Back to Journals » Journal of Pain Research » Volume 14
Effects of Proprioceptive and Craniocervical Flexor Training on Static Balance in University Student Smartphone Users with Balance Impairment: A Randomized Controlled Trial
Authors Wah SW , Puntumetakul R , Boucaut R
Received 23 March 2021
Accepted for publication 2 June 2021
Published 25 June 2021 Volume 2021:14 Pages 1935—1947
DOI https://doi.org/10.2147/JPR.S312202
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Saw Wah Wah,1,2 Rungthip Puntumetakul,2,3 Rose Boucaut4,5
1Human Movement Sciences, School of Physical Therapy, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, 40002, Thailand; 2Research Center in Back, Neck, Other Joint Pain and Human Performance, Khon Kaen University, Khon Kaen, 40002, Thailand; 3School of Physical Therapy, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, 40002, Thailand; 4International Centre for Allied Health Evidence, University of South Australia, Adelaide, SA, 5001, Australia; 5University of South Australia: Allied Health and Human Performance, Adelaide, SA, 5001, Australia
Correspondence: Rungthip Puntumetakul
Research Center in Back, Neck, Other Joint Pain and Human Performance, KhonKaen University, 123 Mitraphab Road, Muang District, Khon Kaen, 40002, Thailand
Tel +66 83 419 6186
Fax +66 43 2020 856
Email [email protected]
Purpose: University student smartphone users adopt flexed neck postures during smartphone use, creating an increased compressive load on their neck structures. This study was conducted to compare the effects of proprioceptive and craniocervical flexor training with a control group on static balance in a group of university student smartphone users with balance impairment.
Methods: A double-blinded, randomized controlled trial was conducted involving 42 university students (19.67± 1.68 years old) with balance impairment. Participants were randomized into a proprioceptive training (ProT) group (n=14), a craniocervical flexor training (CCFT) group (n=14), and a control group (CG; n=14) for a 6-week intervention. The balance error scoring system (BESS), cervical joint position sense (CJPS), craniocervical flexion (CCF) test, and visual analog scale (VAS) for neck pain were evaluated using univariate analysis of covariance (ANCOVA).
Results: After 6 weeks of intervention, the ProT group showed significantly greater improvement of CJPS than the CG (p=0.000) and the CCFT group significantly improved of CCF test than CG (p=0.002). Findings, at 4 weeks after intervention, were (i) the ProT group had significantly more improvement in BESS than the CCFT group (p=0.014) and CG (p=0.003), (ii) the ProT group had significantly more improvement of CJPS than the CG (right and left rotate) (p=0.001, p=0.016, respectively) and CCFT group (right rotate) (p=0.004), (iii) the CCFT group had significantly more improvement of craniocervical flexor strength than CG (p=0.004), and (iv) the ProT group and CCFT group had significantly more decreased pain than CG (p=0.015, p=0.033, respectively). No adverse effects occurred during or after training in any group.
Conclusion: ProT is important for regaining static balance and CJPS, while CCFT improved craniocervical flexor strength. Moreover, both ProT and CCFT can reduce neck pain. We recommend performing ProT to improve static balance, CJPS and to reduce neck pain in smartphone users with static balance impairment.
Clinical Trail Registration Number: TCTR20190909003.
Keywords: smartphone users, neck pain, cervical proprioception, craniocervical flexion strength
Introduction
The smartphone is an element of a comfortable life today; a smartphone typically has a touchscreen; mobile internet access via Wi-Fi or a cellular network; capacity to install smartphone applications; other functions, such as media players, digital cameras, global positioning system (GPS) based navigation;1 and the ability to send and receive e-mails, store data, and play computer games.2 Because of this convenience, worldwide smartphone use has increased rapidly, but at the same time, the prevalence of neck pain has also increased,3 especially in university students.4
The prevalence of neck pain in 18- to 24-year-old smartphone user university students has been reported as 68.2% in Hong Kong5 and 90% in Thailand.6 Smartphones lead users to adopt awkward static postures and engage in repetitive work.6 More than 91% of university students who are smartphone users adopt a flexed neck posture during smartphone use7 with increased compressive loads on neck structures.8 Prolonged smartphone use not only increases neck pain5,6,9–11 and cervical joint position sense (CJPS) error4,12 but also decreases dynamic balance.1,13,14 University students are reported as having the highest smartphone addiction rates compared with users of other age groups.4 Therefore, we expect that university students may have impaired balance.
Balance is necessary to maintain an upright stance and perform activities of daily living.15 Balance control depends on proprioceptive information from mechanoreceptors, as well as vestibular and visual input to the central nervous system.15 Static balance is the ability to maintain posture and orientation of the center of mass over the base of support with the body at rest.16 Dynamic balance is the ability to maintain posture and orientation of the center of mass over the base of support while the body parts are in motion.17 Many daily activities involve static balance activities with low muscle force.16 A deficiency of cervical proprioceptive inhibition of nociceptors contributes to impaired balance.18
Craniocervical flexor training (CCFT) controls pain and improves muscle strength, function, and balance in patients with cervical degenerative disc disease.19 Strengthening the craniocervical flexors maintains structural integrity.20 The muscle receptors play a major role in detecting joint movement and position sense for appropriate reflexive and voluntary movements.21
Proprioceptive training (ProT) involves fine control of neck movement involving the suboccipital muscles,22 control of muscle tone23 and spatial orientation of voluntary movement.24 The goal of ProT is to improve motor control and cervical proprioception.25,26 ProT is superior to CCFT in reducing neck pain and disability, improving craniocervical flexor strength and neuromuscular coordination,19,25–27 and improving cervical proprioception.22 For university students with impaired balance, there is limited evidence on the effect of ProT on static balance and the comparative effects, if any, between ProT and CCFT.
The main purpose of the current study was to compare the effects of ProT and CCFT on static balance in university student smartphone users with neck pain and balance impairment. The secondary purpose was to compare the effects of ProT and CCFT on cervical joint repositioning sense error, craniocervical flexor strength, and pain in this cluster of smartphone users. The hypothesis was that the ProT group would be superior to or equal to the CCFT group and superior to the control group (CG) in terms of static balance.
Materials and Methods
Study Design
A double-blinded, stratified randomized controlled trial was conducted involving 42 neck pain university student smartphone users with impaired balance.
Participants
The study was conducted after gaining approval from the Khon Kaen University Ethics Committee for Human Research following the Declaration of Helsinki (HE 612,374); the Yangon University of Medical Technology, Myanmar (IRB Approval No. 1/2019-1); the University of Public Health-Institutional Review Board, Myanmar (UPH-IRB 2019/Research/35); and the Thai Clinical Trial Registry (TCTR20190903003). University students who met the eligibility criteria were recruited for the study. The inclusion criteria were as follows: 1) Neck pain with recurrent episodes and low-grade neck dysfunction not having regular treatment in the last 7 days; 2) Age of 18–25 years; 3) Balance impairment as determined by a balance error scoring system (BESS) score ≥ 15;28–30 4) Body mass index (BMI) ≤ 30 kg/m2; 5) Experience of smartphone use for more than 6 months and smartphone screen time of at least 2 hours per day; 6) Neck disability index score between 5/50 and 14/50; 7) Pain rated as mild to moderate on a visual analog scale (VAS; 5–74 mm); and 8) Voluntary participation and ability to read and understand English.
Participants with any one of the following conditions were excluded: 1) Visual, auditory, vestibular, or neurological deficits; 2) Traumatic injuries or surgical interventions of the spine or lower limbs within the prior year; 3) Medical conditions that could have a negative effect on balance; 4) Chronic musculoskeletal diseases, lower limb fractures, and injuries; 5) Participation in any neck muscle strengthening and balance training over the past 12 months; 6) A Beck Depression Inventory (BDI) score > 30/63; 7) A Dizziness Handicap Inventory (DHI) score > 30/100; and 8) Taking any sedative drug or alcohol within the past 48 hours.
The DHI is the most widely used self-report measure of dizziness disability and has been translated into more than 17 languages.31 It has 25 items with three response levels, which are sub-grouped into functional, emotional, and physical domains. A total DHI score of 0–30 indicates mild, 31–60 moderate, and 61–100 severe dizziness handicap.32 The DHI is a reliable (r = 0.92–0.97), valid, comprehensive, and clinically useful tool to measure self-perceived handicap associated with dizziness symptoms from a variety of causes.33 It also has good correlation with specific objective balance measures.32,34,35
The BDI is a 21-item self-report rating inventory that assesses characteristic attitudes and depressive symptoms, with each corresponding to the major depressive symptoms in the preceding 2 weeks.36 Internal consistency of the BDI ranges from 0.73 to 0.92 with a mean of 0.86.36,37 In addition, the BDI demonstrates high internal consistency values of 0.86 and 0.81 for the psychiatric and nonpsychiatric population, respectively.36
Screening Procedure
Participants were recruited via posted invitations on notice boards at Yangon University of Medical Technology. Each participant was screened by a general physician from that university and a research assistant based on a checklist for neck pain signs and symptoms38 and eligibility criteria. Before the study, informed written consent was provided by all eligible participants. A researcher (SWW) assessed each participant using a self-report questionnaire that was tailored specifically for smartphone users and evaluated with outcome measures, including the BESS, CJPS, craniocervical flexion (CCF) test, and VAS.
Randomization Procedure
A researcher (RP) generated a random allocation sequence to assign participants to the intervention. Participants were stratified randomly using block sizes of 6 and 9 created by a randomization list (Sealed EnvelopeTM) program. Following Iverson et al, stratification was performed using BESS scores as follows: 15–17, 18–23, and above 24.28 Each participant selected a preferred pre-coded envelope to allocate them into one of the three following groups: the ProT group, CCFT group, or CG.
Interventions
Before the intervention, a researcher (SWW) provided 3 hours of training about the relevant interventions to three assistant researchers, each with at least 5 years of musculoskeletal physiotherapy experience. Each researcher gave relevant interventions to their participants for 6 weeks. Finally, assessment was conducted at 4 weeks after the intervention—that is, after the end of the 6-week intervention—to determine any lasting effects on balance control in the ProT group, CCFT group, and CG; the results were statistically analyzed by the researcher (SWW; Figure 1). Participants in the ProT and CCFT groups were trained to perform their 20-minute exercise routine on three alternate days per week for up to 6 weeks according to their allocated intervention group. Participants from each group were requested to perform their daily home exercise session twice per day for 6 weeks. Exercise compliance was monitored in each of the three groups; participants were asked to record their exercise details in a booklet over the duration of the study. A weekly phone call was made to all participants (by researcher SWW) to encourage them to continue their home exercises during the trial period.
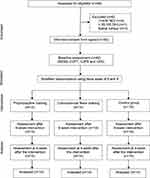 |
Figure 1 Study flow diagram and follow-up evaluation. |
Proprioceptive Training (Experimental Group)
ProT targets the improvement of overall proprioceptive function by using somatosensory signals from the suboccipital muscles to improve both conscious perceptual and unconscious proprioception. A training period of 6 weeks is enough to produce improvement in cervical proprioception.26
In the present study, ProT consisted of the three following types of tailored active exercises: CJPS (head relocation), cervical movement sense (kinesthetic), and oculomotor control (gaze stability). Exercises were started sitting on a chair in an erect posture, with a laser attached to the center of a forehead band, which enabled participants to use their head to point to a target located on a wall 90 cm away as the natural head posture (NHP). This starting position was used for all participants in this group.39
In weeks 1 and 2, the CJPS exercises were performed by the participants relocating their heads back to the NHP from active neck movements (flexion, extension, rotation, and lateral flexion) with eyes open, using feedback from the laser attached to their heads. During weeks 3 and 4, exercises were progressed with pupillary glasses preventing pupillary excursion, and finally, the exercises were done with participants’ eyes closed during weeks 5 and 6 for three sets of five repetitions to all weeks.39
During weeks 1 and 2, the cervical movement sense exercises involved tracing a line with laser feedback in a vertical and horizontal plane with the eyes open. During weeks 3 and 4, exercise progressed to slowly tracing a figure eight with laser feedback, and finally, to tracing a figure eight with laser feedback with increased speed during weeks 5 and 6 for three sets of five repetitions to all weeks.39
The oculomotor control (gaze stability) exercises were started with eyes following a target on the outstretched hand at slow speed while keeping the head still (vertical/horizontal) during weeks 1 and 2. It involved head moving while fixing eyes on a target (vertical/horizontal) during weeks 3 and 4, and eyes and head moving together in the same direction on a target (vertical/horizontal) during weeks 5 and 6 for three sets of five repetitions to all weeks.39
Craniocervical Flexor Training (Experimental Group)
CCFT improves tonic postural function of the deep cervical flexors and minimizes activation of the superficial cervical flexors.40 It focuses on motor control training of the deep craniocervical flexors, decreases neck pain,19,41 and improves cervical proprioception after 6 weeks of training.22,41
The CCFT exercise program consisted of co-contraction of the deep and superficial cervical flexors, strengthening exercises of the cervical flexors, and strength and endurance of the CCF movement pattern. Initially, each patient was taught to perform the CCF movement in a slow and controlled manner in crook lying. The physiotherapist identified the target level that the patient could hold steadily for 5 seconds without any retraction, use of the superficial neck flexors, or quick and jerky CCF. The target goal of exercise progression was based on the timeframe and exercise program of Izquierdo et al,25 but it was individually tailored based on the participant’s performance pressure score to prevent treatment soreness and adverse effects. Tailored home instructions prescribed by the physiotherapist were given to each participant.
Co-contraction exercises of the deep and superficial cervical flexors started from a slow and controlled nodding action facilitated by eye movement in crook lying during weeks 1 and 2. During weeks 3 and 4, the exercise progressed in a controlled head movement pattern through a range of extension and returned to neutral in sitting. Finally, isometric resisted CCF was given with the strength of the participant’s right thumb at the inferior aspect of the chin with cervical extension in sitting during weeks 5 and 6.
Strengthening exercises of the cervical flexors started in supine lying on the plinth, with hands resting beside the body. Exercises were progressed by lifting the head off 2 pillows during weeks 1 and 2, lifting the head off one pillow in supine lying during weeks 3 and 4, and lifting the head off the plinth without a pillow during weeks 5 and 6.
Strength/endurance of the CCF movement pattern was started as a sustained CCF movement with pressure biofeedback under the neck at 20–22 mmHg in crook lying for weeks 1 and 2. For each target level, the contraction was increased to 10 seconds hold, 5 seconds rest between each of the 10 repetitions. One set of 10 repetitions of 10 seconds was achieved at one target level twice a day. This exercise progressed as a repeated and sustained CCF movement with pressure biofeedback under the neck at 24–26 mmHg in crook lying for weeks 3 and 4 and 28–30 mmHg in crook lying for weeks 5 and 6.20,25
Advice Group (Control Group)
All participants in the CG received individual instruction once per week from an experienced physiotherapist on their specific neck exercise program. The program consisted of free active neck range of motion exercises, stretching cervical erector spinae, and neck extensor strengthening.42 Participants were also given advice to perform these exercises twice daily for 6 weeks without any provocation of neck pain. Participants were instructed to stop the exercises if pain was provoked or any adverse effects occurred during or after exercise and to inform the researcher SWW if this was the case. A booklet of the specific sequential neck exercise program with diagrams (neck range of motion exercises followed by stretching and strengthening exercises of neck extensors) was provided to each participant. The exercise program was an individualized, progressive, submaximal program designed to improve each participant’s ability. All participants were provided with an explanation of the benefits of their specific neck exercise program and how to use an ice pack if pain provocation of more than 50/100 mm (VAS) occurred. They were also given instructions about how and what to record in their exercise diaries—the intensity, duration, and frequency of exercise and pain, as well as the frequency of ice application if used.
Free active neck range of motion exercises were performed in the pain-free range by rotating the head to left and right; flexing the head forward and backward; and side flexing the head to left and right. Exercise setting and intensity of free active neck range of motion exercises were the same during weeks 1 to 6 (10 repetitions for each movement, rest 10 seconds between each movement, twice daily for 6 weeks).
Stretching the cervical erector spinae was started by upright sitting on a chair, bending the head slightly to the front with both hands behind the occiput and within the limits of pain. The exercise intensity was set as hold 10 seconds, rest 10 seconds for three repetitions, twice daily for the whole 2-week period. The exercise program progressed up to hold 20 seconds, rest 10 seconds, three repetitions, twice daily for weeks 3 and 4 and hold 30 seconds, rest 10 seconds for three repetitions twice daily during weeks 5 and 6.
Neck extensor strengthening exercises were started by firmly pushing the head down over a pillow for 5 seconds to strengthen the neck extensors in supine lying during weeks 1 and 2. This exercise was progressed to pushing the head firmly against a wall for 5 seconds in erect standing with the upper back touching the wall and feet apart during weeks 3 and 4. During weeks 5 and 6, exercise progressed to prone lying over a pillow under the abdomen while lifting the head from the bed for 5 seconds to strengthen the neck extensors. The exercise intensity was set as hold 5 seconds, rest 10 seconds, 10 repetitions, twice daily during weeks 1–6.
Outcome Measurements
Static Balance
Static balance, the primary outcome measure, was assessed using the BESS score. BESS is a continuously scaled outcome measure; lower scores indicate better static balance control, and higher scores indicate worse control from possible scores of 0–60. The total BESS score of ≥ 15 is the cutoff point of balance impairment for participants aged 18–25 years.28 BESS is becoming the gold standard measure in clinical settings to evaluate balance deficits.43,44 BESS has shown adequate to excellent criterion validity when used to measure force-plate target sway (r = 0.31 to 0.79), test–retest reliability, intraclass correlation coefficient (ICC) = 0.70 (Bell et al, 2011) and intra-rater reliability (intraclass coefficient [ICC] = 0.92).30
For the BESS assessment, barefoot participants were instructed to maintain standing balance (stand with hands on the hips) while performing the six BESS sub-tests with eyes closed for 20 seconds. Participant position was double-leg stance with feet together, single-leg stance on the non-dominant foot, and tandem stance with the non-dominant foot behind on firm and foam surfaces for each of three tests. Each condition was scored by counting the errors of the participant’s deviation from the designated test position. If several errors occurred at the same time, the inability to remain in the proper test position for more than 5 seconds was counted as the error. Errors consisted of moving hands off the iliac crest, opening eyes, footstep stumble or fall, hip abduction or flexion more than 30 degrees, lifting the forefoot or heel off the test surface, or inability to remain in the designated test position for more than 5 seconds. A total BESS score comprised the addition of the number of errors per condition in each of the six conditions.28,45 In the current study, the ICC for the intra-rater reliability measures ranged from 0.98 to 0.99, and the inter-examiner reliability ranged from 0.97 to 0.99.
Cervical Joint Position Sense
CJPS, or cervical proprioception, was assessed using a special type of cervical range of motion (CROM) measurement device (CROM Deluxe USA, EN-121,156).46 CROM has been shown to have excellent concurrent validity (r = 0.93–0.98), excellent test–retest reliability (ICC = 0.89–0.98) using 3D Fastrack,47 as well as excellent correlation (r = 0.78–0.86) and substantial to excellent test–retest reliability (ICC = 0.74–0.96) using the VICON motion capture system.48 A CJPS error ≥ 4.5 degrees indicates impaired CJPS.39
To assess CJPS, the CROM device was aligned on the nose bridge and ears, and it was fastened to the participant’s head with a Velcro strap; the head was positioned with the horizontal, sagittal, and compass meters each reading zero degrees. The participant was blindfolded and sitting upright on a chair, hands on thighs, feet resting on the floor, with hip and knee flexion at 90 degrees. Practice recognizing the CJPS and the NHP was performed for 3 seconds before the test trials.49,50 The average of three trials to relocate the NHP from each cervical movement was recorded in degrees of CJPS.46 In the present study, the ICC for the intra-rater reliability measures ranged from 0.78 to 0.99 and inter-examiner reliability from 0.77 to 0.99.
Craniocervical Flexion Test
The craniocervical flexion (CCF) test was used to evaluate craniocervical flexor function, endurance or strength, and coordination of the deep and superficial neck flexors.20 The activation pressure score was the highest pressure that the participant could achieve and maintain for 10 seconds while properly performing the CCF, from a baseline of 20 mmHg. The performance pressure score was the highest target pressure that the participant could achieve and hold for 10 seconds, starting at a baseline of 20 mmHg and increasing by 2 mmHg at each phase, with a total of five phases and a top value of 30 mmHg (target pressures of 22, 24, 26, 28, and 30 mmHg). For example, if a participant could achieve the second level of the test (24 mmHg) and perform six repetitions of 10-second holds with the correct action of CCF, then the performance index was 4×6 = 24 mmHg. Possible performance pressure scores of CCF ranged from 0 to 100 mmHg.20,51,52 Dysfunction of the craniocervical flexors was indicated by a decreased performance pressure score of CCF ≤ 24 mmHg.53
To assess CCF, the participant was positioned in crook lying (supine with 45° hip flexion and 90º knee flexion). A feedback device “stabilizer” (Chattanooga Group, Inc., Hixson, TN, USA) and a towel were placed under the patient’s suboccipital region. CCF was described by the physiotherapist as flexion of the head from the upper cervical region without any flexion of the middle or lower cervical region. Two CCF items were measured in two tests—activation pressure score and performance pressure score.20 In this study, ICC for the intra-rater reliability measures ranged from 0.91 to 0.99 and inter-examiner reliability from 0.88 to 0.99.
Visual Analog Scale
A VAS of 100 mm in length was used as a self-reported measure of pain intensity. This scale is simple to use (Hawker et al, 2011), with a higher VAS score indicating greater pain intensity. The categories used were no pain (0–4 mm), mild pain (5–44 mm), moderate pain (45–74 mm), and severe pain (75–100 mm).54,55
To assess the VAS, participants were asked to place a line perpendicular to the VAS line at the point that represented their pain intensity. The VAS score was recorded as the distance (mm) from the zero anchor.56,57
Sample Size
The sample size estimation was conducted based on detecting a mean difference of the BESS scores of 2 points and a pooled variance estimation (σ2 = 2.3892) between the ProT and CCFT groups in the 6-week intervention after the pilot study. Jirawatkul’s formula58 was used with a statistical power of 80% and an alpha level of 0.05. Allowing for a dropout rate of 10%, we required at least 14 participants for each group; the total sample size in this study was 42.
Statistical Analysis
Data were analyzed using STATA version 13.1 (STATA, College Station, TX, USA). All analyses were performed on an intention-to-treat basis. The descriptive statistics are presented as frequency and percentage for categorical variables and as mean and standard deviation for continuous variables. The data distribution was assessed using the Kolmogorov–Smirnov goodness-of-fit test, and data were measured repeatedly at the following points: at baseline, after the 6-week intervention, and at 4 weeks after the intervention (follow-up after the end of the 6-week intervention). Univariate analysis of covariance (ANCOVA; adjusted for baseline) was used to determine differences between groups with a 95% confidence interval (CI) for all outcome measures. Bonferroni corrections were used. A p-value < 0.05 was considered statistically significant normality.
Results
A total of 48 smartphone users with balance impairment were screened for eligibility. Reasons for exclusion of six participants are depicted in Figure 1. There were 14 participants in each group after stratified randomization. Recruitment began on March 22, 2019; follow-up of all 42 participants was completed on August 30, 2020, when the trial ended. Participants performed the 20-minute exercise program three times per week for up to 6 weeks, and they were measured at 4 weeks after the intervention (follow-up). All 14 participants in each group completed the study (Figure 1). Except for gender, the demographic characteristics of the participants were similar in each group. Two male participants were included in both the ProT and CCFT groups, but no male participant was allocated to the CG because of the randomization process. Participants’ stage of neck pain, daily smartphone use hours, years, daily visual display terminal (VDT) use hours, and BESS scores are described in Table 1.
 |
Table 1 Participants Characteristics (n=42) |
Static balance in the ProT group improved significantly (shown by reduced balance error score) compared with the CG (p = 0.003) and the CCFT group (p = 0.014) after 4 weeks of intervention (Table 2).
 |
Table 2 Results After 6 Weeks Intervention and Post-Intervention 4 Weeks Between Groups (n=42) |
Error in CJPS (right rotation) reduced significantly more in the ProT group compared with the CCFT group after 4 weeks (p = 0.004). Error in CJPS (right rotation) was significantly reduced in the ProT group compared with the CG after the 6-week intervention (p = 0.005) and at the 4-week follow-up (p = 0.001). Error in CJPS (left rotation) reduced significantly more in the ProT group compared with the CCFT group after the 6-week intervention (p = 0.001). Error in CJPS (left rotation) reduced significantly more in the ProT group compared with the CG after the 6-week intervention (p = 0.000) and 4-week follow-up (p = 0.016; Table 2). The CCF test results improved significantly in the CCFT group compared with the CG after the 6-week intervention (p = 0.002) and at the 4-week follow-up (p = 0.004; Table 2). VAS scores in the ProT group and CCFT group decreased significantly more than those of the CG at the 4-week follow-up (p = 0.015 and p = 0.033, respectively; Table 2).
Discussion
The results after the 6-week intervention demonstrated the following: (i) the ProT group improved in CJPS significantly more than the CG (p = 0.000) and the CCFT group did (p = 0.001), and (ii) the CCFT group improved in craniocervical flexor strength significantly more than the CG did (p = 0.002). The 4-week follow-up results showed the following: (i) the ProT group’s BESS improved significantly compared with the CG (p = 0.003) and the CCFT group (p = 0.014); (ii) the ProT group’s CJPS improved significantly compared with the CG on both sides of rotation (Rt. rotate, p = 0.001; Lt. rotate, p = 0.016) and CCFT group only in right rotation (p = 0.004); (iii) the ProT and CCFT groups reported significantly reduced pain compared with the CG (p = 0.015 and p = 0.033, respectively); and (iv) the CCFT group significantly improved in craniocervical flexor strength compared with the CG (p = 0.004). All the findings supported our primary and secondary objectives and revealed that the BESS and CJPS outcomes of the ProT group were superior to those of the CCFT group in university student smartphone users with neck pain and balance impairment.
The ProT group was superior to the CCFT group and the CG in improving static balance. Balance control depends on proprioceptive information from mechanoreceptors and vestibular and visual input to the central nervous system.15 Several possible mechanisms may explain the improvement of balance control in the ProT group. Cervical muscle spindles are the important proprioceptors in maintaining balance control.59 The enhancement of suboccipital muscles on ProT is responsible for accurate kinesthesia and proprioception.22 Suboccipital muscles modulate the postural reflexes, which are important for eye–head coordination and balance control.60 The suboccipital muscles are part of the same superficial back line of myofascial chains with the hamstring and calf muscles; all these muscles are involved in maintaining balance control.61 Training the suboccipital muscles has been recommended for improving gait in children with cerebral palsy because of the coordinated movement of the suboccipital muscles with the hamstring and the calf muscles.62 This study demonstrated that ProT targeting the suboccipital muscles was effective in improving static balance control at the 4-week follow-up in persons with neck pain.
Additional reasons for the superior and significant improvement of balance in the ProT group over the CCFT group at the 4-week follow-up may have arisen from the progressive exercise programs of the ProT group during weeks 5 and 6 of the intervention. The CJPS exercise progressed from relocating the neutral head posture with eyes open to eyes closed during weeks 5 and 6. ProT targets improvement of proprioceptive afferents in the absence of vision to improve conscious perceptual and unconscious sensorimotor function.26 The ProT progressed from a slow speed to a very fast speed during weeks 5 and 6. Very fast movements principally rely on feedforward control mechanisms, whereas slower movements also include feedback control. The frontal and parietal areas of the brain control balance.63
Possible reasons for the lack of significant difference between participant balance control in the ProT and CCFT groups on balance control after 6 weeks of intervention are as follows: (i) suboccipital muscles provide postural stability from ProT,60 whereas craniocervical flexors provide segmental stability from the CCFT,64 and (ii) suboccipital muscles regulate eye–head coordination,60 whereas craniocervical flexor muscles improve coordination of the deep and superficial cervical flexors.20
The ProT group showed improved CJPS both after 6 weeks of intervention and at the 4-week follow-up compared with the CCFT group and CG. These findings following ProT (head relocation) could reflect that relocation practice in the proprioceptive training program directly trained the impairment41 and the outcome measure of cervical joint positioning error. Second, the program addressed the cervical afferent input in its functional role by the inclusion of eye movement exercises.22 Generally, specific ProT was designed to target the deep muscles, particularly the suboccipital muscle,22 because this muscle has the highest density of receptors relative to other parts of the body. Because of this important feature, it has an important role in control and spatial orientation.65 Thus, proprioceptive retraining incorporating eye–head coordination may specifically influence the suboccipital cervical receptors and muscles.22
Improvement in the CCF test was found when comparing the CCFT group and CG. Several mechanisms may explain improvements in craniocervical flexor muscle strength following CCFT. First, CCFT directly activates the deep cervical flexor musculature,64,66 and the repeated contractions in CCFT may improve muscle spindle function. Second, the CCFT biofeedback was provided by a pressure sensor under the neck as the participant practiced precise holding of progressive inner range positions. Third, CCFT decreases stress placed on the joints and other structures of the cervical region19,20,67 and restores normal cervical curvature.19,20,67 Researchers have found that CCFT improves muscle strength and function, alters ascending pain signals, and modifies motor neuronal discharge.19
There was no significant difference in pain after the 6-week intervention between the CG and both training groups. This is consistent with findings from a prior study,68 which showed that strengthening exercises, endurance exercises, and stretching exercises of the cervical muscles each proved effective in reducing neck pain. Another consideration may be that the intervention duration was not prolonged in the current study. In addition, the CG participants were provided with diagrams and a text explanation of their program, as well as ice packs.
All interventions chosen for this study proved equally effective in reducing pain for up to 6 weeks of intervention. These results are in accordance with some prior studies22,39,69 but conflict with other studies that showed superior effects of ProT on VAS scores.25,70
The ProT group showed superior effects to the CCFT group and CG in terms of improving BESS, our key outcome. This may be explained in that ProT reduced pain and muscle tension via changes in suboccipital muscle spindle activity;22 consequently, CJPS also improved. In addition, there are many receptors in the deep suboccipital muscles that create reflex and central communication with vestibular, visual, and postural control systems.71 Thus, the results of the current study were considered generalizable to university student smartphone users with neck pain and balance impairment aged 18–25 years.
Limitations
The DHI and BDI questionnaires were used as inclusion screening criteria; in hindsight, applying them for outcome measurement may have provided more evidence. For additional objective findings, future investigation is suggested to compare the muscle properties of suboccipital muscles and the craniocervical flexors using electromyography (EMG) or rehabilitative ultrasound imaging (RUSI) in smartphone users with balance impairment. Additional male volunteers in this study would have provided a more balanced participant gender ratio.
Conclusion
Both ProT and CCFT are important for static balance control. The ProT group was significantly superior to the CCFT group and CG in improving static balance and cervical proprioception. We recommend performing ProT to improve static balance, craniocervical flexor function, and cervical proprioception and to reduce neck pain in people with neck pain and static balance impairment.
Data Sharing Statement
The authors will allow sharing of non-identified participants’ data related to our outcomes. The data will be available for anyone who wishes to access it. The data will be accessible immediately following publication until 6 months after publication. Contact should be made via the researcher RP as [email protected].
Acknowledgments
We acknowledge all participants of the study and thank them for their voluntary participation. Deepest thanks to the Research Center in Back, Neck, Other Joint Pain and Human Performance (BNOJPH), Khon Kaen University, Thailand and University of Medical Technology, Yangon, Myanmar.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Hyong IH. The effects on dynamic balance of dual-tasking using smartphone functions. J Phys Ther Sci. 2015;27(2):527–529. doi:10.1589/jpts.27.527
2. Long J, Cheung R, Duong S, Paynter R, Asper L. Viewing distance and eyestrain symptoms with prolonged viewing of smartphones. Clin Exp Optom. 2017;100(2):133–137. doi:10.1111/cxo.12453
3. Park J, Kim J, Kim J, et al. The effects of heavy smartphone use on the cervical angle, pain threshold of neck muscles and depression. Adv Sci Technol Lett. 2015;91(3):12–17.
4. Lee J, Seo K. The comparison of cervical repositioning errors according to smartphone addiction grades. J Phys Ther Sci. 2014;26(4):595–598. doi:10.1589/jpts.26.595
5. Woo EHC, White P, Lai CWK. Effects of electronic device overuse by university students in relation to clinical status and anatomical variations of the median nerve and transverse carpal ligament. Muscle Nerve. 2017;56(5):873–880. doi:10.1002/mus.25697
6. Namwongsa S, Puntumetakul R, Neubert MS, Chaiklieng S, Boucaut R, Stoffregen TA. Ergonomic risk assessment of smartphone users using the Rapid Upper Limb Assessment (RULA) tool. PLoS One. 2018;13(8):e0203394. doi:10.1371/journal.pone.0203394
7. Gold JE, Driban JB, Thomas N, Chakravarty T, Channell V, Komaroff E. Postures, typing strategies, and gender differences in mobile device usage: an observational study. Appl Ergon. 2012;43(2):408–412. doi:10.1016/j.apergo.2011.06.015
8. Hansraj KK. Assessment of stresses in the cervical spine caused by posture and position of the head. Surg Technol Int. 2014;25:277–279.
9. Berolo S, Wells RP, Amick BC. Musculoskeletal symptoms among mobile hand-held device users and their relationship to device use: a preliminary study in a Canadian university population. Appl Ergon. 2011;42(2):371–378. doi:10.1016/j.apergo.2010.08.010
10. Shan Z, Deng G, Li J, Li Y, Zhang Y, Zhao Q. Correlational analysis of neck/shoulder pain and low back pain with the use of digital products, physical activity and psychological status among adolescents in Shanghai. PLoS One. 2013;8(10):e78109. doi:10.1371/journal.pone.0078109
11. Stalin P, Abraham SB, Kanimozhy K, Prasad RV, Singh Z, Purty AJ. Mobile phone usage and its health effects among adults in a Semi-Urban Area of Southern India. J Clin Diagn Res. 2016;10(1):Lc14–Lc16. doi:10.7860/JCDR/2016/16576.7074
12. Kim Y-G, Kang M-H, Kim J-W, Jang J-H, Oh J-S. Influence of the duration of smartphone usage on flexion angles of the cervical and lumbar spine and on reposition error in the cervical spine. Phys Ther Korea. 2013;20(1):10–17. doi:10.12674/ptk.2013.20.1.010
13. Cho S-H, Choi M-H, Goo B-O. Effect of smart phone use on dynamic postural balance. J Phys Ther Sci. 2014;26(7):1013–1015. doi:10.1589/jpts.26.1013
14. Azab Doaa Rafat E, Amin D, Mohamed GI. Effect of smart phone using duration and gender on dynamic balance. Int J Med Health Res. 2017;6:42–49.
15. Hrysomallis C. Relationship between balance ability, training and sports injury risk. Sports Med. 2007;37(6):547–556. doi:10.2165/00007256-200737060-00007
16. Juul-Kristensen B, Clausen B, Ris I, et al. Increased neck muscle activity and impaired balance among females with whiplash-related chronic neck pain: a cross-sectional study. J Rehabil Med. 2013;45(4):376–384. doi:10.2340/16501977-1120
17. O’Sullivan SB, Schmitz TJ, Fulk G. Physical Rehabilitation. FA Davis; 2019.
18. McPartland JM, Brodeur RR, Hallgren RC. Chronic neck pain, standing balance, and suboccipital muscle atrophy-a pilot study. J Manipulative Physiol Ther. 1997;20(1):24–29.
19. Zakaria H, Badawy W, Ibrahim O. Effect of craniocervical flexion training on postural stability in patients with cervical degenerative disc disease: a randomized controlled trial. Int J Ther Rehabil. 2017;6:16.
20. Jull GA, O’Leary SP, Falla DL. Clinical assessment of the deep cervical flexor muscles: the craniocervical flexion test. J Manipulative Physiol Ther. 2008;31(7):525–533. doi:10.1016/j.jmpt.2008.08.003
21. Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. doi:10.2522/ptj.20060083
22. Jull G, Falla D, Treleaven J, Hodges P, Vicenzino B. Retraining cervical joint position sense: the effect of two exercise regimes. J Orthop Res. 2007;25(3):404–412. doi:10.1002/jor.20220
23. Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86(1):89–154. doi:10.1152/physrev.00028.2005
24. Bove M, Bonzano L, Trompetto C, Abbruzzese G, Schieppati M. The postural disorientation induced by neck muscle vibration subsides on lightly touching a stationary surface or aiming at it. Neuroscience. 2006;143(4):1095–1103. doi:10.1016/j.neuroscience.2006.08.038
25. TGallego I, Pecos-Martin D, Girbés EL, et al. Comparison of cranio-cervical flexion training versus cervical proprioception training in patients with chronic neck pain: a randomized controlled clinical trial. J Rehabil Med. 2016;48:48–55. doi:10.2340/16501977-2034
26. Aman JE, Elangovan N, Yeh IL, Konczak J. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci. 2014;81075. doi:10.3389/fnhum.2014.01075
27. Amiri AS, Ghamkhar L, Kahlaee AH. The relevance of proprioception to chronic neck pain: a correlational analysis of flexor muscle size and endurance, clinical neck pain characteristics, and proprioception. Pain Med. 2018;19(10):2077–2088. doi:10.1093/pm/pnx331
28. Iverson GL, Kaarto ML, Koehle MS. Normative data for the balance error scoring system: implications for brain injury evaluations. Brain Inj. 2008;22(2):147–152. doi:10.1080/02699050701867407
29. Broglio SP, Monk A, Sopiarz K, Cooper ER. The influence of ankle support on postural control. J Sci Med Sport. 2009;12(3):388–392. doi:10.1016/j.jsams.2007.12.010
30. Erkmen N, Takin H, Kaplan T, Saniolu A. The effect of fatiguing exercise on balance performance as measured by the balance error scoring system. Isokinet Exerc Sci. 2009;17:121–17127.
31. Van De Wyngaerde KM, Lee MK, Jacobson GP. The component structure of the dizziness handicap inventory (dhi): a reappraisal. Otol Neurotol. 2019;40(9):1217–1223. doi:10.1097/MAO.0000000000002365
32. Whitney SL, Marchetti GF, Morris LO. Usefulness of the dizziness handicap inventory in the screening for benign paroxysmal positional vertigo. Otol Neurotol. 2005;26(5):1027–1033. doi:10.1097/01.mao.0000185066.04834.4e
33. Enloe LJ, Shields RK. Evaluation of health-related quality of life in individuals with vestibular disease using disease-specific and general outcome measures. Phys Ther. 1997;77(9):890–903. doi:10.1093/ptj/77.9.890
34. Kaufman KR, Brey RH, Chou L-S, Rabatin A, Brown AW, Basford JR. Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Med Eng Phys. 2006;28(3):234–239. doi:10.1016/j.medengphy.2005.05.005
35. Treleaven J. Dizziness handicap inventory (DHI). Aust J Physiother. 2006;52(1):67. doi:10.1016/S0004-9514(06)70070-8
36. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi:10.1001/archpsyc.1961.01710120031004
37. Marnat G. Current status and future directions of psychological assessment: introduction. J Clin Psychol. 1999;55(7):781–785. doi:10.1002/(SICI)1097-4679(199907)55:7<781::AID-JCLP1>3.0.CO;2-2
38. Paulus I, Brumagne S. Altered interpretation of neck proprioceptive signals in persons with subclinical recurrent neck pain. J Rehabil Med. 2008;40(6):426–432. doi:10.2340/16501977-0189
39. Revel M, Minguet M, Gregoy P, Vaillant J, Manuel JL. Changes in cervicocephalic kinesthesia after a proprioceptive rehabilitation program in patients with neck pain: a randomized controlled study. Arch Phys Med Rehabil. 1994;75(8):895–899. doi:10.1016/0003-9993(94)90115-5
40. Falla D, O’Leary S, Farina D, Jull G. The change in deep cervical flexor activity after training is associated with the degree of pain reduction in patients with chronic neck pain. Clin J Pain. 2012;28(7):628–634. doi:10.1097/AJP.0b013e31823e9378
41. Falla D, Jull G, Russell T, Vicenzino B, Hodges P. Effect of neck exercise on sitting posture in patients with chronic neck pain. Phys Ther. 2007;87(4):408–417. doi:10.2522/ptj.20060009
42. Martel J, Dugas C, Dubois J-D, Descarreaux M. 1sA randomised controlled trial of preventive spinal manipulation with and without a home exercise program for patients with chronic neck pain. BMC Musculoskelet Disord. 2011;12:1–13.
43. Bell DR, Guskiewicz KM, Clark MA, Padua DA. Systematic review of the balance error scoring system. Sports Health. 2011;3(3):287–295. doi:10.1177/1941738111403122
44. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19–25.
45. Guskiewicz KM. Balance assessment in the management of sport-related concussion. Clin Sports Med. 2011;30(1):89–102, ix. doi:10.1016/j.csm.2010.09.004
46. Burke S, Lynch K, Moghul Z, Young C, Saviola K, Schenk R. The reliability of the cervical relocation test on people with and without a history of neck pain. J Man Manip Ther. 2016;24(4):210–214. doi:10.1179/2042618615Y.0000000016
47. Audette I, Dumas JP, Côté JN, De Serres SJ. Validity and between-day reliability of the cervical range of motion (CROM) device. J Orthop Sports Phys Ther. 2010;40(5):318–323. doi:10.2519/jospt.2010.3180
48. Inokuchi H, Tojima M, Mano H, Ishikawa Y, Ogata N, Haga N. Neck range of motion measurements using a new three-dimensional motion analysis system: validity and repeatability. Eur Spine J. 2015;24(12):2807–2815. doi:10.1007/s00586-015-3913-2
49. Reddy RSY, Alahmari K, Silvian P. Test-retest reliability of assessing cervical proprioception using cervical range of motion device. Saudi J Sports Med. 2016;16:118.
50. Pinsault N, Vuillerme N, Pavan P. Cervicocephalic relocation test to the neutral head position: assessment in bilateral labyrinthine-defective and chronic, nontraumatic neck pain patients. Arch Phys Med Rehabil. 2008;89(12):2375–2378. doi:10.1016/j.apmr.2008.06.009
51. Jull G, Barrett C, Magee R, Ho P. Further clinical clarification of the muscle dysfunction in cervical headache. Cephalalgia. 1999;19(3):179–185. doi:10.1046/j.1468-2982.1999.1903179.x
52. James G, Doe T. The craniocervical flexion test: intra-tester reliability in asymptomatic subjects. Physiother Res Int. 2010;15(3):144–149. doi:10.1002/pri.456
53. Wing Chiu TT, Hung Law EY, Fai Chiu TH. Performance of the craniocervical flexion test in subjects with and without chronic neck pain. J Orthop Sports Phys Ther. 2005;35(9):567–571. doi:10.2519/jospt.2005.35.9.567
54. Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407–414. doi:10.1016/S1526-5900(03)00716-8
55. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18(6):927–932. doi:10.1016/j.jse.2009.03.021
56. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63(Suppl):11S240–252.
57. Burckhardt C, Dupree JK. Adult measures of pain: the McGill Pain Questionnaire (MPQ), Rheumatoid Arthritis Pain Scale (RAPS), Short-Form McGill Pain Questionnaire (SF-MPQ), Verbal Descriptive Scale (VDS), Visual Analog Scale (VAS), and West Haven-Yale Multidisciplinary Pain Inventory (WHYMPI). Arthritis Care Res. 2003;49:S96–S104.
58. Jirawatkul A. Biostatistic.
59. Treleaven J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther. 2008;13(1):2–11. doi:10.1016/j.math.2007.06.003
60. Humphreys BK. Cervical outcome measures: testing for postural stability and balance. J Manipulative Physiol Ther. 2008;31(7):540–546. doi:10.1016/j.jmpt.2008.08.007
61. Ledin T, Kronhed AC, Möller C, Möller M. Effects of balance training in elderly evaluated by clinical tests and dynamic posturography. J Vestib Res. 1990;1(2):129–138.
62. Aparicio EQ, Quirante LB, Blanco CR, Sendín FA. Immediate effects of the suboccipital muscle inhibition technique in subjects with short hamstring syndrome. J Manipulative Physiol Ther. 2009;32(4):262–269. doi:10.1016/j.jmpt.2009.03.006
63. Goble DJ, Coxon JP, Van Impe A, et al. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci. 2011;31(45):16344–16352. doi:10.1523/JNEUROSCI.4159-11.2011
64. Falla D, Jull G, Edwards S, Koh K, Rainoldi A. Neuromuscular efficiency of the sternocleidomastoid and anterior scalene muscles in patients with chronic neck pain. Disabil Rehabil. 2004;26(12):712–717. doi:10.1080/09638280410001704287
65. Mazidi M, Letafatkar A, Hadadnejad M, Rajabi S. The effects of neck muscular fatigue on static and dynamic postural control in elite male volleyball players. Hormozgan Med J. 2017;20(6):407–415. doi:10.18869/acadpub.hmj.20.6.407
66. Falla D, Farina D. Neuromuscular adaptation in experimental and clinical neck pain. J Electromyogr Kinesiol. 2008;18(2):255–261. doi:10.1016/j.jelekin.2006.11.001
67. Cheng CH, Chien A, Hsu WL, Yen LW, Lin YH, Cheng HY. Changes of postural control and muscle activation pattern in response to external perturbations after neck flexor fatigue in young subjects with and without chronic neck pain. Gait Posture. 2015;41(3):801–807. doi:10.1016/j.gaitpost.2015.02.007
68. Gross AR, Paquin JP, Dupont G, et al. Exercises for mechanical neck disorders: a cochrane review update. Man Ther. 2016;24:25–45.
69. Jull G, Trott P, Potter H, et al. A randomized controlled trial of exercise and manipulative therapy for cervicogenic headache. Spine. 2002;27(17):
70. Arami J, Rezasoltani A, Eghlidi J, Ebrahimabadi Z, Ylinen J. The applicability of proprioceptive and endurance measurement protocols to treat patients with chronic non-specific neck pain. El Med J. 2014;2:227.
71. Letafatkar K, Alizadeh MH, Kordi MR. The effect of exhausting exercise induced muscular fatigue on functional stability. J Soc Sci. 2009;5(4):416–422.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
