Back to Journals » Clinical Interventions in Aging » Volume 18
Effects of High-Intensity Resistance Training on Visceral Adipose Tissue and Abdominal Aortic Calcifications in Older Men with Osteosarcopenia – Results from the FrOST Study
Authors Knauer K , Chaudry O, Uder M, Kohl M , Kemmler W , Bickelhaupt S, Engelke K
Received 10 September 2022
Accepted for publication 6 December 2022
Published 19 January 2023 Volume 2023:18 Pages 71—80
DOI https://doi.org/10.2147/CIA.S388026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Kira Knauer,1,2 Oliver Chaudry,2,3 Michael Uder,1 Matthias Kohl,4 Wolfgang Kemmler,1,2 Sebastian Bickelhaupt,1 Klaus Engelke2,3
1Institute of Radiology, Friedrich-Alexander-Universität Erlangen-Nürnberg and University Hospital Erlangen, Erlangen, 91054, Germany; 2Institute of Medical Physics, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, 91052, Germany; 3Department of Medicine III, Friedrich-Alexander University of Erlangen-Nürnberg, University Hospital Erlangen, Erlangen, 91054, Germany; 4Faculty Medical and Life Sciences, University of Furtwangen, Villingen-Schwenningen, 78054, Germany
Correspondence: Kira Knauer, Institute of Radiology, Friedrich-Alexander University of Erlangen-Nürnberg, University Hospital Erlangen, Maximiliansplatz 3, Erlangen, 91054, Germany, Tel +49 9131/85-36065, Email [email protected]
Purpose: To evaluate the effect of a high-intensity resistance training (HIT-RT) on visceral adipose tissue (VAT) and abdominal aortic calcifications (AAC).
Patients and Methods: We conducted a post hoc analysis of the Franconian Osteopenia and Sarcopenia Trial (FrOST). 43 community-dwelling men with osteosarcopenia aged 72 years and older were randomly allocated to a supervised high-intensity resistance training (HIT-RT) twice weekly for 18 months (EG; n=21) and a non-training control group (CG; n=22). Non-contrast enhanced 2-point Dixon MRI scans covering mid L2 to mid L3 were acquired to measure VAT volume inside the abdominal cavity. Volume of AAC and hard plaques in renal arteries, truncus celiacus and superior mesenteric artery was measured by computed tomography (CT) scans covering mid T12 to mid L3. Intention-to-treat analysis with imputation for missing data was used to determine longitudinal changes in VAT and AAC volume. Correlations were used to determine associations between VAT and AAC.
Results: Significant reduction of VAT volume in the EG (− 7.7%; p< 0.001) combined with no change in the CG (− 1.3%; p=0.46) resulted in a significant 6.4% between group effect (p=0.022). We observed a significant increase of AAC volume in EG (+10.3%; p< 0.001) and CG (12.0%; p< 0.001). AAC differences between groups were not significant (p=0.57). In vascular outlets increases in volume of the hard plaques were observed in both groups, however, not all of them were significant. There was no significant correlation between changes in VAT and AAC volumes.
Conclusion: The study confirmed a positive impact of HIT-RT on the metabolic and cardiovascular risk profile with respect to reduction of VAT volume. No positive exercise effect on AAC was observed. However, there was a further progression of AAC volume independent of group affiliation. Whether different exercise regimen may show a positive effect on AAC remains subject to further studies.
Keywords: HIT-RT, VAT, AAC, osteopenia, sarcopenia
Introduction
Demographic changes and the ever increasing rate of metabolic and cardiovascular diseases are challenging individual health and healthcare systems worldwide. In general exercise is considered as an effective intervention to positively affect cardiometabolic and cardiovascular risk factors in various populations. There is a considerable body of evidence that regular exercise affects parameters such as the metabolic syndrome (MetSyn) as a cluster of cardiometabolic and cardiovascular risk factors,1 predominately based on positive changes in visceral adipose tissue (VAT).2 However, the effect of exercise on other hallmarks of metabolic and cardiovascular diseases such as aortic calcifications is less clear. The complex interaction between the different risk factors has not been fully deciphered so far, however, it has been shown that different body composition features are specifically associated with cardiovascular events. Subjects with more advanced abdominal aortic calcifications (AAC) have a higher risk for cardiovascular events, especially those with a fatal outcome.3 It has also been shown that AAC is influenced by visceral adipose tissue inducing lipotoxic effects on the atherosclerotic processes, while subcutaneous adipose tissue may play a rather protective role.4
However, apart from positive effect on metabolic and cardiovascular risk factors, exercise also improves musculoskeletal diseases such as osteoporosis and sarcopenia that often occur in parallel in older peoples.5 This might explain the attractiveness of exercise as a multi-purpose prevention tool in the elderly. However in detail, exercise effects on various outcomes will differ dependent on the type of exercise and the strain composition applied. This might particularly refer to older physically limited cohorts. The FrOST-study, an 18 months high-intensity resistance training (HIT-RT) protocol reported positive effects on musculoskeletal6 and cardiometabolic risk7 in a population of older, community dwelling men with osteosarcopenia. Of importance, a high proportion of this cohort suffered from overweight/obesity, MetSyn and related parameters causative for cardiometabolic and cardiovascular risk. Considering further the high relevance of VAT and AAC for the latter aspect, led us to expand our research on the effects of HIT-RT on both risk factors in this older cohort at increased cardiometabolic and cardiovascular risk.
The hypotheses for this post hoc analysis were (1) that HIT-RT positively affect VAT and (2) the volume of AAC and hard plaques in the renal arteries, truncus celiacus and superior mesenteric artery in comparison with a non-exercising control group.
Materials and Methods
Study Design
FrOST is a randomized controlled longitudinal exercise study with 18 months of training. The project was initiated and performed by the Institute of Medical Physics (IMP), Friedrich-Alexander-University of Erlangen-Nürnberg (FAU), Germany. The project was registered under ClinicalTrials.gov: NCT03453463. Comprehensive details on study design, participants, exercise regimen, assessments and results have been published previously.6,8,9 Here we will only repeat information necessary to understand the specific post hoc analysis reported here.
Participants
In brief, one hundred and eighty community dwelling men 72 years and older were eligible and willing to participate in FrOST. Inclusion criteria were: a) morphometric sarcopenia (skeletal muscle mass index (SMI) ≤ 7.26 kg/m2,10,11 and b) osteopenia or osteoporosis according to WHO.12 Exclusion criteria were: a) secondary osteoporosis, b) a history of hip fracture, c) (osteo)anabolic and antiresorptive pharmaceutical therapy, c) glucocorticoid therapy > 7.5 mg/d for more than four weeks during the last 2 years, d) participation in resistance type exercise (>60 min/week) during the last 2 years, e) cognitive impairments, f) limitations or problems that inhibit intense exercise and g) alcohol abuse of more than 60 g/d ethanol. 43 men were eligible and randomly assigned either to an exercise (EG, n=21) or a control (CG, n=22) group (Figure 1). Apart from study intervention described below all participants were encouraged to maintain their normal physical activity and exercise habits beside the study. Furthermore, they were asked not to change their dietary routines.
Resistance Exercise Training
The exercise group underwent a progressive, single-set HIT-RT at a local, well-equipped gym (Kieser-Training, Erlangen, Germany) twice weekly consistently supervised by licensed instructors. During the training period, 5 consecutive phases with a duration of 8–12 weeks were carried out in linear periodization. According to the approach of Steele et al,13 our training concept was based on a non-repetition maximum (nRM) and a repetition maximum (RM) as endpoints of the sets. The intensity of the exercises was determined by a range of repetitions per set (ie 8–10 or 5–7), aiming at different relative intensities between 65 and 80% 1RM. The first twelve weeks were characterized by a phase of briefing and initial familiarization eg learning of proper lifting techniques followed by conditioning of the practiced exercises. Subsequently in Phase 2, 14–15 out of 18 exercises (back extension, bench press, butterfly with extended arms, calf raises, crunches, hip extension, inverse fly, lateral crunches, lateral raises, latissimus front pulleys, leg adduction and abduction, leg curls, leg extension, leg press, pullovers, rowing, shoulder press) were performed on resistance-devices (MedX, Ocala, Florida, USA). Phase 3 was extended by an explosive movement in the concentric phase and the initiation of the repetition maximum approach.13 In the further course, the superset approach (phase 4) and drop sets (phase 5) were introduced in order to intensify the training protocol. For more details on the training procedure, please refer to Kemmler et al.14
Dietary Supplementation
According to recent recommendations15 individual supplementation of whey protein (Active PRO80, inkospor, Roth, Germany) was provided to all participants to generate a total protein intake of 1.5–1.6 in the EG and 1.2–1.3 g/kg body mass per day in the CG.
To ensure a daily calcium uptake of ≈1000 mg16 each participant was provided with calcium capsules (Sankt Bernhard, Bad Dietzenbach, Germany) based on a dietary calcium questionnaires (Rheumaliga, Switzerland) if calcium intake fell below 950 mg per/day.
On the basis of measured and analyzed 25-Hydroxy-Vitamin-D 3 (25-OH D3) concentrations of blood samples, participants with levels <30 ng/mL were provided with 10,000 IE per week (MYVITAMINS, Manchester, UK). Participants with 30–40 ng/mL were requested to ingest 5000 IE/week.
MRI to Assess VAT
MRI scans were acquired at baseline and after 16 months of HIT-RT. All scans were performed on a 3T scanner (MAGNETOM Skyrafit, Siemens Healthineers AG, Erlangen, Germany) using a non-contrast enhanced 2-point Dixon Gradient Echo Volumetric Interpolated Breath-hold Examination (VIBE) sequence (TE: 1.29 ms; TR: 3.97 ms; matrix: 320x260; resolution: 1.2×1.2 mm2; slice thickness: 3.5 mm; slice gap: 0.7 mm). Twelve slices covered a total length of approximately 5 cm from mid L2 to mid L3.
Image analysis was performed by a single reader (KK, medical student) under the supervision of a board certified radiologist (SB, >10 years of experience) using MIAF (Medical Image Analysis Framework, University of Erlangen) as described before.17 The body surface was determined automatically. The contour of the abdominal cavity was manually segmented slice by slice using open source software 3D Slicer.18 Finally, VAT inside the abdominal cavity was automatically separated from inner organs such as kidney or intestines, and blood vessels using a threshold automatically calculated with the Otsu-Method19 form the intensity histogram (Figure 2). Volume of VAT was measured in cm³.
CT to Assess AAC
CT scans were acquired at baseline and after 12 months. All scans were performed on a Somatom Force (Siemens, Erlangen, Germany) using 120 kV, CAREDose 3D with an effective 100 mAs; 1 mm slice thickness, BR40s kernel and a field of view of 20 cm. The scans covered mid T12 to mid L3.
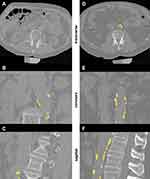
Calcifications were assessed by the same reader (KK) under the supervision of the same radiologist (SB). Segmentation of the abdominal aorta, the renal arteries, truncus celiacus and superior mesenteric artery was performed with 3D Slicer using an individually adapted threshold ranging from 145 to 160 Hounsfield Units (HU). The respective threshold in a patient was selected based on an Osteo phantom included in the images, which is typically used for calibration of bone mineral density. Identical thresholds were used for the BL and FU images of a given participant. For this purpose, BL and FU images of a given participant were segmented side by side. First the calcifications were cut out manually using the scissor function of 3D Slicer and then remaining artefacts were removed slice by slice using the eraser function. Subsequently, the segmented volume of the plaques was measured in the respective section in mm³ (Figure 3).
Statistical Analysis
Pearson correlations were used to describe associations between VAT and AAC volume as well as between both parameters and obesity and MetSyn. An intention-to-treat (ITT) with imputation for missing data analysis was performed. R statistics software (R Development Core Team Vienna, Austria) in combination with Amelia II20 was used to apply repeatedly imputations. For ITT, the complete data set was utilized and imputed 100 times, which worked well in all cases. A normal distribution was tested using qq-plots. An ANCOVA adjusted for baseline values was performed to detect differences between the changes in the training and the control group. Results of the ITT analysis were compared with those of a per-protocol (PP) analysis, significances were similar. Therefore, the PP analyses are omitted in the following. All applied tests were 2-tailed with an accepted significance of p < 0.05.
Results
Baseline Characteristics
Table 1 shows participant characteristics at BL. Apart from body height and protein intake parameters were not significantly different in the two groups. Per inclusion all participants had osteosarcopenia. Accepting that a body fat rate of 27%–30% constitutes obesity in men21 most participants (75%–94%) can be considered as (osteosarcopenic) obese and one third of the subjects of the CG and EG respectively were affected by the MetSyn.22 At baseline VAT volume of the combined group was significantly correlated with BMI (r = 0.66) and MetSyn Z-Score7 (r = 0.70). In contrast there were no significant correlations between AAC volume of the combined group with BMI (r = 0.1) and MetSyn Z-Score (r = 0.05). VAT volume was also not significantly correlated with AAC volume (r = 0.18).
 |
Table 1 Baseline Characteristics of Control (CG) and Exercise Group (EG) |
Participants attended the HIT-RT sessions at a high rate of 95% ± 4% indicating the high attractiveness and feasibility of the training protocol. No serious adverse effects or injuries were observed during the study period.
Study Endpoints
Table 2 shows VAT results. After 16 months of training, VAT volume significantly decreased (−7.7%; p<0.001) in the EG, while in the CG VAT volume did not considerably change (−1.3%; p=0.46). Absolute changes in VAT volume were significantly higher in the EG compared with the CG (p=0.022).
 |
Table 2 Absolute and Relative Changes of Visceral Adipose Tissue (VAT) in Control (CG) and Exercise Group (EG) After 16 Months of High Intensity Resistance Training |
Table 3 shows AAC results. We observed a significant increase in AAC volume in the EG (+10.3%; p<0.001) as well as in the CG (+12.0%; p<0.001). The between group difference was not significant (p=0.57). Between-group differences were also not significant for the vascular branches of the abdominal aorta (Right renal artery p=0.52; Left renal artery p=0.60; Truncus celiacus p=1.00; Superior mesenteric artery p=0.64). The individual vascular outlets showed both significant and non-significant increases in hard plaques.
There was no significant correlation between changes in VAT volume and changes in AAC volume.
Discussion
The current post hoc analysis of the effect of HIT-RT on metabolic and cardiovascular risk factors in FrOST confirmed the hypothesis of positive training effects on VAT in elderly men with osteosarcopenia. The second hypothesis was rejected – positive effects on AAC were not observed in the observation period. Numerous metabolic and cardiovascular risk factors exist that contribute to a negative impact on health. For the FrOST study, we had previously investigated parameters of adiposity and the effect of HIT-RT on body composition and showed a positive training effect on total body fat mass and abdominal body fat percentage.7,23
It is known that an enhanced VAT volume contributes to the development of obesity and that it is a more sensitive marker to detect changes in adiposity than total body weight or waist circumference.24 Here we showed that at baseline VAT volume was moderately correlated with the MetSyn and obesity and that the positive effect of the HIT-RT on body composition reported earlier also applied to VAT. This was confirmed by a significant decrease in VAT in the EG (p<0.001) and a significant between-group difference (p=0.022).
Our results are consistent with other studies. Arciero and colleagues25 reported a significant reduction of about −8% in VAT in an exercise group and a non-significant decrease in the CG group. The participants were middle-aged and of mixed gender and were also provided with whey protein, but in a smaller quantity than ours. Only 16 weeks of the intervention took place, but 4 times a week instead of 2 times a week as in our study. Meta-analyses showed that aerobic endurance training with moderate or high intensity had a greater effect on VAT than resistance training.26,27 However, there is a great heterogeneity among strength training studies in terms of training protocols, cohort characteristics and study designs.26 Furthermore, Vissers et al included fewer strength training than aerobic exercise studies in their meta-analysis.26
A more recent CT study of subjects of mixed gender and an average age in the early sixties by Yan et al28 showed that both strength and aerobic exercise led to reductions in VAT volume over a 12-month period with no statistical difference between the different training groups. The change in VAT volume within one year was consistent with our results.
In contrast using single slice CT at the L3 level, Turcotte and colleagues29 did not observe significant changes in VAT (−1.9%) during 18 months of progressive resistance training. Their sample size of male-only participants, ranging in age from 50–79 years was larger than ours but the training protocols were similar. Potentially the larger age range, particularly including younger subjects and a lower total body fat mass at baseline, could explain the different outcome compared to the studies discussed above.
An important point regarding VAT is precision of longitudinal assessments due to motion of internal organs, partly caused by respiration. In contrast to other studies29 that only used a single slice we employed a volumetric analysis, which reduces the movement effect of the internal organs. We used a total scan length of 5 cm between vertebrae L2 and L3 as suggested by others.30–32 Meta-analyses showed that many previous exercise studies only used a single-slice measurement of VAT,27 which simplified imaging but increased precision errors.
Studies on the effect of physical activity on vascular calcification (eg in coronary arteries) are inconclusive.33
While results on VAT are in line with other studies, the investigation of exercise on AAC is novel. While our study showed changes of AAC volume over time our study data did neither demonstrate a significant effect of training on AAC nor on hard plaque calcifications in the small arterial outlets of the abdominal aorta. Over the observation period, both groups demonstrated a similar progression without significant differences, however, with considerable inter individual differences. It is also interesting that in contrast to earlier studies4,34 in the FrOST cohort AAC volume was not correlated with MetSyn or with obesity and there was no correlation between AAC and VAT volume although AAC volume was similar to volumes of 1950 ± 2300 mm³ reported in by Fuji et al35 in subjects with comparable age (but a different gender distribution).
Limited literature is available for longitudinal volumetric assessments of AAC. Particularly with higher age, there is an increase in the prevalence and the severity of plaques.36–38 In our study, the average age was 77.8 ± 3.6 years in EG and 79.2 ± 4.7 years in CG. It has been further suggested, that the age related increase of AAC in men is more pronounced than in women.37,39 Graffy et al40 reported an increase in the age-specific Agatston score of 10% per year in an observational study without training. This is comparable to the change of 12.0% in the CG of our study. However, Graffy’s study was a mixed female-male cohort with an average age younger than ours. Another study described a progression of plaques of around 8% in one year measured by MRI, slightly lower than our observation.41 A lack of positive effects on AAC progression using training intervention, however, with a different training protocol, has previously also been described by Zhou et al38 demonstrating a significant progression despite training, as in our results. Whilst Zhou et al did only use radiography and no control group our study included CT and a control group allowing for a more detailed analysis. One might argue, that the calcium substitution may have influenced our results since it has been reported to influence calcified vascular plaques42 but the majority of studies do not describe a significantly increased risk of cardiovascular outcomes due to calcium substitution43 and substitution was only at nutritional recommendation levels.
Study Strengths and Limitations
Strengths of the study are the randomized study design, the long duration of the HIT-RT exercise program and the quantitative assessment of VAT and AAC volume using advanced 3D imaging techniques. The main limitations of the study are the relatively small number of participants and the post hoc approach of the project. Furthermore, some study and participant characteristics (e.g, high age, osteosarcopenic status, male gender, protein supplementation, specific exercise protocol) might prevent a proper generalization of our results to other (older) cohorts and/or conditions. No evaluation of reproducibility of the segmentations was conducted. Imaging was performed in a limited body area, thus we cannot draw conclusions eg with regards to the influence of other vessels or fat tissue locations in the body. No contrast agents were given limiting the assessment of soft plaques within the AAC and the outlets, which apparently differs in contrast and non-contrast enhanced CT scans.44
Conclusion
HIT-RT is an attractive, time effective and feasible training modality for elderly men with osteosarcopenia in terms of VAT reduction. However, the progression of AAC and its vascular branches could not be prevented in the FrOST study. Therefore, in combination with the results published earlier7,23 we conclude that HIT-RT has a positive effect on the metabolic and cardiovascular risk profile but at least in the FrOST study not on AAC. Perhaps a variation of the exercise protocol with further emphasis on endurance may be more effective for targeting AAC resulting in further optimization of an integrated and cost-effective exercise approach for the elderly.
Abbreviations
HIT-RT, high-intensity resistance training; VAT, visceral adipose tissue; AAC, abdominal aortic calcifications; EG, exercise group; CG, control group; MRI, magnetic resonance imaging; CT, computed tomography; FrOST, Franconian osteopenia and sarcopenia trial; DXA, dual x-ray absorptiometry; BMD, bone mineral density; SMI, skeletal muscle mass index; MetSyn, metabolic syndrome; IMP, Institute of Medical Physics; FAU, Friedrich-Alexander-University of Erlangen-Nürnberg; WHO, world health organization; nRM, non-repetition maximum; RM, repetition maximum; TE, time to echo; TR, repetition time; ITT, intention-to-treat; PP, per-protocol; BL, baseline; FU, follow-up; SD, standard deviation.
Data Sharing Statement
Data sharing has not been approved by the study participants.
Ethics Approval and Informed Consent
The FrOST study was approved by the University of Erlangen Ethics Committee (number 67_15b and 4464b) and the Federal Bureau of Radiation Protection (BfS, number Z 5 – 2246212-2017-002). All study participants gave their written informed consent. The trial fully complies with the Helsinki Declaration.45
Consent for Publication
The current contribution does not include any details that would allow conclusions to be made about the identity of the participants.
Acknowledgments
The present contribution was performed in fulfilment of the requirements for obtaining the degree “Dr. med.” for the first author Kira Knauer. The authors also acknowledge the strong support of Kieser Training (Erlangen, Germany) that provided the training facilities.
Funding
The FrOST study was not funded by any commercial, non-profit or public organizations.
Disclosure
Dr Sebastian Bickelhaupt reports personal fees, non-financial support from Siemens Healthineers, outside the submitted work; In addition, Dr Sebastian Bickelhaupt has a pending patent in the field of MRI. The other authors report no conflicts of interest in this work.
References
1. Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi:10.1016/S0140-6736(05)67402-8
2. Dutheil F, Lac G, Lesourd B, et al. Different modalities of exercise to reduce visceral fat mass and cardiovascular risk in metabolic syndrome: the RESOLVE randomized trial. Int J Cardiol. 2013;168(4):3634–3642. doi:10.1016/j.ijcard.2013.05.012
3. Leow K, Szulc P, Schousboe JT, et al. Prognostic Value of Abdominal Aortic Calcification: a Systematic Review and Meta-Analysis of Observational Studies. J Am Heart Assoc. 2021;10(2):e017205. doi:10.1161/JAHA.120.017205
4. Goldenberg L, Saliba W, Hayeq H, Hasadia R, Zeina A-R. The impact of abdominal fat on abdominal aorta calcification measured on non-enhanced CT. Medicine. 2018;97(49):e13233. doi:10.1097/MD.0000000000013233
5. Nielsen BR, Abdulla J, Andersen HE, Schwarz P, Suetta C. Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med. 2018;9(4):419–434. doi:10.1007/s41999-018-0079-6
6. Kemmler W, Kohl M, Fröhlich M, et al. Effects of High-Intensity Resistance Training on Osteopenia and Sarcopenia Parameters in Older Men with Osteosarcopenia-One-Year Results of the Randomized Controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). J Bone Miner Res. 2020;35(9):1634–1644. doi:10.1002/jbmr.4027
7. Kemmler W, Kohl M. Effect of high-intensity resistance exercise on cardiometabolic health in older men with osteosarcopenia: the randomised controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). BMJ Open Sport Exerc Med. 2020;6(1):e000846. doi:10.1136/bmjsem-2020-000846
8. Lichtenberg T, von Stengel S, Sieber C, Kemmler W. The Favorable Effects of a High-Intensity Resistance Training on Sarcopenia in Older Community-Dwelling Men with Osteosarcopenia: the Randomized Controlled FrOST Study. Clin Interv Aging. 2019;14:2173–2186. doi:10.2147/CIA.S225618
9. Kemmler W, Weineck M, Kohl M, et al. High Intensity Resistance Exercise Training to Improve Body Composition and Strength in Older Men With Osteosarcopenia. Results of the Randomized Controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). Front Sports Act Living. 2020;2:4. doi:10.3389/fspor.2020.00004
10. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi:10.1093/oxfordjournals.aje.a009520
11. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
12. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129.
13. Steele J, Fisher J, Giessing J, Gentil P. Clarity in reporting terminology and definitions of set endpoints in resistance training. Muscle Nerve. 2017;56(3):368–374. doi:10.1002/mus.25557
14. Kemmler W, Kohl M, Jakob F, Engelke K, von Stengel S. Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study. Nutrients. 2020;12(8):548. doi:10.3390/nu12082341
15. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–559. doi:10.1016/j.jamda.2013.05.021
16. DVO. Prophylaxe, Diagnostik und Therapie der Osteoporose bei postmenopausalen Frauen und bei Männern. Schattauer. 2017;1:548.
17. Chaudry O, Grimm A, Friedberger A, et al. Magnetic Resonance Imaging and Bioelectrical Impedance Analysis to Assess Visceral and Abdominal Adipose Tissue. Obesity. 2020;28(2):277–283. doi:10.1002/oby.22712
18. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. doi:10.1016/j.mri.2012.05.001
19. Otsu N, Threshold Selection A. Method from Gray-Level Histograms. IEEE Trans Syst Man Cybern. 1979;9(1):62–66. doi:10.1109/tsmc.1979.4310076
20. Honaker J, King G, Blackwell M. Amelia II: a Program for Missing Data. J Stat Soft. 2011;45(7):1–47. doi:10.18637/jss.v045.i07
21. Donini LM, Busetto L, Bauer JM, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–2388. doi:10.1016/j.clnu.2019.11.024
22. Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi:10.1111/j.1464-5491.2006.01858.x
23. Kemmler W, Kohl M, Fröhlich M, Engelke K, von Stengel S, Schoene D. Effects of High-Intensity Resistance Training on Fitness and Fatness in Older Men With Osteosarcopenia. Front Physiol. 2020;11:1014. doi:10.3389/fphys.2020.01014
24. Verheggen RJHM, Maessen MFH, Green DJ, Hermus ARMM, Hopman MTE, Thijssen DHT. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17(8):664–690. doi:10.1111/obr.12406
25. Arciero PJ, Baur D, Connelly S, Ormsbee MJ. Timed-daily ingestion of whey protein and exercise training reduces visceral adipose tissue mass and improves insulin resistance: the PRISE study. J Appl Physiol. 2014;117(1):1–10. doi:10.1152/japplphysiol.00152.2014
26. Vissers D, Hens W, Taeymans J, Baeyens J-P, Poortmans J, van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8(2):e56415. doi:10.1371/journal.pone.0056415
27. Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13(1):68–91. doi:10.1111/j.1467-789X.2011.00931.x
28. Yan J, Dai X, Feng J, et al. Effect of 12-Month Resistance Training on Changes in Abdominal Adipose Tissue and Metabolic Variables in Patients with Prediabetes: a Randomized Controlled Trial. J Diabetes Res. 2019;2019:8469739. doi:10.1155/2019/8469739
29. Turcotte A-F, Kukuljan S, Dalla Via J, Gagnon C, Abbott G, Daly RM. Changes in spinal bone density, back muscle size, and visceral adipose tissue and their interaction following a multi-component exercise program in older men: secondary analysis of an 18-month randomized controlled trial. Osteoporos Int. 2020;31(10):2025–2035. doi:10.1007/s00198-020-05484-z
30. Demerath EW, Shen W, Lee M, et al. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr. 2007;85(2):362–368. doi:10.1093/ajcn/85.2.362
31. Linder N, Schaudinn A, Garnov N, et al. Age and gender specific estimation of visceral adipose tissue amounts from radiological images in morbidly obese patients. Sci Rep. 2016;6:22261. doi:10.1038/srep22261
32. Schweitzer L, Geisler C, Pourhassan M, et al. Estimation of Skeletal Muscle Mass and Visceral Adipose Tissue Volume by a Single Magnetic Resonance Imaging Slice in Healthy Elderly Adults. J Nutr. 2016;146(10):2143–2148. doi:10.3945/jn.116.236844
33. Aengevaeren VL, Mosterd A, Sharma S, et al. Exercise and Coronary Atherosclerosis: observations, Explanations, Relevance, and Clinical Management. Circulation. 2020;141(16):1338–1350. doi:10.1161/CIRCULATIONAHA.119.044467
34. Qin Z, Jiang L, Sun J, et al. Higher visceral adiposity index is associated with increased likelihood of abdominal aortic calcification. Clinics. 2022;77:100114. doi:10.1016/j.clinsp.2022.100114
35. Fujii H, Kono K, Watanabe K, et al. Evaluation of aortic calcification using a three-dimensional volume-rendering method in patients with end-stage kidney disease. J Bone Miner Metab. 2020. doi:10.1007/s00774-020-01172-4
36. Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68(5):271–276. doi:10.1007/BF02390833
37. Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–336. doi:10.1161/01.ATV.0000110786.02097.0c
38. Zhou Y, Hellberg M, Hellmark T, Höglund P, Clyne N. Twelve months of exercise training did not halt abdominal aortic calcification in patients with CKD - a sub-study of RENEXC-a randomized controlled trial. BMC Nephrol. 2020;21(1):233. doi:10.1186/s12882-020-01881-y
39. Chuang ML, Massaro JM, Levitzky YS, et al. Prevalence and distribution of abdominal aortic calcium by gender and age group in a community-based cohort (from the Framingham Heart Study). Am J Cardiol. 2012;110(6):891–896. doi:10.1016/j.amjcard.2012.05.020
40. Graffy PM, Liu J, O’Connor S, Summers RM, Pickhardt PJ. Automated segmentation and quantification of aortic calcification at abdominal CT: application of a deep learning-based algorithm to a longitudinal screening cohort. Abdom Radiol. 2019;44(8):2921–2928. doi:10.1007/s00261-019-02014-2
41. Yonemura A, Momiyama Y, Fayad ZA, et al. Effect of lipid-lowering therapy with atorvastatin on atherosclerotic aortic plaques: a 2-year follow-up by noninvasive MRI. Eur J Cardiovasc Prev Rehabil. 2009;16(2):222–228. doi:10.1097/HJR.0b013e32832948a0
42. Daly RM, Ebeling PR. Is excess calcium harmful to health? Nutrients. 2010;2(5):505–522. doi:10.3390/nu2050505
43. Spence LA, Weaver CM. Calcium intake, vascular calcification, and vascular disease. Nutr Rev. 2013;71(1):15–22. doi:10.1111/nure.12002
44. Buijs RVC, Leemans EL, Greuter M, Tielliu IFJ, Zeebregts CJ, Willems TP. Quantification of abdominal aortic calcification: inherent measurement errors in current computed tomography imaging. PLoS One. 2018;13(2):e0193419. doi:10.1371/journal.pone.0193419
45. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
46. Kemmler W, Weineck J, Kalender WA, Engelke K. The effect of habitual physical activity, non-athletic exercise, muscle strength, and VO2max on bone mineral density is rather low in early postmenopausal osteopenic women. J Musculoskelet Neuronal Interact. 2004;4(3):325–334.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.