Back to Journals » Clinical Ophthalmology » Volume 16
Effects of Ketoconazole on the Clinical Recovery in Central Serous Chorioretinopathy
Authors Chantarasorn Y, Rasmidatta K, Pokawattana I, Silpa-archa S
Received 29 March 2022
Accepted for publication 24 May 2022
Published 9 June 2022 Volume 2022:16 Pages 1871—1882
DOI https://doi.org/10.2147/OPTH.S368427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Yodpong Chantarasorn,1 Kochapong Rasmidatta,1 Itsara Pokawattana,1,2 Sukhum Silpa-archa3
1Department of Ophthalmology, Vajira Hospital, Navamindradhiraj University, Bangkok, 10300, Thailand; 2Department of Ophthalmology, H.R.H Maha Chakri Sirindhorn Medical Center, Srinakharinwirot University, Nakhon Nayok, 26120, Thailand; 3Department of Ophthalmology, Rajavithi Hospital, College of Medicine, Rangsit University, Bangkok, 10400, Thailand
Correspondence: Yodpong Chantarasorn, Vajira Hospital, Navamindradhiraj University, 681 Samsen Street, Bangkok, 10300, Thailand, Tel +66 (89) 103-6633, Fax +66 (2) 241-4388, Email [email protected]
Purpose: Patients with hypercortisolism have been associated with a higher prevalence of the pachychoroid spectrum including central serous chorioretinopathy (CSCR), which may explain the inconsistency of therapeutic responses of the mineralocorticoid receptor antagonist because hyperaldosteronism has rarely been detected in patients with CSCR. Therefore, this study aimed to evaluate the effects of ketoconazole, the first-line cortisol inhibitor, on the resolution of subretinal fluid (SRF) in CSCR and to analyze correlations between choroidal thickness and steroid hormones.
Patients and Methods: This retrospective cohort study included 41 naïve CSCR eyes of 41 patients categorized into control (20 eyes) and treatment (21 eyes) groups. Patients in the treatment group were administered oral ketoconazole at a daily dose of 400 or 600 mg for 3– 6 weeks. At week 12, rescue laser therapy was applied to patients exhibiting persistent SRF. Thus, a survival analysis was performed to determine the time interval from presentation to clinical resolution of SRF. Secondary outcomes consisted of eyes with persistent SRF and factors affecting the therapeutic response.
Results: The mean 24-hour urinary free cortisol (UFC) levels were elevated at 181 ± 70 and 150 ± 68 μg/day (range: 20– 150) in the treatment and control groups, respectively (p = 0.21). After controlling for age and gender, baseline UFC levels were significantly associated with choroidal thickness in both eyes (p < 0.05). Ketoconazole significantly increased the CSCR resolution with the median time to resolution of 7 vs 16 weeks (p < 0.01) and decreased the proportion of eyes receiving rescue therapy at 12 weeks (23.8% vs 50%; p = 0.01). Prolonged CSCR durations were likely found in elderly patients with thick choroids in fellow eyes.
Conclusion: Patients with CSCR showed elevated glucocorticoids, which further correlated with their choroidal thickness. Using cortisol blockers may shorten the duration of existing SRF.
Keywords: pachychoroidopathy, cortisol, choroidal thickness, mineralocorticoid receptor antagonist
Plain Language Summary
This study showed an association between elevated cortisol levels and choroidal thickness in patients with central serous chorioretinopathy (CSCR), a common retinal disease affecting the central vision due to abnormal fluid underneath the retina. Using oral ketoconazole, a cortisol blocker, may reinforce faster resolution for subretinal fluid. However, due to its potential side effects, this drug may only be considered for those highly at risk for refractory CSCR.
Introduction
Choroidal hyperpermeability in central serous chorioretinopathy (CSCR) has been associated with increased endogenous corticosteroids.1–6 Nonetheless, most patients with CSCR rarely represent Cushing’s syndrome. Such an elevation of steroids, particularly glucocorticoids, is a production of repetitive hyperactivity in the hypothalamic–pituitary–adrenal axis. Abnormalities in endocrine signaling potentially affect predisposing factors for CSCR including constant exposure to stress, psychiatric problems, and chronic sleep deprivation.2,7 Modification of these factors combined with deferred laser therapy for CSCR still has one major unsolvable problem, ie, ambiguous information regarding features affecting persistent subretinal fluid (SRF). Potential results include some degrees of permanent visual dysfunction even after laser therapy.8,9 This concern has led to an early and noninvasive treatment using oral mineralocorticoid (aldosterone) receptor (MR) antagonists in recent years.10 However, hyperaldosteronism has rarely been detected in patients with CSCR, which may explain the ineffectiveness of MR antagonists.11–13 This was supported by a multicenter, double-blind, randomized, placebo-controlled trial (VICI trial) that ran successfully to a high-quality standard over a 1-year period. The study produced clear, unequivocal findings: aldosterone antagonists (eplerenone) were not superior to placebo for treatment of active CSCR in terms of median best-corrected visual acuity (BCVA) at 12 months or with regards to secondary outcomes including time-to-resolution of SRF and time to recurrence.11 Theoretically, active free cortisol has the same MR affinity as aldosterone but is present in much higher concentrations in plasma. MR antagonists only inhibit the binding of excessive steroids to the MR, whereas most glucocorticoid receptors on the choroid are still engaged by cortisol.14
Ketoconazole is the first-line glucocorticoid inhibitor through an adrenolytic activity at the adrenal cortex. Furthermore, the medication simultaneously blocks glucocorticoid receptors and MR ligands at the cellular level and should prevent further choroidal vasodilation.14,15 These mechanisms are expected to accelerate the SRF resolution and thereby decrease the risk of persistent CSCR. Therefore, this study aimed to evaluate the effects of ketoconazole on clinical outcomes in CSCR eyes and to analyze relationships between choroidal thickness and steroid hormones.
Materials and Methods
This was a three-center, retrospective cohort study conducted at Vajira, Rajavithi, and Srinakharinwirot University Hospitals in Thailand between July 2018 and August 2020. The Institutional Review Boards approved the study (COA No. 30/2020 and 178/2562), and the principles for this research were based on the Declaration of Helsinki. Currently, oral ketoconazole is not yet approved for the treatment of CSCR. All patients were informed about the off-label therapy and signed a written consent before initiating therapy.
Patients aged 20–55 years, who were diagnosed with naïve CSCR and exhibited symptoms within the past 6 months, had been consecutively reviewed. In bilateral CSCR, the eye with shorter disease duration was selected as the study eye. Simultaneous fluorescein and indocyanine green angiography (Spectralis, Heidelberg engineering, Germany) images were obtained to eliminate the possibility of choroidal neovascularization. Patients with the following conditions were excluded: high myopia or hyperopia (>4 diopters), other conditions manifesting serous macular detachment, nephrotic syndrome, chronic kidney disease, liver disease, conditions depending on a long-term steroid use, and women during pregnancy and lactation. Urine pregnancy test was performed for all female patients who were uncertain of their pregnancy status prior to drug administration. Further, patients taking drugs that may interrupt a cytochrome-dependent metabolism and patients taking antacid or H2 blockers were excluded from the study.
Regarding our hospitals’ checklists and guidelines for patients with CSCR, a discontinuation of smoking and steroid usage was routinely advised. A psychiatrist consultation had been prescribed as indicated. Patients must have completed the STOP-BANG questionnaire, a quick screening tool for obstructive sleep apnea (OSA).16 All high-risk patients were required to undergo sleep laboratory tests.
Reviewed patients were classified into those receiving ketoconazole and those who did not (ketoconazole-treated and control group, respectively). In the ketoconazole-treated group, oral ketoconazole at a daily dose of 400 (BW <50 kg) or 600 mg (BW >50 kg) was administered for a maximum duration of 6 weeks. Treatments could be terminated if any evidence of dry fovea is detected in the optical coherence tomography (OCT) (clinical resolution of CSCR). The dosage could be decreased by 200 mg in patients with clinical improvement at 3 weeks. Placebos were not administered to the control group. Treatment allocations were based on patients’ decisions after thoroughly explaining differences in risks and benefits between oral ketoconazole and conservative treatment. Different treatment possibilities, such as bevacizumab and MR antagonists were discussed in brief. Of note, we did not offer anti-vascular endothelial growth factor (VEGF) as the first-line therapy to the patients due to its unclear benefits.17,18 The medications also were not accessible at the baseline visit because of policy-related reimbursement issues. Rescue laser therapies using laser photocoagulation or half-dose photodynamic therapy (PDT) were administered to individuals with SRF that included the fovea on OCT scanning at the 12-week follow-up. We used 12 weeks as the cut-off value, as the guidelines for the rescue interventions since patients who failed to have a spontaneous SRF resolution within 3 months after the disease onset tended to have persistent SRF accompanying retinal pigment epithelium (RPE) alteration, which may lead to permanent damage to the photoreceptors and a decrease in long-term visual quality.19 Although half-dose PDT has been proved to be better than micropulse laser for treating active CSCR,20 eligibility for verteporfin was strictly limited at that time. Hence, PDT was selected exclusively in patients with the following conditions: ill-defined hyperfluorescent areas on fluorescein angiography, multifocal leakage points, or leakage within 500 µm from the foveal center. Off-labeled anti-VEGF was administered to eyes with thin choroid and diffuse fluorescein leakage involving the fovea.
Symptom durations were recorded based on patients’ history. The primary outcome was the time interval from SRF presentation to resolution in foveal areas. Secondary outcomes consisted of eyes with persistent SRF requiring rescue interventions at the 12-week follow-up and predictors of such occurrences. Due to disrupted circadian rhythms in most patients with CSCR, periodic collections of urine specimen throughout the 24-h period are more reliable samples to detect free cortisol than a single-timed morning serum.21 Therefore, baseline hormonal profile of endogenous steroids selected in this study comprised 24-h urinary free cortisol (UFC), serum aldosterone, and total testosterone. Subfoveal choroidal thickness (SFCT) was measured from the back surface of the RPE to chorioscleral interface using a horizontal scan of enhanced-depth imaging OCT (Spectralis OCT, Heidelberg engineering, Germany). A 1-mm central subfield thickness (CST) and ocular examination were collected at baseline and 1, 3, and 6 months. A flow diagram showing the study profile summary is shown in Supplementary Figure S1.
In the ketoconazole-treated group, potential adverse effects of the drug were monitored and recorded every visit, including but not limited to stomach pain, skin rash, headache, dizziness, breast swelling, impotence, and elevated liver enzymes.15
Statistical Analysis
Skewed data were logarithmically transformed before the analysis (Table 1). P values for age and symptom duration were calculated using two sample t-test. Multivariate regression models controlling for age were separately performed to compare SFCT and 1-mm CST between the two groups at the baseline visit. After validating the implemented model, multiple regression analysis adjusted for age and symptom duration was performed to detect relationships between baseline SFCT and each endogenous steroid level (Figure 1). Regarding the 6-month results including visual acuity and changes in SFCT, covariate adjustment was applied for each analysis by adding baseline confounders (age, symptom duration, and baseline CST) and significant predictors into the multivariate regression model (Table 2).
 |
Table 1 Baseline Clinicodemographic and Hormonal Profiles of All 41 Study Patients with Central Serous Chorioretinopathy (CSCR) |
 |
Table 2 Clinical Outcomes of Study Patients in Both Groups at 6 Months |
Survival analysis was performed to determine the time distribution to SRF resolution using the p-sample Log rank test (Figure 2). Observations were censored at the first detection of dry macula in all eyes including those receiving rescue therapy at 12 weeks. Sample size was calculated based on the primary outcome (median time to resolution of foveal SRF). The study power was performed using an unconditional, two-sided exponential test with an alpha of 0.05. We expected to detect a difference in time to resolution from 16 (by observation) to 8 weeks, with estimated hazards ratio of 3.0. The time estimated was based on the previous studies evaluating the effects of PDT for the treatment of CSCR. The mean time from baseline to initial complete resolution of SRF ranged from 6 to 12 weeks after a single session of half-dose PDT.18,22–25 Regarding these assumptions, a total sample size of 20 eyes from 20 patients in each group would yield 80% power of difference detection.
A Cox proportional hazards regression was used to calculate hazard ratios of predictors in the 12-week persistent SRF (Wald p-value). Covariate adjustments were performed on the included potential predictors in the model (Table 3). Lastly, whether any violations of proportional hazards assumptions exist on each covariate was assessed. The two-sided p-value of <0.05 was considered statistically significant. The Stata version 15.0 (StataCorp, College Station, TX) was used for all computations.
 |
Table 3 A Cox Regression Analysis Demonstrating Predictors for Rapid Subretinal Fluid Resolution in the Control Group (20 Eyes) |
Results
After excluding 13 eyes from the study because of incomplete imaging, a total of 41 eyes from 41 patients had been categorized into 21 and 20 eyes in the treatment and control groups, respectively. Baseline clinical settings and 6-month results are presented in detail in Tables 1 and 2, respectively.
For baseline characteristics, ketoconazole-treated patients had significantly longer symptom duration than the controls (19.8 ± 3.9 and 8.5 ± 2.7 weeks; p = 0.02), baseline 1-mm CST was comparable between the two groups (440 ± 167 and 432 ± 143 µm in the treatment and control groups, respectively; p = 0.90). Baseline SFCTs of CSCR eyes in the treated patients were slightly thicker than that in the control patients (515 ± 170 and 461 ± 79 µm; p = 0.22). Both groups had slight elevations 24-h UFC at 181 ± 70 and 150 ± 68 µg/day in the treatment and control groups, respectively (range = 20–150 µg/day; p = 0.21). Serum aldosterone and total testosterone levels of both groups were within the normal range. After controlling for age, gender, and symptom duration, hormonal profiles of each endogenous steroid were not different between them (Table 1). The baseline mean arterial pressure was normal in all patients (95.5 ± 12.1 mmHg) and not correlated with SFCT.
After adjusting for age and symptom duration, the scatter plots of data from all patients at baseline showed a significant association between the 24-h UFC level and SFCT. Such relationship analyzed in the fellow non-CSCR eyes presented stronger and had smaller p-values than that in the CSCR eyes (Figure 1). Similar associations were not detected in models using serum aldosterone or testosterone. The formulas are displayed as follows:24 - h UFC = 118 - 0.66 x age + 0.18 x SFCT (CSCR eyes); p = 0.0424 - h UFC = 69 - 0.67 x age + 0.28 x SFCT (fellow eyes); p = 0.011
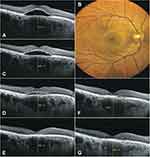
The proposed formulas may not be similar to those acquired from different techniques due to varied reference intervals. For the primary outcome, Kaplan–Meier estimates displayed in Figure 2 showed that ketoconazole significantly accelerated the CSCR resolution with the median time to complete SRF absorption of 7 vs 16 weeks in the treatment and control groups, respectively (p = 0.01; Log rank test). Figure S2 demonstrates an example patient with CSCR who is responsive to ketoconazole.
After controlling for age, symptom duration, and baseline CST, ketoconazole decreased the proportion of eyes requiring rescue therapy at 12 weeks from 50% to 23.8% in the treatment and control groups, respectively (p = 0.01) and also resulted in a more improved Early Treatment Diabetic Retinopathy Study letter at 6 months (11.5 ± 8.9 and 5.8 ± 9.3) letters in the treatment and control groups, respectively (p = 0.01). Furthermore, after controlling for age and mean arterial pressure, SFCT reduction in the ketoconazole-treated eyes was greater than that in the controls at 3 months (9.3 ± 10.2 vs 4 ± 13.8 µm; p = 0.20). However, differences observed in the overall SFCT reduction between the two groups did not reach a significant level throughout the 6-month follow-up (9.9 ± 24.4 and 9.5 ± 26.4 µm; p = 0.96) (Table 2).
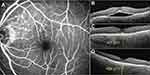
Regarding the STOP-BANG questionnaire, abnormal sleep behavior had been detected in 26 (63.4%) patients. Sleep laboratory tests revealed 7 (17%) patients with moderate-to-severe OSA (respiratory disturbance index > 15/hour), all requiring positive airway pressure therapy. Of all 32 patients with body mass index of ≥25 kg/m2, any OSA stages were detected in 12 patients (37.5%). Figure 3 shows a patient with chronic CSCR who was later diagnosed with severe OSA.
In the control group, Cox regression analysis revealed that patients’ advanced age and thick choroid in the fellow non-CSCR eyes predisposed persistent CSCR (Table 3). Despite the absence of appropriate data to validate the c-statistic and cut-off values, the 75 percentiles of data, choroidal thickness of ≥450 µm, and age of ≥45 may be the reasonable cut-off points. Figure 4 shows a patient with high-risk features for persistent CSCR.
In this study, four (19%) patients reported minor side effects, including stomach problems or skin rashes. In all reported patients, symptoms disappeared within 1 week after treatment discontinuation. Liver enzymes were elevated in one (4.7%) patient at 3-week post-treatment. After the immediate termination of the ketoconazole treatment, his liver enzymes returned to normal. These five patients were included in all analyses.
Discussions
In our study, ketoconazole significantly reduced the duration of CSCR course and likelihoods of persistent SRF. Correspondingly, the SFCT reduction was greater in the treatment arm during the first 3 months (Table 2), which may have reflected a temporary decrease in choroidal hydrostatic pressure by ketoconazole and, thus, facilitating the fluid absorption across RPE. These findings suggest that early intervention is required to minimize the chance of developing high-viscosity SRF, which will decelerate the SRF pumping process.26 It should be noted that the laser treatment at 12 weeks follow-up could potentially affect the overall duration of active CSCR in both groups. Nevertheless, most ketoconazole-treated patients (16 of 21 cases, 76.2%) achieved the resolution of SRF before the second intervention (Figure 1 and Table 2). Furthermore, the proportion of eyes requiring rescue therapy in the control group was significantly larger than that in the treatment group (50% vs 23.8%). Hence, the confounding effects resulting from laser photocoagulation or PDT should favor the overall time to disease resolution in the control group, rather than the ketoconazole-treated group.
To discuss visual results, ketoconazole-treated patients who initially had poorer BCVA than the control group showed more letter gain and achieved comparable final BCVA at approximately 20/32 for 6 months (Table 2). It is to emphasize that such difference in baseline visual acuity that was not statistically significant (mean Snellen equivalence of 20/50 vs 20/40 in the ketoconazole-treated group and control group, respectively) could have become significant with a sufficient sample size. Therefore, the greater number of BCVA ETDRS letters gained in the treatment group may not be solely due to the medication effect, but instead could stem from the consequences of a larger margin for BCVA improvement in those eyes.
Regarding the baseline characteristics, the longer symptom duration in the treatment group than in the control group may dispute this study’s results based on its primary outcome. However, instead of a naturally spontaneous SRF resolution, the equivalences in both baseline macular thickness and SFCT should support the effects of medication in shortening the disease course. Additionally, a difference in time to submacular fluid resolution remained significant after including age, baseline CST, and symptom duration into the covariate adjustment. Though not significant, the ketoconazole group had a greater proportion of patients with chronic CSCR, diffuse patterns of fluorescein leakage, and eyes with slightly worse presenting BCVA than the control group (Table 1). These three factors are likely to be associated with persistent symptoms and could have affected the patients’ decision on whether to initiate oral medications. Some previous studies reported inconsistency in the key hormone responsible for the CSCR development.1,12,27 Karahan et al reported no significant correlation between choroidal thickness and serum cortisol level (p = 0.14). Nevertheless, assessment of the serum cortisol at a single time point has many limitations for CSCR interpretation.21 Their study also conducted methodological challenges by enrolling only healthy participants, making the data less distributed.27
By demonstrating a significant association between elevated UFC level and choroidal thickness, this study supports the hypothesis that increased plasma cortisol levels predispose CSCR; these associations were not observed in the serum aldosterone and total testosterone levels. The magnitude of correlation between cortisol level and SFCT was even stronger in the fellow non-CSCR eyes (Figure 1B). We speculate that the decompressed choroid in CSCR eyes makes its thickness less predictable. Subsequently, we selected these fellow eyes to further analyze the potential predictors of persistent CSCR (Table 3). Thus, the significant associations between 24-h UFC and SFCT support the principle behind our study that application of a pan-glucocorticoid inhibitor should alleviate choroidal congestion during the acute CSCR stage.
When measuring free cortisol levels, despite the reliability of the 24-h urine specimen, its downsides include incomplete urinary collection and the requirement for cold storage. In particular, given a rare association between CSCR and persistent hypercortisolism, routine measurement of UFC in patients with CSCR would not be practical or beneficial. Alternatively, a direct assessment of bioavailable cortisol reflecting the hormonal activity at the target tissue (choroidal) level may provide ancillary benefits in monitoring hormonal activity with chorioretinal findings. Unfortunately, this test is not yet clinically available.21,28
Only two studies have evaluated the effects of ketoconazole on CSCR. First, a controlled study performed by Golshahi et al showed no difference in visual outcomes after treating patients with acute CSCR treated with 200 mg/day of ketoconazole for 4 weeks.29 The indifference in visual outcome may be due to inadequate dosage, short follow-up intervals, and favorable natural course of acute CSCR. Regarding the protocol differences between this study and ours, higher doses were administered with longer treatment durations. Another small case series conducted by Meyerle et al reported anatomic improvement in five patients with chronic CSCR at the 8-week follow-up after treating patients with 600 mg/day ketoconazole for 4 weeks.30
Our study’s predictors for persistent CSCR correspond well with those from previous ones.31 Daruich et al concluded that SFCT of >500 µm (p = 0.01) and patients’ age of >40 years (p = 0.01) were significantly associated with longer CSCR episodes.32 These cut-off values are close to the 75th percentiles of our study’s predictive values: choroidal thickness of >450 µm and age of >45 years. Inconsistent with some reports, either baseline BCVA or CST was not associated with the disease episode length.33,34
Nonetheless, pharmacological therapy for CSCR is also associated with infrequent but serious adverse effects including impotence and transaminitis. One (4.7%) patient in the treatment group experienced transient elevation of liver enzymes at 3 weeks post-treatment. Compared with all patients receiving ketoconazole for Cushing’s disease, those developing hepatic failure have reportedly received higher doses and longer duration than our protocol.35,36 Given that most CSCR has a favorable natural history, balancing side effects and therapeutic advantages of the medication remain crucial to individualize treatments. In the present study, ketoconazole appeared to accelerate the resolution of SRF but did not produce superior final visual acuity compared to the conventional approach at 6 months.
Therefore, for the practical aspects, such oral medications may be administered to only patients highly at risk for persistent subretinal fluid, those in relatively advanced age group with thick choroid in the fellow eyes (>450 µm); this unique set of patients with CSCR likely possess high magnitude of choroidal hydrostatic pressure and relatively decreased RPE function. However, additional therapeutic advantages on visual functions and long-term recurrences should be further investigated to justify the usage of medication.
Apart from the pharmacological treatment, CSCR risk factors should be modified on the individual level to prevent disease recurrences. Our study found that correspondingly 26.8%, 63.4%, and 17% of all patients had a history of steroid use, abnormal sleep behavior, and OSA requiring airway pressure therapy. These results are supported by a previous meta-analysis and large cohort study pointing that poor sleep quality (hazards ratio = 1.08) and sleep apnea (odds ratio = 1.56) significantly increase the CSCR risk in the general population.7,37,38 These risk factors also are known to primarily affect the central nervous system that results in the release of glucocorticoids.39,40 We recommend that such features should be considered, and plans on how we can eliminate them should be established. For instance, referring specialists, such as sleep specialist and respiratory clinics, could help patients minimize the use of inhaled steroids or provide a definite treatment for sleep apnea.
Our study limitations include the small sample size and several methodological shortcomings inherent to retrospective design: a lack of placebo controls, potential selection bias, variation in treatment regimens, differences in symptom duration between the two groups, and a lack of treatment randomization. In addition, effects of involving the pigment epithelial detachment were not evaluated in the Cox regression model, making the study results less generalizable, in particular for a multifactorial disease like CSCR that can clinically manifest in various ways. Lastly, this study may have missed certain effects of disease duration precisely on the likelihood of spontaneous resolution because of heterogeneity in the study population, where both acute and chronic CSCR were mixed in the same group. Well-designed randomized controlled clinical trials are required to warrant the benefits of ketoconazole by shortening the disease duration as compared to the natural recovery. Furthermore, a larger number of patients exposed to the medication is needed to detect unexpected and severe adverse events.
Conclusion
This study addressed the association between elevated glucocorticoid levels and thickening of the macular choroid in patients with CSCR. Prolonged CSCR episodes were more likely to occur in elderly persons with thickened choroids in fellow eyes. Properly powered, randomized controlled trials are required to confirm whether proactive interventions using cortisol blockers can reinforce faster subretinal fluid resolution.
Abbreviations
CSCR, central serous chorioretinopathy; MR, mineralocorticoid receptor; SRF, subretinal fluid; UFC, urinary free cortisol; OCT, optical coherence tomography; OSA, obstructive sleep apnea; SFCT, subfoveal choroidal thickness; RPE, retinal pigment epithelium; CST, central subfield thickness; BCVA, best-corrected visual acuity.
Data Sharing Statement
Data supporting the results reported in the article are not public but can be accessed after communicating with the corresponding author.
Ethics Approval and Informed Consent
The current study followed the tenets of the Declaration of Helsinki, and all patients provided informed consent after an explanation of the study protocol. The Institutional Review Board at Vajira Hospital (030/2020) approved this retrospective study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The funding for open-access publication charge is supported by Navamindradhiraj University.
Disclosure
The authors report no conflicts of interest in relation to this work. No financial disclosures or conflicting relationship exists for any authors. Oral ketoconazole is not yet approved for the treatment of CSCR.
References
1. Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81(11):962–964. doi:10.1136/bjo.81.11.962
2. van Haalen FM, van Dijk EHC, Dekkers OM, et al. Cushing’s syndrome and hypothalamic-pituitary-adrenal axis hyperactivity in chronic central serous chorioretinopathy. Front Endocrinol (Lausanne). 2018;9:39. doi:10.3389/fendo.2018.00039
3. Eymard P, Gerardy M, Bouys L, et al. Choroidal imaging in patients with Cushing syndrome. Acta Ophthalmol. 2021;99(5):533–537. doi:10.1111/aos.14664
4. Wang E, Chen S, Yang H, Yang J, Li Y, Chen Y. Choroidal thickening and pachychoroid in Cushing syndrome: correlation with endogenous cortisol level. Retina. 2019;39(2):408–414. doi:10.1097/IAE.0000000000001956
5. Karaca C, Karaca Z, Kahraman N, Sirakaya E, Oner A, Mirza GE. Is there a role of ACTH in increased choroidal thickness in Cushing syndrome? Retina. 2017;37(3):536–543. doi:10.1097/IAE.0000000000001198
6. Abalem MF, Machado MC, Santos HN, et al. Choroidal and retinal abnormalities by optical coherence tomography in endogenous Cushing’s syndrome. Front Endocrinol (Lausanne). 2016;7:154. doi:10.3389/fendo.2016.00154
7. Ji Y, Li M, Zhang X, et al. Poor sleep quality is the risk factor for central serous chorioretinopathy. J Ophthalmol. 2018;2018:9450297. doi:10.1155/2018/9450297
8. Gawecki M, Jaszczuk-Maciejewska A, Jurska-Jasko A, et al. Impairment of visual acuity and retinal morphology following resolved chronic central serous chorioretinopathy. BMC Ophthalmol. 2019;19(1):160. doi:10.1186/s12886-019-1171-5
9. Baran NV, Gurlu VP, Esgin H. Long-term macular function in eyes with central serous chorioretinopathy. Clin Exp Ophthalmol. 2005;33(4):369–372. doi:10.1111/j.1442-9071.2005.01027.x
10. Petkovsek DS, Cherfan DG, Conti FF, et al. Eplerenone for the treatment of chronic central serous chorioretinopathy: 3-year clinical experience. Br J Ophthalmol. 2020;104(2):182–187. doi:10.1136/bjophthalmol-2019-314047
11. Lotery A, Sivaprasad S, O’Connell A, et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):294–303. doi:10.1016/S0140-6736(19)32981-2
12. Ciloglu E, Unal F, Dogan NC. The relationship between the central serous chorioretinopathy, choroidal thickness, and serum hormone levels. Graefes Arch Clin Exp Ophthalmol. 2018;256(6):1111–1116. doi:10.1007/s00417-018-3985-x
13. Gergely R, Kovacs I, Schneider M, et al. Mineralocorticoid receptor antagonist treatment in bilateral chronic central serous chorioretinopathy: a comparative study of exudative and nonexudative fellow eyes. Retina. 2017;37(6):1084–1091. doi:10.1097/IAE.0000000000001303
14. Odermatt A, Atanasov AG. Mineralocorticoid receptors: emerging complexity and functional diversity. Steroids. 2009;74(2):163–171. doi:10.1016/j.steroids.2008.10.010
15. Shalet S, Mukherjee A. Pharmacological treatment of hypercortisolism. Curr Opin Endocrinol Diabetes Obes. 2008;15(3):234–238. doi:10.1097/MED.0b013e3282fc7025
16. Nagappa M, Liao P, Wong J, et al. Validation of the STOP-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10(12):e0143697. doi:10.1371/journal.pone.0143697
17. Unlu C, Erdogan G, Aydogan T, et al. Intravitreal bevacizumab for treatment of central serous chorioretinopathy. J Ophthalmic Vis Res. 2016;11(1):61–65. doi:10.4103/2008-322X.180700
18. Lu HQ, Wang EQ, Zhang T, Chen YX. Photodynamic therapy and anti-vascular endothelial growth factor for acute central serous chorioretinopathy: a systematic review and meta-analysis. Eye. 2016;30(1):15–22. doi:10.1038/eye.2015.208
19. Breukink MB, Dingemans AJ, den Hollander AI, et al. Chronic central serous chorioretinopathy: long-term follow-up and vision-related quality of life. Clin Ophthalmol. 2017;11:39–46. doi:10.2147/OPTH.S115685
20. van Rijssen TJ, van Dijk EHC, Scholz P, et al. Long-term follow-up of chronic central serous chorioretinopathy after successful treatment with photodynamic therapy or micropulse laser. Acta Ophthalmol. 2021;99(7):805–811. doi:10.1111/aos.14775
21. Bansal V, El Asmar N, Selman WR, Arafah BM. Pitfalls in the diagnosis and management of Cushing’s syndrome. Neurosurg Focus. 2015;38(2):E4. doi:10.3171/2014.11.FOCUS14704
22. Ozkaya A, Alkin Z, Ozveren M, et al. The time of resolution and the rate of recurrence in acute central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy: a case-control study. Eye. 2016;30(7):1005–1010. doi:10.1038/eye.2016.79
23. van Rijssen TJ, van Dijk EHC, Tsonaka R, et al. Half-dose photodynamic therapy versus eplerenone in chronic central serous chorioretinopathy (SPECTRA): a randomized controlled trial. Am J Ophthalmol. 2022;233:101–110. doi:10.1016/j.ajo.2021.06.020
24. Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol. 2014;92(8):e594–e601. doi:10.1111/aos.12482
25. Lai FH, Ng DS, Bakthavatsalam M, et al. A multicenter study on the long-term outcomes of half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2016;170:91–99. doi:10.1016/j.ajo.2016.07.026
26. Schwartz R, Habot-Wilner Z, Martinez MR, et al. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol. 2017;95(7):e610–e618. doi:10.1111/aos.13491
27. Karahan E, Zengin MO, Aydin R, et al. Correlation of choroidal thickness with serum cortisol level. Clin Exp Optom. 2015;98(4):362–365. doi:10.1111/cxo.12254
28. Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability–effects on hormone measurement and action. Nat Rev Endocrinol. 2012;8(12):717–727. doi:10.1038/nrendo.2012.134
29. Golshahi A, Klingmuller D, Holz FG, Eter N. Ketoconazole in the treatment of central serous chorioretinopathy: a pilot study. Acta Ophthalmol. 2010;88(5):576–581. doi:10.1111/j.1755-3768.2008.01467.x
30. Meyerle CB, Freund KB, Bhatnagar P, et al. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007;27(7):943–946. doi:10.1097/IAE.0b013e318050ca69
31. Romano MR, Parolini B, Allegrini D, et al. An international collaborative evaluation of central serous chorioretinopathy: different therapeutic approaches and review of literature. The European Vitreoretinal Society central serous chorioretinopathy study. Acta Ophthalmol. 2019;98:e549–58.
32. Daruich A, Matet A, Marchionno L, et al. Acute central serous chorioretinopathy: factors influencing episode duration. Retina. 2017;37(10):1905–1915. doi:10.1097/IAE.0000000000001443
33. Parajuli A, Joshi P. Factors influencing the episode duration and the anatomical and functional outcome in cases of acute central serous chorioretinopathy. BMJ Open Ophthalmol. 2020;5(1):e000540. doi:10.1136/bmjophth-2020-000540
34. Suwal B, Khadka D, Shrestha A, et al. Baseline predictive factors of visual outcome and persistence of subretinal fluid based on morphologic changes in spectral domain optical coherence tomography in patients with idiopathic central serous chorioretinopathy. Clin Ophthalmol. 2019;13:2439–2444. doi:10.2147/OPTH.S233273
35. Greenblatt HK, Greenblatt DJ. Liver injury associated with ketoconazole: review of the published evidence. J Clin Pharmacol. 2014;54(12):1321–1329. doi:10.1002/jcph.400
36. Banankhah PS, Garnick KA, Greenblatt DJ. Ketoconazole-associated liver injury in drug-drug interaction studies in healthy volunteers. J Clin Pharmacol. 2016;56(10):1196–1202. doi:10.1002/jcph.711
37. Wu CY, Riangwiwat T, Rattanawong P, et al. Association of obstructive sleep apnea with central serous chorioretinopathy and choroidal thickness: a systematic review and meta-analysis. Retina. 2018;38(9):1642–1651. doi:10.1097/IAE.0000000000002117
38. Pan CK, Vail D, Bhattacharya J, et al. The effect of obstructive sleep apnea on absolute risk of central serous chorioretinopathy. Am J Ophthalmol. 2020;218:148–155. doi:10.1016/j.ajo.2020.05.040
39. Chopra S, Rathore A, Younas H, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102(9):3172–3181. doi:10.1210/jc.2017-00619
40. Kritikou I, Basta M, Vgontzas AN, et al. Sleep apnoea and the hypothalamic-pituitary-adrenal axis in men and women: effects of continuous positive airway pressure. Eur Respir J. 2016;47(2):531–540. doi:10.1183/13993003.00319-2015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.