 every 6 hours in the ward.
Methods of Remifentanil Administration
C group (control group): A continuous intraoperative infusion of remifentanil at a rate of 0.3 μg/kg/min was discontinued at the end of the suture. Patients were given a pump infusion of 20 mL of saline for 30 minutes immediately after tracheal extubation.
P group (postoperative pump infusion of remifentanil group): Remifentanil was continuously infused at a rate of 0.3 μg/kg/min intraoperatively, and was stopped at the end of the suture. Immediately after the patient’s tracheal extubation, 1μg/kg of remifentanil was diluted in 20 mL of saline and pumped continuously for 30 minutes.
G group (gradual withdrawal of remifentanil group): Remifentanil was continuously infused at a rate of 0.3 μg/kg/min intraoperatively. The rate was adjusted to 0.2 μg/kg/min 30 minutes before the expected end of the operation, and then to 0.1 μg/kg/min after 15 minutes, and was discontinued at the end of the suture. The patient was given a pump infusion of 20 mL of saline for 30 minutes immediately after tracheal extubation.
GP group (gradual withdrawal plus postoperative pump infusion of remifentanil group): Remifentanil was continuously infused at a rate of 0.3 μg/kg/min intraoperatively. The rate was adjusted to 0.2 μg/kg/min 30 minutes before the expected end of the operation, and then to 0.1 μg/kg/min after 15 minutes, and was discontinued at the end of the suture. Immediately after the patient’s tracheal extubation, 1ug/kg of remifentanil was diluted in 20 mL of saline and pumped continuously for 30 minutes.
Outcome Measures
Primary Outcomes
The mechanical pain thresholds of the medial forearm were recorded in the four groups of patients at 1, 6, 24, and 48 hours postoperatively, with an interval of 30 seconds between each measurement, and the average value was taken after repeating the measurement five times.
Secondary Outcomes
The mechanical pain thresholds around the incision (2 cm directly below the umbilical incision) at 1, 6, 24, and 48 hours postoperatively in all four groups. The pain NRS scores at rest and during coughing among the four groups of patients immediately, at 10 minutes, 20 minutes, 30 minutes after tracheal extubation and at 1, 6, 12, 24, and 48 hours after surgery (the highest recorded NRS score was used as the outcome measure). Fentanyl consumption in the PACU, flurbiprofen axetil use within 48 hours after surgery, and adverse events were recorded. We compared the patients’ Sedation-Agitation Scale (SAS) agitation scores during the awakening period and Ramsay sedation scores of the patients immediately, at 10 minutes, 20 minutes, 30 minutes and at 1 hour after tracheal extubation. The 15-item quality of recovery (QoR-15) survey was administered at 24 and 48 hours postoperatively (The QoR-15 is a patient-reported outcome questionnaire that measures the quality of recovery after surgery and anaesthesia,19 with higher scores indicating better quality of recovery for patients). The time to first flatus and the total length of the postoperative hospital stay were also documented.
Sample Size
The sample size was calculated based on the primary outcome (the mechanical pain thresholds of the medial forearm at 1 hour postoperatively in each group). We assumed that there would be no interaction between the two methods. The differences in pain thresholds for each group were obtained from the pretest portion of the data. The mean ± SD of the mechanical pain thresholds at 1 hour postoperatively was 6.8 ± 4.237 in group C, 8.9 ± 4.321 in group P, 15.7 ± 3.81 in group G, and 22.4 ± 3.95 in group GP (10 patients in each group). The sample size was calculated according to a test level of 0.05 and a power of 0.80, using the PASS v21.0.3 software (NCSS, LLC, Kaysville, Utah, USA), with a 1:1:1:1 allocation ratio, resulting in a required sample size of 116 cases (29 cases in each group). Considering a 20% dropout rate, 37 patients were included in each group.
Statistical Analysis
The SPSS v25.0 (IBM, Armonk, NY, USA) and Prism v8.0.1 (GraphPad, La Jolla, CA, USA) software programs were used for statistical analyses. Data normality was measured with the Shapiro–Wilk test. The Levene test was used to verify the homogeneity of variance. Normally distributed data were reported as the mean and standard deviation, and non-normally distributed data were presented as the median and interquartile range. Analysis of variance (ANOVA) was chosen for normally distributed and variance-aligned data, and the Kruskal–Wallis test (multi-sample rank-sum test) was chosen for non-normally distributed data. Repeated-measures data were analyzed using ANOVA for repeated measures, and the Bonferroni correction was applied for post hoc comparisons. Non-normally distributed data collected at different time points (such as pain NRS scores) were analyzed using a generalized estimating equation. Categorical variables were described as numbers (%) and were analyzed with the chi-squared test or Fisher’s exact test. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant for the differences tested.
Results
A total of 160 patients were screened and evaluated for enrollment, of whom 12 were excluded (7 not meeting inclusion criteria, 3 meeting exclusion criteria, 2 refused participation). A total of 148 were randomized to the four groups with 37 per group. One patient had intraoperative ovarian cancer conversion to laparotomy, 2 had additional procedures, and five withdrew. Finally, 140 patients completed the trial for analysis. The flow chart of patient selection is shown in Figure 1.
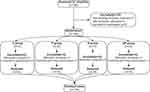 |
Figure 1 Flow diagram of the study. |
No significant inter-group differences were discerned with respect to baseline characteristics, intraoperative parameters including operative duration, anesthesia time, time to awakening, tracheal extubation time, PACU length of stay, medication use, or total postoperative hospitalization (all P > 0.05), as delineated in Table 1. Likewise, no hemodynamic differences were observed between groups at any timepoint as assessed by MAP and HR (all P > 0.05), as shown in Figure 2.
 |
Table 1 Baseline Patient Characteristics and Anesthetic Management Parameters |
Analysis of outcomes revealed no significant interaction between gradual cessation of remifentanil and postoperative infusion on mechanical pain thresholds of the medial forearm or surgical incision at any postoperative juncture (medial forearm threshold: 1 hour, P = 0.473; 6 hours, P = 0.284; 24 hours, P = 0.476; 48 hours, P = 0.843. Incisional threshold: 1 hour, P = 0.609; 6 hours, P = 0.612; 24 hours, P = 0.909; 48 hours, P = 0.313). Examining the independent effects of each intervention, gradual remifentanil discontinuation conferred significant elevations in postoperative pain thresholds compared to abrupt cessation (P < 0.05), whereas the effect of postoperative infusion did not differ significantly from absence of infusion (P > 0.05), as delineated in Table 2.
 |
Table 2 Mechanical Pain Thresholds in the Medial Forearm and Around the Incision |
The preoperative thresholds did not differ between groups (P > 0.05). With Bonferroni comparison, the G group had higher medial forearm thresholds at 1 and 6 hours postoperative versus C group (P < 0.05), and the GP group had higher medial forearm thresholds at all timepoints and higher incisional thresholds at 6, 24, and 48 hours postoperative versus C group (P < 0.05). The GP group also had higher medial forearm thresholds at 1 and 6 hours and higher incisional thresholds at 6 hours postoperative versus the P group. All groups except GP had significantly decreased thresholds postoperatively versus preoperatively (P < 0.05), with the C and P group not recovering by 48 hours after surgery (P < 0.05). The G group recovered medial (P > 0.05) but not incisional thresholds (P < 0.05) by 48 hours after surgery, while the GP group maintained medial forearm thresholds with delayed incisional recovery, as shown in Table 3, Figure 3A and B.
 |
Table 3 Comparison of Mechanical Pain Thresholds and NRS Scores in Four Groups of Patients |
Post-extubation pain NRS scores differed significantly between groups over time. The P group had lower scores than the C group at 10 and 20 minutes after tracheal extubation (P < 0.05). The G group had lower scores than the P group at 10 minutes after tracheal extubation (P < 0.05). The GP group had lower scores than the C group and the G group from 10 minutes after tracheal extubation through 48 hours postoperatively (P < 0.05). The GP group also had lower scores than P group from 30 minutes after tracheal extubation through 48 hours postoperatively (P < 0.05), as shown in Table 3.
More patients in the C, P, and G groups required fentanyl in the PACU versus the GP group (P < 0.05). No patients received flurbiprofen axetil in the PACU. All groups used similar flurbiprofen axetil amounts on the ward (P > 0.05). The QoR-15 scores were higher for the GP group versus other groups at 24 and 48 hours postoperatively (P < 0.05). No differences were seen in awakening agitation or sedation scores (P > 0.05). Adverse reaction rates were similar between groups (P > 0.05), as shown in Table 4.
 |
Table 4 Comparison of Postoperative Conditions Among the Four Groups |
Discussion
The heightened risk of OIH with remifentanil versus other opioids potentially relates to its accelerated onset and elimination kinetics.20 Prior reports have documented a 32.7% incidence of RIH in surgeries exceeding 2 hours,21 escalating to 41.8% with infusions surpassing 30 μg/kg.22 In this prospective randomized controlled trial, diminished postoperative pain thresholds in controls confirm that sustained intraoperative remifentanil elicits postoperative nociceptive sensitization, corroborating previous evidence,20 and amplifying the risk of chronic pain.23 Previous studies implemented a three-stage slow withdrawal of remifentanil to alleviate RIH from high-dose infusions.17,24 This gradual withdrawal protocol was also adopted in our study. However, earlier work found that gradual discontinuation did not significantly reduce overall opioid use, pain, sedation, or recovery compared to abrupt discontinuation.25 Enhanced recovery after surgery (ERAS) principles advocate combining techniques for synergistic analgesia, reduced side effects, and improved experience.26 One retrospective study suggested gradual withdrawal of remifentanil plus postoperative infusion reduced rescue analgesia and pain NRS scores versus tapering alone.18 However, randomization was lacking and specific quantitative hyperalgesia assessments were not utilized. Instead, pain was documented using NRS scores and postoperative analgesic use, which can be influenced by factors such as patient mood, education, cognition, and attention.27,28 Therefore, this study prospectively investigated if combining gradual withdrawal of remifentanil and postoperative pump infusion of remifentanil, alone and together, relieves RIH in laparoscopic hysterectomy using mechanical pain thresholds and pain scores, examining their interaction and adverse effects.
The lack of a significant interaction between remifentanil tapering and pumping for postoperative hypersensitivity may relate to their different mechanisms. The use of remifentanil in vivo leads to acute depression of synaptic strength in C-fibers; upon withdrawal, synaptic strength not only quickly returns to normal but becomes potentiated for prolonged periods of time.29 Abrupt withdrawal induced spinal synaptic LTP whereas tapering did not, suggesting tapering prevents RIH.30 In their research, Koppert et al observed that remifentanil significantly decreased pain ratings and puncture hyperalgesia only during the period of infusion.15 Post-infusion evaluations revealed an increase in pain scores and areas of puncture hyperalgesia around puncture sites, surpassing baseline levels, with the peak analgesic effect noted approximately 30 minutes following the cessation of remifentanil administration. Subsequently, a gradual reduction in pain was noted, yet it remained elevated compared to the control group figures. Consequently, this study aims to extend the analgesic duration of remifentanil after surgery by employing a 30-minute pump infusion strategy. This approach seeks to not only prolong remifentanil’s pain-relieving effect but also mitigate remifentanil-induced hyperalgesia (RIH), thereby building upon the foundational findings of Saxena25 and further exploring the insights from Huang’s study.18 Postoperative pump infusion of remifentanil may extend analgesia duration and mitigate RIH progression. Analysis of outcomes revealed a significant main effect of tapering, which markedly increased postoperative thresholds versus controls, supporting the hyperalgesia reduction conferred by gradual withdrawal as aligned with prior reports.24 In contrast, postoperative infusion alone did not relieve hypersensitivity. In summary, while postoperative remifentanil infusion in isolation did not impact hyperalgesia, the strategy of gradual perioperative withdrawal uniquely attenuated postoperative hypersensitivity, potentially by averting LTP of spinal nociceptive circuits.
Tröster noted the emergence of RIH in the PACU following the discontinuation of remifentanil.31 Additionally, the significance of RIH appears linked to the initial hour post-surgery in clinical environments.17 A majority of RIH cases were observed in the PACU soon after the discontinuation of remifentanil.31 Therefore, in this study, the mechanical pain threshold in the first hour after surgery was used as the primary outcome, and the follow-up period was extended to 48 hours after surgery to observe the duration of postoperative hyperalgesia. Primary hyperalgesia stemming from peripheral sensitization occurs at the site of injury (such as surgical trauma), whereas secondary hyperalgesia manifesting in areas distant from the lesion represents central sensitization.32 Having a wider area of hyperalgesia increases the likelihood of a diagnosis of RIH.33 In this context, decreased medial forearm thresholds distal to the incision imply centrally-mediated sensitization. In this study, the difference between postoperative and preoperative mechanical pain thresholds in the medial forearm in the combined group was not significant, and the duration of hyperalgesia around the incision was significantly shorter, suggesting that peripheral and central sensitization can be well relieved. The gradual withdrawal of remifentanil in combination with postoperative pump infusion group exhibited increased postoperative thresholds compared to controls or either intervention alone, averted declines versus baseline, and abbreviated incisional hyperalgesia duration, consistent with concurrent central and peripheral hyperalgesia relief. The mechanism of gradual withdrawal of remifentanil may involve the gradual reversion of μ-opioid receptors from intracellular to membrane locations upon discontinuation, preventing sudden concentrated membrane exposure and hyperalgesia.34,35 Prolonged postoperative pump infusion of remifentanil may extend analgesia duration. However, the precise mechanisms require deeper investigation.
Lower postoperative pain NRS scores in the postoperative pump infusion of remifentanil group and gradual withdrawal of remifentanil group versus control group suggest attenuated pain. This demonstrates that remifentanil tapering and postoperative pump infusion of remifentanil significantly reduces postoperative pain compared to patients in the control group, which is consistent with previous study reports.24,36 In this study, there was a continued requirement for postoperative analgesic intervention, evidenced by a prevalence of 15% in P group and 19% in G group. This outcome, which showed no substantial deviation from the results of the control group, implies the necessity of exploring different analgesic techniques. The combined tapering and postoperative pump infusion group exhibited the nadir pain scores and rescue analgesic utilization postoperatively, underscoring the synergistic analgesia afforded by this multimodal approach. This finding concurs with previous findings.18 Similarly, the quality of recovery was the highest with the combination approach, aligning with the hyperalgesia and pain effects. In summation, coupling perioperative remifentanil tapering with postoperative low-dose pump infusion confers maximal amelioration of postoperative hyperalgesia, pain, and analgesic requirements. The convergence of results across quantitative sensory thresholds, patient-reported pain scores, analgesic consumption, and recovery quality indicates that this novel therapeutic paradigm meaningfully improves multiple clinically relevant postoperative outcomes.
Fading of anesthesia causing negative reactions is a primary cause of emergence agitation. This study discerned no significant differences between cohorts in time to awakening, duration of tracheal extubation, PACU length of stay, or validated agitation and sedation scale scores, suggesting the gradual withdrawal of remifentanil will not lead to a weakening of the anesthetic effect. There were no statistically significant differences between the four groups in terms of MAP and HR at any time point, indicating that the gradual withdrawal of remifentanil combined with postoperative pump infusion did not affect patients’ hemodynamics. The drugs commonly used in clinical practice to prevent agitation during the awakening period of general anesthesia (such as colistin, propofol, midazolam, tramadol) have drawbacks like prolonged awakening and respiratory issues.37 In our study, patients underwent gradual withdrawal of remifentanil and received postoperative pump infusion did not experience respiratory depression agitation during awakening, or prolonged awakening time both in the PACU and on the ward postoperatively. This result was consistent with previous reports25,38 with continuous infusion of remifentanil persistently in the PACU after thyroid surgery and laparoscopic-assisted vaginal hysterectomy. Gradual withdrawal of remifentanil combined with postoperative pump infusion showed no hemodynamic differences, allowing smooth emergence without agitation.
This study conducted an exhaustive assessment of postoperative adverse events, which occurred in all groups. The incidence of postoperative nausea and vomiting (PONV) aligned with established rates following laparoscopic surgery of approximately 50–70%.39 Relative to other groups, the combined gradual withdrawal and postoperative pump infusion group exhibited moderately lower rates of nausea, vomiting, as well as dizziness. This group also demonstrated the most expeditious return of postoperative bowel function, as measured by time to first flatus. However, no statistically significant differences were discerned, potentially owing to insufficient statistical power from the modest sample size. The addition of postoperative remifentanil pump infusion to gradual intraoperative tapering did not heighten the incidence of adverse reactions and may confer modest benefits in select measures of postoperative recovery.
This study represents the inaugural investigation of a novel perioperative analgesic paradigm that integrates gradual withdrawal of remifentanil with low-dose postoperative infusion. This first-in-field study examined the efficacy and safety of this unique dual-phase technique. It elucidates a promising alternative strategy to remifentanil monotherapy for attenuating postoperative hyperalgesia. Additionally, this work characterized the effects on postoperative pain and functional recovery, providing an early evidence base that can guide potential clinical implementation. The duration and extent of RIH may be associated with factors such as extended administration, high infusion rates, or high doses of remifentanil.40 In cases of prolonged surgery and anesthesia (such as musculoskeletal, thoracic, and abdominal surgeries), efforts to gradually discontinue remifentanil, combined with a postoperative infusion, are also necessary to prevent postoperative hyperalgesia.
Nevertheless, the limitations of this single-center study with a modest sample size exclusively undergoing laparoscopic hysterectomy necessitate acknowledgment. Broader validation via large multicenter trials across heterogeneous surgical populations is imperative to corroborate generalizability. Moreover, the exclusive focus on acute postoperative pain fails to illuminate the long-term risks or benefits of this protocol. Extended follow-up in expansive cohorts is essential to ascertain the durability and safety of treatment effects. Moreover, in order to meet the analgesic needs of the patients, analgesics were administered postoperatively. The inclusion of these patients could have introduced a confounding factor in our study, potentially impacting the results. And we did not conduct patient satisfaction surveys, which may have compromised our results. Finally, some evidence implicates certain hormones and inflammatory cytokines in postoperative hyperalgesia,41 but these were not evaluated here.
Conclusion
The combination of gradually withdrawal of remifentanil and postoperative pump infusion, as a unique method of remifentanil administration, can significantly reduce the incidence of postoperative hyperalgesia in patients undergoing laparoscopic hysterectomy. It also diminishes the intensity of postoperative pain and the need for analgesia, and enhance the quality of postoperative recovery. This combination approach is superior to using a single agent and does not increase the number of adverse effects, making it a valuable addition to clinical practice.
Abbreviations
RIH, remifentanil-induced hyperalgesia; OIH, Opioid-induced hyperalgesia; NSAIDs, non-steroidal anti-inflammatory drugs; PACU, postanesthesia care unit; NRS, numeric rating scale; HR, heart rate; BP, blood pressure; PETCO2, partial pressure of end-tidal carbon dioxide; BIS, bispectral index; SAS, Sedation -Agitation Scale; QoR-15, 15-item quality of recovery; ERAS, Enhanced recovery after surgery; LTP, long-term potentiation; BMI, body mass index; ASA, American Society of Anesthesiologist.
Data Sharing Statement
Please contact the corresponding author if you would like access to the datasets used or analyzed in this research.
Ethics Approval and Informed Consent
This trial was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (no. XYFY2023-KL049-02). The trial was registered before patient enrollment with the Chinese Clinical Trial Registry (ChiCTR2300074524). Written informed consent was obtained from all patients. This is in accordance with the Declaration of Helsinki.
Acknowledgments
The authors thank the Affiliated Hospital of Xuzhou Medical University for support with this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Jiangsu Provincial Health Commission Medical Research Key Project, ZD2021015.
Disclosure
The work described has not been submitted elsewhere for publication, in whole or in part, and all the authors listed have approved the enclosed manuscript. The authors report no conflicts of interest in this work.
References
1. Santonocito C, Noto A, Crimi C, Sanfilippo F. Remifentanil-induced postoperative hyperalgesia: current perspectives on mechanisms and therapeutic strategies. Local Region Anesthesia. 2018;11:15–23. doi:10.2147/lra.S143618
2. Vanderah TW, Suenaga NM, Ossipov MH, et al. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–286. doi:10.1523/jneurosci.21-01-00279.2001
3. Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi:10.1097/00000542-200008000-00019
4. Thomas J, Mustafa S, Johnson J, Nicotra L, Hutchinson M. The relationship between opioids and immune signalling in the spinal cord. Handbook Exp Pharmacol. 2015;227:207–238. doi:10.1007/978-3-662-46450-2_11
5. Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT. Nociceptin/Orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev. 2016;68:419–457. doi:10.1124/pr.114.009209
6. Tsai RY, Tai YH, Tzeng JI, et al. Ultra-low dose naloxone restores the antinociceptive effect of morphine in pertussis toxin-treated rats by reversing the coupling of mu-opioid receptors from Gs-protein to coupling to Gi-protein. Neuroscience. 2009;164:435–443. doi:10.1016/j.neuroscience.2009.08.015
7. Gao Y, Zhan W, Jin Y, et al. KCC2 receptor upregulation potentiates antinociceptive effect of GABAAR agonist on remifentanil-induced hyperalgesia. Molecular Pain. 2022;18:17448069221082880. doi:10.1177/17448069221082880
8. Recker DC, Perry PM. Postsurgical pain syndromes: chronic pain after hysterectomy and cesarean section. Tech Reg Anesthesia Pain Manage. 2011;15:133–139. doi:10.1053/j.trap.2011.08.002
9. Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–2225. doi:10.1016/s0140-6736(11)60245-6
10. Koo C-H, Yoon S, Kim B-R, et al. Intraoperative naloxone reduces remifentanil-induced postoperative hyperalgesia but not pain: a randomized controlled trial. Br J Anaesth. 2017;119(6):1161–1168. doi:10.1093/bja/aex253
11. Yamashita S, Yokouchi T, Tanaka M. Effects of intraoperative high-dose vs low-dose remifentanil for postoperative epidural analgesia after gynecological abdominal surgery: a randomized clinical trial. J Clin Anesthesia. 2016;32:153–158. doi:10.1016/j.jclinane.2016.02.024
12. Joly V, Richebe P, Guignard B, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–155. doi:10.1097/00000542-200507000-00022
13. Lenz H, Raeder J, Draegni T, et al. Effects of COX inhibition on experimental pain and hyperalgesia during and after remifentanil infusion in humans. Pain. 2011;152:1289–1297. doi:10.1016/j.pain.2011.02.007
14. Echevarría G, Elgueta F, Fierro C, et al. Nitrous oxide (N(2)O) reduces postoperative opioid-induced hyperalgesia after remifentanil-propofol anaesthesia in humans. Br J Anaesth. 2011;107:959–965. doi:10.1093/bja/aer323
15. Koppert W, Sittl R, Scheuber K, et al. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99:152–159. doi:10.1097/00000542-200307000-00025
16. Song JW, Lee YW, Yoon KB, Park SJ, Shim YH. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesthesia Analg. 2011;113:390–397. doi:10.1213/ANE.0b013e31821d72bc
17. Comelon M, Raeder J, Stubhaug A, et al. Gradual withdrawal of remifentanil infusion may prevent opioid-induced hyperalgesia. Br J Anaesth. 2016;116:524–530. doi:10.1093/bja/aev547
18. Huang YH, Lee MS, Lin YT, et al. Postoperative drip-infusion of remifentanil reduces postoperative pain-a retrospective observative study. Int J Environ Res Public Health. 2021:18. doi:10.3390/ijerph18179225
19. Kleif J, Waage J, Christensen KB, Gögenur I. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth. 2018;120:28–36. doi:10.1016/j.bja.2017.11.013
20. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393:1558–1568. doi:10.1016/s0140-6736(19)30430-1
21. Ma JF, Huang ZL, Li J, Hu SJ, Lian QQ. Cohort study of remifentanil-induced hyperalgesia in postoperative patients. Zhonghua Yi Xue Za Zhi. 2011;91:977–979. Chinese. doi:10.3760/cma.j.issn.0376-2491.2011.14.010
22. Yu EH, Tran DH, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia. 2016;71:1347–1362. doi:10.1111/anae.13602
23. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393:1537–1546. doi:10.1016/s0140-6736(19)30352-6
24. An XJ, Liu RJ, Yang J, et al. Effects of remifentanil gradual withdrawal on remifentanil induced postoperative hyperalgesia. Zhonghua Yi Xue Za Zhi. 2019;99:1298–1301. Chinese. doi:10.3760/cma.j.issn.0376-2491.2019.17.005
25. Saxena S, Gonsette K, Terram W, et al. Gradual withdrawal of remifentanil delays initial post-operative analgesic demand after thyroid surgery; double-blinded, randomized controlled trial. BMC Anesthesiol. 2019;19:60. doi:10.1186/s12871-019-0731-9
26. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surgery. 2017;152:292–298. doi:10.1001/jamasurg.2016.4952
27. Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153:1583–1592. doi:10.1016/j.pain.2012.02.028
28. Wilder-Smith OH, Tassonyi E, Crul BJ, Arendt-Nielsen L. Quantitative sensory testing and human surgery: effects of analgesic management on postoperative neuroplasticity. Anesthesiology. 2003;98:1214–1222. doi:10.1097/00000542-200305000-00025
29. Sandkühler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol. 2012;12:18–27. doi:10.1016/j.coph.2011.10.018
30. Drdla R, Gassner M, Gingl E, Sandkühler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi:10.1126/science.1171759
31. Tröster A, Sittl R, Singler B, et al. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology. 2006;105:1016–1023. doi:10.1097/00000542-200611000-00024
32. Mercieri M, Palmisani S, De Blasi RA, et al. Low-dose buprenorphine infusion to prevent postoperative hyperalgesia in patients undergoing major lung surgery and remifentanil infusion: a double-blind, randomized, active-controlled trial. Br J Anaesth. 2017;119:792–802. doi:10.1093/bja/aex174
33. Malik OS, Kaye AD, Urman RD. Perioperative hyperalgesia and associated clinical factors. Curr Pain Headache Rep. 2017;21:4. doi:10.1007/s11916-017-0602-3
34. Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci. 2000;20:8578–8584. doi:10.1523/jneurosci.20-23-08578.2000
35. Nowoczyn M, Marie N, Coulbault L, et al. Remifentanil produces cross-desensitization and tolerance with morphine on the mu-opioid receptor. Neuropharmacol. 2013;73:368–379. doi:10.1016/j.neuropharm.2013.06.010
36. Wu T-S, Wu H-C, Wu Z-F, Huang Y-H. Nalbuphine sebacate interferes with the analgesic effect of fentanyl. J Med Sci. 2020;40:101–102. doi:10.4103/jmedsci.jmedsci_150_19
37. Li D, Q LIN. Preemptive analgesia effect and safety of dezocine for postoperative agitation patients after remifentanil—based anesthesia. Clin Med Eng. 2012;19:2182–2183. doi:10.3969/j.issn.1674-4659.2012.12.2182
38. Lee JJ, Hwang SM, Lee JS, et al. Continuous infusion of two doses of remifentanil immediately after laparoscopic-assisted vaginal hysterectomy. Korean J Anesthesiol. 2010;58:537–541. doi:10.4097/kjae.2010.58.6.537
39. Zheng XZ, Cheng B, Luo J, et al. The characteristics and risk factors of the postoperative nausea and vomiting in female patients undergoing laparoscopic sleeve gastrectomy and laparoscopic gynecological surgeries: a propensity score matching analysis. Eur Rev Med Pharmacol Sci. 2021;25:182–189. doi:10.26355/eurrev_202101_24383
40. Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004. doi:10.1093/bja/aeu137
41. Zhao J, Shi W, Lu Y, et al. Alterations of monoamine neurotransmitters, HPA-axis hormones, and inflammation cytokines in reserpine-induced hyperalgesia and depression comorbidity rat model. BMC Psychiatry. 2022;22:419. doi:10.1186/s12888-022-04065-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


