Back to Journals » Nutrition and Dietary Supplements » Volume 9
Effects of p-synephrine in combination with caffeine: a review
Authors Stohs SJ , Ratamess NA
Received 24 June 2017
Accepted for publication 3 August 2017
Published 13 September 2017 Volume 2017:9 Pages 87—96
DOI https://doi.org/10.2147/NDS.S144761
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Chandrika J Piyathilake
Sidney J Stohs,1,2 Nicholas A Ratamess3
1School of Pharmacy and Health Professions, Creighton University, Omaha, NE, 2Kitsto Consulting LLC, Frisco, TX, 3Department of Health and Exercise Science, College of New Jersey, Ewing, NJ, USA
Abstract: This review summarizes the current research involving p-synephrine in combination with caffeine. Over 30 clinical human studies with in excess of 700 subjects, and animal and in vitro studies have assessed the safety, efficacy, and mechanism of action of Citrus aurantium (bitter orange) extract and its primary active constituent p-synephrine. Approximately 35% of these human subjects concurrently consumed caffeine, a stimulant thermogenic agent. In these clinical investigations, no serious adverse effects were reported or observed when using the combination of p-synephrine with caffeine. Animal studies with exceedingly high doses of p-synephrine in combination with caffeine support and affirm the human studies. The results of studies conducted to date indicate that p-synephrine does not augment the cardiovascular effects of caffeine or produce cardiovascular effects at commonly used doses. These observations can be explained on the basis of in vitro mechanistic studies.
Keywords: caffeine, p-synephrine, bitter orange extract, Citrus aurantium, mechanisms, cardiovascular effects
Introduction
p-Synephrine (Figure 1) is a naturally occurring phenylethylamine derivative (protoalkaloid) present in Citrus aurantium and some other Citrus spp.1 Bitter orange extract (BOE) has been widely used for ~20 years as the patented, standardized aqueous/ethanol extract of the dried immature fruit. BOE has been consumed in dietary supplements for weight management, sport performance, appetite suppression, energy, and focus.2–4
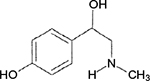  | Figure 1 Structure of p-synephrine. |
In traditional Chinese medicine, the peel and/or whole dried immature fruit of C. aurantium (BO) is used for a variety of health applications, including indigestion, diarrhea, dysentery, and constipation, and as an expectorant.5,6 BO has been used in South American folk medicine to treat insomnia, anxiety, and epilepsy. BO is also known as Seville orange, because it has been grown in Seville, Spain, for approximately 800 years, where the oranges are used in various food products, including marmalade, syrup, and juice.7,8
p-Synephrine constitutes ~90% of the protoalkaloids in BOE and to which extracts are standardized. Various studies indicate that p-synephrine enhances energy production and sport performance by acting as a thermogenic agent.9 p-Synephrine augments lipolysis, energy expenditure, and fat oxidation at rest and during the latter stages of recovery (ie, 30 minutes) following acute resistance exercise.4,10 It has also been shown to increase energy and appetite control.11
Caffeine (trimethylxanthine) (Figure 2) is the world’s most widely consumed stimulant. Coffee and tea are the most widely consumed caffeine-containing beverages, and it is estimated that over 80% of adults consume caffeine regularly in the US.12 Caffeine has been consumed for over 1,000 years, and is present in coffee, tea, chocolate, and many other plant sources.13 Coffee and tea are also the most widely consumed beverages worldwide, with an estimated 80% of the world’s population consuming caffeine on a regular basis in various foods and beverages, with few side effects.14,15
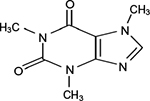  | Figure 2 Structure of caffeine. |
Caffeine has been shown to increase alertness and wakefulness, feelings of well-being, concentration and focus, and mood.16 In addition, caffeine decreases mental and physical fatigue, and enhances physical and motor performance, as well as cognitive function.17 These effects are achieved with few adverse effects.18 The widespread use of caffeinated products is due to the actions of caffeine as a stimulant thermogenic agent.9,19 Various authors have summarized the effects of caffeine and coffee on the cardiovascular system, nervous system, and carbohydrate and lipid metabolism, and questions have been raised regarding the safety of consuming caffeine in combination with p-synephrine.18,20–22
p-Synephrine is regarded as a nonstimulant thermogenic agent, due to its lack of effects on the cardiovascular system,9 while caffeine is a stimulant thermogenic.9,19 The two exhibit different mechanisms of action, and p-synephrine as BOE is frequently combined with caffeine in dietary supplements. This review specifically addresses the studies that have been conducted to date regarding the safety of BO (C. aurantium) extract (p-synephrine) and caffeine in combination. This review is based on a search of the literature in PubMed and Google Scholar for all published p-synephrine (BO, C. aurantium extract) plus caffeine studies in humans, animals, and in vitro systems, including case studies and reviews up to the date of submission.
Safety studies
Human studies
Recent studies have provided a growing body of information on the safety of BOE and p-synephrine and their mechanisms of action. Reviews of the scientific literature based on human studies indicate that commonly consumed doses of p-synephrine do not produce significant adverse effects and are free of stimulant activity.2,3,7,8 Stohs et al2 and Stohs and Shara3 summarized published and unpublished human clinical studies up to 2012, involving over 360 subjects who received a product containing a C. aurantium extract and p-synephrine alone or in combination with other ingredients. Over half the subjects consumed p-synephrine (up to 80 mg/day) in combination with caffeine (up to 528 mg/day).
Since the publication of these reviews, a number of other safety studies have been conducted, with the majority of these on patented BOE standardized to p-synephrine, alone or in combination with caffeine. Human studies involving p-synephrine and caffeine are summarized in Table 1. Several studies that have been conducted and presented at various scientific meetings but not published in peer-reviewed journals are summarized in Table 2. Information regarding these latter studies was obtained online or directly from the authors. Approximately 35% of the subjects in all studies consumed a product that contained caffeine in addition to p-synephrine.20 These studies did not determine how much caffeine was consumed daily in the form of coffee, tea, and other caffeine-containing products.
The Natural Health Products Directorate of Health Canada released an extensive review and health-risk assessment on p-synephrine, p-octopamine, and caffeine in 2011. The 49-page report also defined guidelines for the use of these three natural ingredients.21 Health Canada concluded that the use of up to 50 mg/day of p-synephrine alone in healthy adults “is not likely to cause any adverse health consequences”. A similar conclusion was drawn with respect to products containing ≤40 mg of p-synephrine when combined with ≤320 mg/day of caffeine. Dosing guidelines for p-octopamine were identical to p-synephrine.21 Health Canada is comparable to the US Food and Drug Administration (FDA). This report was prepared before various studies described herein were published demonstrating safety at higher levels.
Intertek Scientific and Regulatory Consultancy, a supplier of scientific, toxicology, and regulatory services, is known for its conservative approach to dietary supplements. Its reports are widely used as a basis for making recommendations for the use and safety of supplements. Intertek conducted an in-depth scientific literature review and issued a report on BOE (p-synephrine) and caffeine.22 This report defined guidelines for the use of p-synephrine, the dominant protoalkaloid in BOE and caffeine, and noted that “p-synephrine is unlikely to have significant effects on inotropy, vasoconstriction, or blood pressure”.22
The Intertek report further stated that dosages “not likely to cause adverse effects” included 70 mg p-synephrine alone or 40 mg in combination with 320 mg of caffeine.22 Furthermore, if taken as divided doses spaced out over the course of the day, 100 mg p-synephrine alone or 70 mg p-synephrine in combination with 400 mg caffeine is unlikely to be associated with adverse health effects. The report stated that “the use of p-synephrine alone or in combination with caffeine, within the specified limits, is not considered to pose significant concerns”.22 This report was also published before various more recent studies were published demonstrating safety at higher doses.
The European Food Safety Authority (EFSA) has published a scientific opinion on the safety of caffeine.23 The extensive review states that “single doses of caffeine up to 200 mg (about 3 mg/kg body weight for a 70 kg adult) do not give rise to safety concerns”. Furthermore, “habitual caffeine consumption up to 400 mg per day does not give rise to safety concerns for non-pregnant adults”.
In a study involving male athletes, each subject was randomly assigned (in double-blind manner) to a treatment sequence consisting of the use of three supplements in the form of two “chews”: 100 mg p-synephrine, 100 mg p-synephrine with 100 mg caffeine, or a placebo.4,10 The supplements were consumed for 3 days prior to testing. Each supplement treatment was separated by a 1-week washout period. All subjects performed a control resistance-exercise protocol. No adverse effects were observed or reported with respect to p-synephrine in the presence or absence of caffeine, while p-synephrine increased lipolysis, energy expenditure, fat oxidation, and maximum oxygen intake (V02max), as well as carbohydrate mobilization, primarily during selected segments of resting conditions before and 30 minutes after resistance exercise. Furthermore, an increase in numbers of repetitions and volume load occurred in response to p-synephrine compared to placebo, while the addition of caffeine increased the velocity of performance and mean power.4
A study examined the safety of a preworkout dietary supplement with and without p-synephrine.24 In a randomized, double-blinded, placebo-controlled manner, 25 healthy males daily received a placebo, a preworkout supplement containing 284 mg caffeine, or the caffeine-containing preworkout supplement, which also contained 20 mg p-synephrine, with a 1-week washout between testing sessions. Ingestion of the preworkout supplement with and without p-synephrine had no adverse effects on liver enzymes, kidney function, blood chemistries, or muscle enzymes relative to baseline or placebo. No clinically significant effects were observed among the treatment groups with respect to heart rate, blood pressure, electrocardiography (ECG), or blood chemistry panels.24
In a second study by the same investigators, the effects of ingesting the same preworkout dietary supplement for 8 weeks containing 284 mg caffeine with or without 20 mg p-synephrine were determined.25 The group receiving the product with p-synephrine contained 27 male subjects, while the other two groups contained similar numbers of subjects. No statistically significant effects were observed between groups with time regarding blood pressure, heart rate, or blood chemistry panels.
A study recently examined the acute cardiovascular effects of five supplements containing p-synephrine, caffeine, or a combination of both in different doses compared to a placebo. In a double-blind, randomized, within-subject research design, eight healthy human subjects were given 103 mg of p-synephrine (50% p-synephrine; ~1.3 mg/kg of body mass), 233 mg caffeine + 104 mg p-synephrine, 240 mg caffeine, 337 mg caffeine + 46 mg p-synephrine, 325 mg caffeine, or a placebo with a 1-week washout period between trials. Subjects reported to the laboratory in a fasted state (at a standard time of day early in the morning) and were assessed during a quiet resting (sitting) protocol for 3 hours following consumption of the supplement.
p-Synephrine consumption on its own did not significantly affect systolic blood pressure or heart rate at any time point. Interestingly, the p-synephrine alone resulted in small but significantly lower diastolic blood pressure at 1 and 2 hours postconsumption compared to the other supplements. Only the two trials consisting of higher caffeine doses resulted in significantly elevated systolic blood pressure. No augmented blood pressure or heart rate responses were observed when p-synephrine was added to caffeine beyond the effects of caffeine alone. In fact, no changes in heart rate were observed during consumption of any of these supplements. Responses were similar in both habitual caffeine consumers and those who did not routinely consume caffeine. These data support previous studies and indicate that p-synephrine on its own does not induce cardiovascular stress at commonly used doses, nor does it augment cardiovascular effects when combined with caffeine.2,3,20
In addition to these scientific studies, tens of millions of doses of p-synephrine-containing products have been consumed in the US and internationally by millions of individuals in combination with coffee, caffeinated beverages, and caffeine without report of serious incidents.2,3 Furthermore, orange juice of various types (mandarins, Marrs sweet oranges, clementines) has been shown to deliver up to 20–25 mg p-synephrine per 8 oz (~237 mL) glass.26,27 Millions of individuals consume p-synephrine on a daily basis in the form of juice and orange-related food products, such as marmalade, alone and in combination with caffeine from various beverages without adverse events. No serious adverse events have ever been directly attributable to p-synephrine or BOE.
In addition to studies involving p-synephrine (BOE) and caffeine, various other human studies have assessed the safety of p-synephrine. In a double-blinded, placebo-controlled safety study by Kaats et al,28 46 healthy human subjects were given BOE in capsule form (49 mg p-synephrine) twice a day (total of 98 mg/day) for 60 days, while 23 subjects received the placebo. No adverse effects were observed with respect to heart rate, blood pressure, blood chemistry, or blood cell count with differentials, indicating the safety of p-synephrine and BOE. No differences were found between the treated and placebo groups, and the subjects reported no adverse events. Caffeine was not included in the study protocol.
A placebo-controlled, double-blind, crossover safety study involving 18 male and female subjects examined the effects of BOE containing a dose of 49 mg p-synephrine in capsule form on ECG, heart rate, blood pressure, and blood chemistry over a 24-hour period.29 No significant changes occurred in ECG, heart rate, diastolic blood pressure, serum chemistry, or blood cell count, and no adverse effects were reported or observed. A small, clinically insignificant decrease in diastolic blood pressure was observed 60 minutes after consumption of the p-synephrine-containing product.
In this study,29 caffeine and p-synephrine blood levels were determined 2 hours after product administration for both control and p-synephrine-treated subjects to confirm that the subjects had consumed the BOE and to determine whether they were consuming caffeinated beverages. The results indicated compliance by all subjects regarding consumption of the BOE (p-synephrine). Levels of caffeine in blood varied from zero (only two non-caffeine-consuming subjects) to levels indicating a high, regular consumption of caffeinated beverages among most participants, without adverse cardiovascular effects being observed. Levels of caffeine in blood averaged about 900 ng/mL. Levels 2 hours after a 49 mg dose of p-synephrine were about 10.3 ng/mL (0.06 µM).29
In a follow-up placebo-controlled, double-blind, crossover study, BOE at a dose of 49 mg p-synephrine daily in capsule form for 15 days to eight male and eight female subjects was shown to have no significant effects on ECG, heart rate, blood pressure, serum chemistry, or blood cell count, and no adverse effects were reported or observed by any of the subjects.30 Average blood levels of p-synephrine 4 hours after administration on days 7 and 14 were ~2.6 ng/mL. At this time point, blood caffeine levels averaged about 800 ng/mL, indicating that most subjects regularly consumed caffeinated beverages.
In a study that assessed safety, energy, and appetite control, chocolate-flavored chews containing 51.5 mg p-synephrine (BOE standardized to 50% p-synephrine) or placebo were consumed 15–30 minutes before the two largest meals of the day for 15 or 30 days.11 As such, subjects consumed 103 mg p-synephrine or placebo daily. No caffeine was given to the participants, although a majority consumed caffeine on a daily basis. No changes in heart rate or blood pressure were noted, and no adverse effects were reported for the p-synephrine treated group (103 mg p-synephrine per day) or the placebo control group. Statistically significant increases in energy and a decrease in appetite/eating were reported with respect to the p-synephrine chew compared to the placebo control chew.11
One case study concerned an ascending aortic dissection in a male who consumed a preworkout supplement of unidentified composition, which was reported to contain caffeine and p-synephrine.31 The authors suggested that p-synephrine caused undetected aortopathy, although they did not know how much p-synephrine or caffeine was in the product. In actuality, the product contained 135 mg caffeine, 10 mg p-synephrine, 1.5 g β-alanine, Mucuna pruriens extract standardized for l-3,4-dihydroxyphenylalanine, and other ingredients. The authors did not review the literature and cited various poorly written case reports and speculative review articles. They failed to note that no controlled studies have ever shown adverse cardiovascular effects associated with p-synephrine, and that this amount of p-synephrine is widely consumed on a daily basis by many individuals in citrus juices and other food products without adverse effects.31
Thomas et al32 reported a case study of ST-segment elongation myocardial infarction in a 24-year-old male who had consumed a product containing p-synephrine and caffeine along with a caffeine energy drink on an empty stomach. The individual then proceeded to initiate a rigorous workout. The composition of the product was not given, but contained at least six alkaloidal ingredients, including 20 mg p-synephrine and 200 mg of caffeine. The amount of caffeine in the energy drink was not disclosed. The authors acknowledged that caffeine is known to “directly stimulate cardiac function”. However, they concluded that although the product “contains a variety of sympathomimetic and stimulant compounds, the most likely culprit for the induction of the coronary artery spasm is synephrine”, in spite of the fact that they could cite no controlled studies in the scientific literature that p-synephrine exerts cardiovascular effects when given orally at this dose or higher doses.
Another case study reported that apical ballooning syndrome occurred in a young woman who consumed a dietary supplement that contained synephrine and caffeine.33 No evidence or information was provided substantiating their claim, other than reference to several other poorly written case studies. The authors failed to review the current scientific literature. The authors did not indicate the names or composition of the products being taken, the amounts of the various ingredients, how many different dietary supplements were being taken, the amounts of the products being taken by the subject, or the conditions under which the supplements were being taken. No evidence directly linking supplement use with the observed syndrome was provided. As a consequence, it is not possible to establish a cause-and-effect relationship.
Well-researched and documented case studies should raise levels of awareness, while poorly written case studies can raise unfounded concerns. As discussed earlier, caffeine is known to exhibit cardiovascular activity, while p-synephrine is not. The results of these studies indicate that BOE and p-synephrine do not cause significant adverse effects, such as cardiovascular and central nervous system stimulation, at the doses commonly used under the conditions that have been employed to date, and do not augment the cardiovascular effects of caffeine. The effects resulting from chronic use of the combination are not known.
Animal studies
Several animal studies have been conducted by the National Center for Toxicological Research in conjunction with the FDA regarding the safety of BOE and p-synephrine.34–36 In a study that examined the developmental toxicity of C. aurantium in rats, the authors concluded that doses of up to 100 mg p-synephrine/kg body weight did not produce developmental toxicity.34 At this dose, there were no adverse effects with respect to embryo lethality, fetal weight, or incidence of gross, skeletal, or visceral abnormalities. This dose in rats is equivalent to a dose of p-synephrine of ~1.3 g in an 80 kg human, an amount that is 26 times greater than a typical 50 mg dose, thus demonstrating a very high degree of safety.
These authors examined the physiological effects of administering p-synephrine in the form of BOE and isolated p-synephrine to rats for 28 days at doses of up to 50 mg/kg with and without caffeine at 25 mg/kg.35 Minimal, clinically insignificant effects were produced by these high doses of p-synephrine with respect to heart rate and blood pressure. As expected, caffeine alone and in combination with p-synephrine produced more pronounced but small increases in heart rate and blood pressure. The 25 mg/kg dose of caffeine in the rats is equivalent to approximately a 325 mg dose in an 80 kg human. Because p-synephrine binds approximately 10 times more readily to adrenergic receptors in rats compared to humans, results of animal studies cannot be directly extrapolated to humans.37
The potential cardiovascular effects of BOE and p-synephrine were also examined in exercised rats given up to 50 mg/kg p-synephrine in the presence and absence of 25 mg/kg caffeine for 28 days.36 Small increases in heart rate and body temperature were reported due to caffeine, while p-synephrine exhibited small, clinically insignificant effects on blood pressure at the high dose. It should be noted that the dose of 50 mg/kg p-synephrine is approximately 13 times higher than a typical 50 mg dose in an 80 kg human in conjunction with a dose of ~325 mg caffeine. It should again be kept in mind that a dose 10 times higher in humans may be required to produce the modest cardiovascular effects that are observed in rats.37
The acute toxicity of BOE and p-synephrine in mice was examined by Arbo et al.38 The administration of BOE containing 2.5% p-synephrine at single oral doses of 300–3,000 mg/kg resulted in reduced locomotor activity (hypoactivity). Doses of 150–2,000 mg/kg of p-synephrine produced reduced locomotion, salivation, gasping, piloerection, and exophthalmia. All effects were reversible and resolved in 3–4 hours. These effects were believed to be due to adrenergic agonist activity. Because p-synephrine is a poor adrenergic agonist (see “Mechanistic studies” section), exceedingly high doses are required to produce these effects.
In another study, mice were treated daily with BOE (7.5% p-synephrine) at doses of 400/2,000/4,000 mg/kg (corresponding to 30/150/300 mg p-synephrine/kg) or 30/300 mg p-synephrine/kg.39 The 300 mg/kg dose is approximately 78 times a typical 50 mg human dose of p-synephrine. This study did not include caffeine. A reduction in body-weight gain was observed at all doses relative to controls. No adverse effects were observed regarding organ weight, biochemical parameters, blood pressure, or heart rate in the treated mice at any of the doses.
In addition, both doses of p-synephrine and the high dose of BOE resulted in increases in the antioxidant and tissue protectant glutathione, while BOE decreased malondialdehyde content (an indicator of lipid peroxidation and oxidative lipid damage) and p-synephrine increased catalase, which neutralizes hydrogen peroxide.39 The results indicated a beneficial effect with respect to weight loss without adverse effects while also providing an antioxidant and tissue-protective effect.
Several unpublished safety studies have also been conducted on the safety of p-synephrine (personal correspondence). A single dose of 10,000 mg/kg of a 6% p-synephrine containing BOE did not cause lethality in rats. Furthermore, 5,000 mg/kg of a 50% p-synephrine-containing extract did not produce deaths in rats, indicating that the LD50 of this extract was >5,000 mg/kg.20 Studies on the LD50 of caffeine in rats have been summarized by Adamson.40 The average LD50 in male albino rats for caffeine over six studies was 367 mg/kg. For female rats, the estimated LD50 was over 300 mg/kg. Cases of caffeine lethality in humans are rare. In summary, animal studies indicate that at the doses of p-synephrine commonly used in dietary supplements, either alone or in conjunction with commonly used doses of caffeine, cardiovascular or other adverse effects would not be expected.
Mechanistic studies
Cardiovascular effects of ligands are associated with adrenergic receptor binding. In general, when ligands bind to α-adrenergic receptors, vasoconstriction occurs, and when binding to β1-adrenergic receptors, cardiovascular contractility and increased heart rate result. Ligand binding to β2-adrenergic receptors is associated with bronchodilation.41 It should be noted that adrenergic agonists with poor adrenergic receptor binding can exert cardiovascular effects through indirect mechanisms, such as the release of norepinephrine and epinephrine.9 Adverse cardiovascular effects are commonly associated with ephedrine, but not p-synephrine.2,3
The reason for the lack of blood pressure and heart rate effects in conjunction with p-synephrine may be due to the fact that p-synephrine binds much more poorly to α1-, α2-, β1-, and β2-adrenergic receptors than other ligands, such as norepinephrine and m-synephrine.42 For example, Brown et al43 observed that p-synephrine was 1,000-fold less active in binding to α1- and α2-adrenergic receptors than norepinephrine, while binding of synthetic m-synephrine (phenylephrine) to these two receptors was 150- and 6-fold less, respectively, than norepinephrine. m-Synephrine does not occur in nature, is not present in BOEs,44 and is not permitted in dietary supplements. Ma et al45 concluded that p-synephrine acts as an antagonist, rather than an agonist, with respect to human α2A- and α2C-adrenergic receptors. Jordan et al46 concluded that p-synephrine bound to β1- and β2-adrenergic receptors about 10,000-fold less actively than norepinephrine.
Various studies have shown that p-synephrine binds to β3-adrenergic receptors, resulting in an increase in the body’s ability to break down fats.37,47,48 Binding to β3-adrenergic receptors does not influence heart rate or blood pressure. Again, because p-synephrine exhibits little or no binding to α1-, α2-, β1-, and β2-adrenergic receptors and does not appear to exert indirect adrenergic effects, cardiovascular effects, such as an increase in heart rate and blood pressure, are not experienced at commonly used doses, unlike a number of other phenylethylamine and phenylpropanolamine derivatives. Small structural changes result in large differences in adrenergic receptor binding and subsequently the ability to produce cardiovascular effects.
p-Synephrine also enhances the body’s utilization of carbohydrates by producing more energy in the form of ATP.49–52 It facilitates cellular uptake of glucose in muscle cells, as well as glycogenolysis, gluconeogenesis, glycolysis, and oxygen uptake. The involvement of both calcium ion and cAMP with adrenergic receptors has been demonstrated to be involved in these biochemical processes, indicating the involvement of multiple mechanisms.
In a study involving the use of NMUR2– and shRNA-knockdown HEK293 cell lines, p-synephrine was shown to bind to this receptor with high efficacy and potency at micromolar concentrations achievable after oral administration.53 NMUR2 is present in the hypothalamic regions of the brain and is associated with regulation of food intake, energy balance, stress, and nociception.
Because p-synephrine is rapidly metabolized, with only about 2.5% being excreted in the urine unchanged, are adequate blood levels achieved to produce potential cardiovascular and thermogenic effects? Several studies have shown that the half-life of p-synephrine is between 2 and 3 hours.54–56 Haller et al55 reported maximum p-synephrine blood concentrations of ~3 ng/mL after a dose of 46.9 mg, with blood levels near baseline after four half-lives. Shara et al30 observed blood concentrations of ~10 ng/mL and 2.6 ng/mL at 2 and 4 hours after consumption of 49 mg. Various effects on lipid metabolism,10,24,25,57 metabolic rate,52,58 sport performance,4 and energy production11 have been demonstrated at these doses, as well as higher doses of p-synephrine that do not produce cardiovascular effects.4,10,11,24,25,52,57,58 Therefore, adequate blood levels are achieved to produce these effects without cardiovascular effects. The question remains whether cardiovascular effects can be attained at doses of p-synephrine >100 mg. To date, doses of up to 100 mg p-synephrine per day for 60 days have been shown to be without adverse effects.28
Caffeine has various effects and mechanisms of action. In vascular smooth muscle cells, caffeine acts predominantly as a competitive inhibitor of the enzyme phosphodiesterase, which is responsible for the breakdown of cAMP. cAMP is an important second messenger and produces diverse signaling responses, including those associated with energy status (AMP:ATP ratio) and the effects of caffeine.59,60
A second mechanism of action of caffeine is based on its binding to and inhibiting adenosine A1 and A2A receptors.61–63 Caffeine inhibition of the A1 receptor activates adenylate cyclase, which results in an increase in cAMP and activity of protein kinase A, the latter being associated with stimulation of the central nervous system.
The resultant downstream pharmacological effects of caffeine include an increase in energy metabolism and vasodilatation and a decrease in smooth muscle contraction. Furthermore, caffeine causes neurotransmitter release, resulting in central nervous system stimulation, positive inotropic and possibly weak chronotropic effects on the heart, and increased blood pressure. In addition, in the kidney caffeine induces diuresis, vasodilatation, and sodium reabsorption as a result of increased glomerular filtration rate secondary to increased blood pressure.61–63 Daily consumption of up to 400 mg of caffeine is believed to be safe and without serious, injurious, long-lasting, or life-threatening adverse health effects.23 Because p-synephrine does not augment the effects of caffeine at commonly used doses or exert cardiovascular effects on its own, recommendations for the use of p-synephrine in combination with caffeine should be a reflection of the recommendations for caffeine.
Conclusion
Over 30 published and unpublished human studies have been conducted on BOE (p-synephrine), involving over 700 subjects, with ~35% of subjects consuming a product that also contained caffeine. The number of subjects in these studies that were regular caffeine consumers is not known, although it is known that ~80% of adults consume caffeine on a regular basis. The highest daily dose of caffeine consumed in a study was over 500 mg, while the highest daily dose of p-synephrine in these studies was 104 mg. p-Synephrine applications include weight loss/weight management, sport performance, appetite control, energy, mental focus, and cognition. Various studies indicate that p-synephrine exerts these effects through such mechanisms as binding to β3-adrenergic receptors, which regulate lipid and carbohydrate metabolism. p-Synephrine also enhances cellular uptake of glucose and mitochondrial production of ATP. Because p-synephrine exhibits little or no binding to α1-, α2-, β1-, and β2-adrenergic receptors and no indirect adrenergic effects, it exerts metabolic enhancement without acting as a central nervous system or cardiovascular stimulant, and thus does not increase heart rate or blood pressure.
Caffeine is a stimulant thermogenic agent with mechanisms of action that differs from p-synephrine. It is a competitive inhibitor of the enzyme phosphodiesterase, and also binds to and inhibits adenosine A1 and A2A receptors. Caffeine is widely used in combination with p-synephrine. Studies in humans and animals to date have shown that the combination of p-synephrine with caffeine is safe and free of adverse events. A recent study in humans specifically addressed the cardiovascular effects of caffeine and p-synephrine alone and in combination. Caffeine at high doses increased systolic blood pressure, while p-synephrine exhibited a small decrease in diastolic blood pressure and did not enhance the cardiovascular effects of caffeine.
Taken together, human, clinical, animal, and in vitro studies indicate that p-synephrine does not act as a cardiovascular stimulant at commonly used doses, nor does it augment the cardiovascular effects of caffeine. This lack of cardiovascular stimulation by p-synephrine can be readily explained by its poor adrenergic receptor binding and lack of indirect adrenergic effects. It should be noted that published studies involving caffeine and p-synephrine have been short term in nature. Longer term studies are needed to assess both the efficacy and the tolerability of the combination of these two ingredients.
Disclosure
SJS has served as a consultant for Novel Ingredients, a company that markets bitter orange (C. aurantium) extracts. NAR reports no conflicts of interest in this work.
References
Avula B, Upparapalli SK, Navarrete A, Khan IA. Simultaneous quantification of adrenergic amines and flavonoids in C. aurantium, various Citrus species, and dietary supplements by liquid chromatography. J AOAC Int. 2005;88:1593–1606. | ||
Stohs SJ, Preuss HG, Shara M. A review of the human clinical studies of bitter orange (Citrus aurantium) and its primary protoalkaloid p-synephrine. Int J Med Sci. 2012;9:527–538. | ||
Stohs SJ, Shara M. A review of the safety and efficacy of bitter orange (Citrus aurantium) and its primary protoalkaloid, p-synephrine, in weight management. In: Bagchi D, Preuss HG, editors. Obesity: Epidemiology, Pathophysiology, and Prevention. 2nd ed. Boca Raton, FL: CRC Press; 2013:535–554. | ||
Ratamess NA, Bush JA, Kang J, et al. The effects of supplementation with p-synephrine alone and in combination with caffeine on resistance exercise performance. J Int Soc Sports Nutr. 2015;12:35. | ||
Chen JK, Chen TT. Zhi shi (fructus aurantii immaturus). In: Chinese Medical Herbology and Pharmacology. Los Angeles: Art of Medicine Press; 2004:483–485. | ||
Fang YS, Shan DM, Liu JW, et al. Effects of constituents from fructus aurantii immaturus and radix paeoniae alba on gastrointestinal movement. Planta Med. 2009;75:24–31. | ||
Stohs SJ, Preuss HG. The safety of bitter orange (Citrus aurantium) and its primary protoalkaloid p-synephrine. HerbalGram. 2011;89:34–39. | ||
Stohs SJ, Preuss HG, Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res. 2011;25:1421–1428. | ||
Stohs SJ, Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother Res. 2016;30:732–740. | ||
Ratamess NA, Bush JA, Kang J, et al. The effects of supplementation with p-synephrine alone and in combination with caffeine on metabolic, lipolytic, and cardiovascular responses during resistance exercise. J Am Coll Nutr. 2016;35:657–669. | ||
Kaats GR, Leckie RB, Mrvichin N, Stohs SJ. Increased eating control and energy levels associated with consumption of bitter orange (p-synephrine) extract: a randomized placebo-controlled study. Nutr Diet Suppl. 2017;9:29–35. | ||
Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010–1022. | ||
Whayne TR Jr. Coffee: a selected overview of beneficial or harmful effects on the cardiovascular system? Curr Vasc Pharmacol. 2015;13:637–648. | ||
Iancu I, Strous RD. [Caffeine intoxication: history, clinical features, diagnosis and treatment]. Harefuah 2006;145:147–151, 163–164. Hebrew. | ||
Muriel P, Arauz J. Coffee and liver diseases. Fitotherapia. 2010;81:297–305. | ||
Nehlig A. Effects of coffee/caffeine on brain health and disease: What should I tell my patients? Pract Neurol. 2016;16:89–95. | ||
Glade MJ. Caffeine: not just a stimulant. Nutrition. 2010;26:932–938. | ||
Cano-Marquinam A, Tarinm JJ, Canom A. The impact of coffee on health. Maturitas. 2013;75:7–21. | ||
Astrup A, Toubro S, Cannon S, Hein P, Breum L, Madsen J. Caffeine: a double-blind, placebo-controlled study of its thermogenesis, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr. 1990;51:759–767. | ||
Stohs SJ. Safety, efficacy and mechanistic studies regarding Citrus aurantium (bitter orange) extract and p-synephrine. Phytother Res. Epub 2017 Jul 8. | ||
Health Canada. Synephrine, octopamine and caffeine health risk assessment (HRA) report. 2011. Available from: http://www.novelingredient.com/wp-content/uploads/2017/03/Health-Canada-May11.pdf. Accessed August 9, 2017. | ||
Lynch B. Review of the safety of p-synephrine and caffeine. 2013. Available from: http://www.novelingredient.com/wp-content/uploads/2017/03/Intertek-Cantox-Apr13.pdf. Accessed August 29, 2017. | ||
European Food Safety Authority. Scientific opinion on the safety of caffeine. EFSA J. 2015;13:4102. | ||
Jung YP, Earnest CP, Koozehchian M, et al. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J Int Soc Sports Nutr. 2017;14:3. | ||
Jung YP, Earnest CP, Koozehchian M, et al. Effects of ingesting a pre-workout dietary supplement with and without synephrine for 8 weeks on training adaptations in resistance-trained males. J Int Soc Sports Nutr. 2017;14:1. | ||
Dragull K, Breksa AP, Cain B. Synephrine content of juice from satsuma mandarins (Citrus unshiu Marcovitch). J Agric Food Chem. 2008;56:8874–8878. | ||
Uckoo RM, Jayaprakasha GK, Nelson DS, Patil BS. Rapid simultaneous determinations of amines and organic acids in citrus using high-performance liquid chromatography. Talanta. 2011;83:948–954. | ||
Kaats GR, Miller H, Preuss HG, Stohs SJ. A 60-day double-blind, placebo-controlled safety study involving Citrus aurantium (bitter orange) extract. Food Chem Toxicol. 2013;55:358–362. | ||
Shara M, Stohs SJ, Mukattash TL. Cardiovascular safety of oral p-synephrine (bitter orange) in human subjects: a randomized placebo-controlled cross-over clinical trial. Phytother Res. 2016;30:842–847. | ||
Shara M, Stohs SJ, Smadi MM. Safety evaluation of bitter orange (p-synephrine) extract following oral administration for 14 days to healthy human subjects: a clinical trial. 2017. In press. | ||
Doctorian T, Do B. Ascending aortic dissection in a young patient using a synephrine-containing workout supplement. J Cardiol Cases. 2017;15:150–152. | ||
Thomas JE, Munir JA, McIntyre PZ, Ferguson MA. STEMI in a 24-year-old man after use of a synephrine-containing dietary supplement: a case report and review of the literature. Tex Heart Inst J. 2009;36:586–590. | ||
Chung H, Kwon SW, Kim TH, et al. Synephrine-containing dietary supplement precipitating apical ballooning syndrome in a young female. Korean J Intern Med. 2013;28:356–360. | ||
Hansen DK, Juliar BE, White GE, Pellicore LS. Developmental toxicity of Citrus aurantium in rats. Birth Defects Res B Dev Reprod Toxicol. 2011;92:216–223. | ||
Hansen DK, George NI, White GE, Pellicore, LS, Abdel-Rahman A, Fabricant D. Physiological effects following administration of Citrus aurantium for 28 days in rats. Toxicol Appl Pharmacol. 2012;261:236–247. | ||
Hansen DK, George NI, White GE, Abdel-Rahman A, Pellicore LS, Fabricant D. Cardiovascular toxicity of Citrus aurantium in exercised rats. Cardiovasc Toxicol. 2013;13:208–219. | ||
Mercader J, Wanecq E, Chen J, Carpéné C. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J Physiol Biochem. 2011;67:442–452. | ||
Arbo MD, Larentis ER, Linck VM, et al. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem Toxicol.2008;46:2770–2775. | ||
Arbo MD, Schmitt GC, Limberger MF, et al. Subchronic toxicity of Citrus aurantium L. (Rutaceae) extract and p-synephrine in mice. Regul Toxicol Pharmacol. 2009;54:114–117. | ||
Adamson RH. The acute lethal dose 50 (LD50) of caffeine in albino rats. Regul Toxicol Pharmacol. 2015;80:274–276. | ||
Inchiosa MA. Evidence (mostly negative) with the use of sympathomimetic agents for weight loss. J Obes. 2011;2011:764584. | ||
Stohs SJ, Preuss HG, Shara M. A review of the receptor-binding properties of p-synephrine as related to its pharmacological effects. Oxid Med Cell Longev. 2011;2011:482973. | ||
Brown CM, McGrath J, Midgley JM, et al. Activities of octopamine and synephrine stereoisomers on α-adrenoreceptors. Br J Pharmacol. 1988;93:417–429. | ||
Pellati F, Benvenuti S. Chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium. J Chromatog A. 2007;1171:71–88. | ||
Ma G, Bavadekar SA, Schaneberg BT, Khan IA, Feller DR. Effects of synephrine and β-phenylephrine on human α-adrenoceptor subtypes. Planta Med. 2010;76:981–986. | ||
Jordan R, Thonoor CM, Williams CM. β-Adrenergic activities of octopamine and synephrine stereoisomers on guinea-pig atria and trachea. J Pharm Pharmacol. 1987;39:752–754. | ||
Carpéné C, Galitzky J, Fontana E, Atgié C, Lafontan M, Berlan M. Selective activation of β3-adrenoreceptors by octopamine: comparative studies in mammalian fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:310–321. | ||
Carpéné MA, Testar X, Carpéné C. High doses of synephrine and octopamine activate lipolysis in human adipocytes, indicating that amines from Citrus might influence adiposity. In: Hayat K, Editor. Citrus. Hauppauge, NY: Nova Science; 2014:141–168. | ||
Hong NA, Cui ZG, Kang HK, Lee DH, Lee YK, Park DB. p-Synephrine stimulates glucose consumption via AMPK in L6 skeletal muscle cells. Biochem Biophys Res Commun. 2012;418:720–724. | ||
Peixoto JS, Comar JF, Moreira CT, Soares AA, de Oliveira AL, Peralta RM. Effects of Citrus aurantium (bitter orange) fruit extracts and p-synephrine on metabolic fluxes in the rat liver. Molecules. 2012;17:5854–5869. | ||
de Oliveira AL, Comar JF, de Sá-Nakanishi AB, Peralta RM, Bracht A. The action of p-synephrine on hepatic carbohydrate metabolism and respiration occurs via both Ca2+-mobilization and cAMP production. Mol Cell Biochem. 2014;388:135–147. | ||
Gougeon R, Harrigan K, Tremblay JF, Hedrei P, Lamarche M, Morais JA. Increase in the thermic effect of food in women by adrenergic amines extracted from Citrus aurantium. Obes Res. 2005;13:1187–1194. | ||
Zheng X, Guo L, Wang D, Deng X. p-Synephrine: a novel agonist for neuromedin U2 receptor. Biol Pharm Bull. 2014;37:764–770. | ||
Hengstmann JH, Aulepp H. [Pharmacokinetics and metabolism of synephrine]. Arzneimittelforschung. 1978;28:2326–2331. German. | ||
Haller CA, Benowitz NL, Peyton J 3rd. Hemodynamic effects of ephedra-free weight loss supplements in humans. Am J Med. 2005;118:998–1003. | ||
Haller CA, Duan M, Peyton J 3rd, Benowitz N. Human pharmacology of a performance-enhancing dietary supplement under resting and exercise conditions. Br J Clin Pharmacol. 2008;65:833–840. | ||
Gutierrez-Hellin J, Coso JD. Acute p-synephrine ingestion increases fat oxidation rate during exercise. Br J Clin Pharmacol. 2016;82:362–368. | ||
Stohs SJ, Preuss HG, Keith SC, Keith PL, Miller H, Kaats GR. Effects of p-synephrine alone and in combination with selected bioflavonoids on resting metabolism, blood pressure, heart rate and self-reported mood changes. Int J Med Sci. 2011;8:295–301. | ||
Montoya GA, Bakuradze T, Eirich M, et al. Modulation of 3’,5’-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br J Nutr. 2014;112:1427–1437. | ||
Nunes AR, Holmes AP, Conde SV, Gauda EB, Monteiro EC. Revisiting cAMP in the carotid body. Front Physiol. 2014;5:406. | ||
Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med. 1990;41:277–288. | ||
Donovan JL, DeVane CL. A primer on caffeine pharmacology and its drug interactions in clinical psychopharmacology. Psychopharmacol Bull. 2001;35:30–48. | ||
Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30. | ||
Colker CM, Kalman DS, Torina GC, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John’s wort on body fat loss, lipid levels, and mood states in overweight healthy adults. Curr Ther Res. 1999;60:145–153. | ||
Kalman DS, Colker CM, Shi QV, Swain MA. Effects of a weight-loss aid in healthy overweight adults: double-blind, placebo-controlled clinical trial. Curr Ther Res. 2000;61:199–205. | ||
Kalman DS, Incledon T, Gaunaurd I, Schwartz H, Krieger D. An acute clinical trial evaluating the cardiovascular effects of an herbal ephedra-caffeine weight loss product in healthy overweight adults. Int J Obes Relat Metab Disord. 2002;26:1363–1366. | ||
Kalman DS. Incledon T, Gaunaurd I, Svhwartz H, Krieger D. An acute clinical trial evaluating the cardiovascular effects of an herbal ephedra-caffeine weight loss product in healthy overweight adults. Int J Obes Relat Metab Disord. 2004;28:1355–1356. | ||
Zenk JL, Leikam SA, Kassen LJ, Kushowski MA. Effect of lean system 7 on metabolic rate and body composition. Nutrition. 2005;21:179–185. | ||
Sale C, Harris RC, Delves S, Corbett J. Metabolic and physiological effects of ingesting extracts of bitter orange, green tea and guarana at rest and during treadmill walking in overweight males. Int J Obes (Lond). 2006;30:764–773. | ||
Hoffman JR, Kang J, Ratamess A, Jennings PF, Mangione G, Faigenbaum AD. Thermogenic effect from nutritionally enriched coffee consumption. J Int Soc Sports Nutr. 2006;3:35–43. | ||
Seifert JG, Nelson A, Devonish J, Burke ER, Stohs SJ. Effect of acute administration of an herbal preparation on blood pressure and heart rate in humans. Int J Med Sci. 2010;8:192–197. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


