Back to Journals » Journal of Inflammation Research » Volume 12
Effects of Exposure to Mild Hyperbaric Oxygen on DSS-Induced Colonic Inflammation and Diarrhea in Rats
Authors Takemura A, Egawa T, Tanaka T , Kuramoto T, Hayashi T , Ishihara A
Received 24 June 2019
Accepted for publication 13 September 2019
Published 25 October 2019 Volume 2019:12 Pages 293—299
DOI https://doi.org/10.2147/JIR.S220586
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ai Takemura,1 Tatsuro Egawa,2 Takuji Tanaka,3 Takashi Kuramoto,4 Tatsuya Hayashi,2 Akihiko Ishihara1
1Laboratory of Cell Biology and Life Science, Graduate School of Human and Environmental Studies, Kyoto University, Kyoto 606-8501, Japan; 2Laboratory of Sports and Exercise Medicine, Graduate School of Human and Environmental Studies, Kyoto University, Kyoto 606-8501, Japan; 3Department of Diagnostic Pathology, Gifu Municipal Hospital, Gifu 500-8513, Japan; 4Department of Animal Science, Faculty of Agriculture, Tokyo University of Agriculture, Atsugi 243-0034, Japan
Correspondence: Ai Takemura
Department of Sports Research, Japan Institute of Sport Sciences, Tokyo 115-0056, Japan
Tel +81-75-753-2996
Fax +81-75-753-6771
Email [email protected]
Purpose: In rodents, dextran sulfate sodium (DSS)-induced diarrhea and colonic inflammation have similar symptoms to those of ulcerative colitis in humans. We examined the effects of exposure to mild hyperbaric oxygen (MHO) at an atmospheric pressure of 1317 hPa with 40% oxygen on DSS-induced diarrhea and colonic inflammation in rats.
Methods: Five-week-old male Kyoto Apc Delta (KAD) rats (n = 12) were administered 2% DSS through drinking water for 1 week. Subsequently, DSS-treated male rats were not subjected to any further treatment (n = 6) or exposed to MHO (n = 6) for 2 weeks. Age-matched KAD rats not subjected to DSS treatment or exposed to MHO were used as the control group (n = 6).
Results: Control rats did not exhibit diarrhea and colonic inflammation. However, DSS-treated rats exhibited diarrhea and colonic inflammation, regardless of exposure to MHO. Exposure to MHO for 2 weeks led to decreased incidence of diarrhea in DSS-treated rats (p < 0.05). Exposure to MHO had no effect on colonic inflammation in DSS-treated rats (p = 0.12).
Conclusion: Exposure to MHO for 2 weeks can improve diarrhea but cannot attenuate colonic inflammation, possibly due to the short exposure duration (2 weeks) used in this study.
Keywords: colonic inflammation, dextran sulfate sodium, Kyoto Apc Delta rats, mild hyperbaric oxygen, oxidative metabolism
Introduction
Globally, at least three million people have from inflammatory bowel disease (IBD), which is roughly divided into two main types, namely, Crohn’s disease and ulcerative colitis (UC).1 Dextran sulfate sodium (DSS) is the most commonly used agent for the development of animal models of IBD; other chemical agents include acetic acid, oxazolone, 2,4,6-trinitrobenzene sulfonic acid, indomethacin, and peptidoglycan polysaccharide.2–5 DSS-treated mice exhibit colonic inflammation accompanied by body weight loss, bloody diarrhea, ulceration, and crypt loss.6,7 In rodents, DSS-induced colonic inflammation exhibits similar morphological and pathophysiological features as UC in humans including, mucosal damage, weakened epithelial barrier function, and ulceration.8–10 DSS treatment mainly induces colonic inflammation in the distal colon,7 and continued treatment for 9 days gradually increases the colonic inflammation grade and mucosal damage through intestinal barrier defects.11 Kyoto adenomatous polyposis coli (Apc) Delta (KAD) rats, a novel homozygous Apc mutant rat strain, have been established by N-ethyl-N-nitrosourea-induced mutagenesis of an inbred F344/NSlc rat strain.12,13 DSS treatment increased the incidence of diarrhea and colon carcinogenesis in KAD rats compared with that in F344 rats.13,14
Chronic exercise can prevent and/or improve inflammatory conditions in several chronic diseases.15 DSS-induced colonic inflammation reduced mitochondrial biogenesis in the intestinal epithelium in mice.16 Exercise increases the oxidative capacity of skeletal muscles due to enhanced mitochondrial biogenesis. Previous studies have demonstrated that exercise activates mitochondrial biogenesis not only in skeletal muscles but also in the intestinal epithelium, and thereby rescues the reduced mitochondrial biogenesis capacity of the intestinal epithelium in DSS-induced colonic inflammation.16–18
High atmospheric pressure and oxygen concentration increase the partial pressure of oxygen and particularly enhance the dissolved oxygen content in blood plasma.19,20 Exposure to mild hyperbaric oxygen (MHO) at an atmospheric pressure of 1266 hPa with 36% oxygen for 50 mins increased the dissolved oxygen content in blood plasma without occurrence of barotrauma and oxidative stress.21 Elevated oxygen levels in blood plasma increase oxidative metabolism in cells and tissues mainly due to enhanced mitochondrial oxidative capacity.22 Previously, we demonstrated that exposure to MHO inhibits and/or improves lifestyle-related diseases, including type 2 diabetes,22,23 diabetes-induced cataract,24 and hypertension,25 in rodents. Furthermore, in a previous study,26 we showed that exposure to MHO improves the mitochondrial oxidative capacity of skeletal muscles in rats with metabolic syndrome and also inhibits hyperglycemia and hyperlipidemia.
We hypothesized that exposure to MHO at an atmospheric pressure of 1317 hPa with 40% oxygen can increase the oxidative capacity of the colonic mucosa as well as skeletal muscles. Therefore, we examined the effects of exposure to MHO on the incidence of diarrhea and colonic inflammation in DSS-treated KAD rats.
Materials and Methods
Animals
Five-week-old male KAD rats (F344-Apcm1Kyo) were randomly divided into control and two experimental groups, DSS and DSS + MHO. Rats in the experimental groups were administered 2% DSS (MW: 36,000–50,000; MP Biochemicals, Burlingame, CA, USA) through drinking water for 7 days. Rats in the DSS + MHO group (n = 6) were exposed to MHO for 2 weeks after DSS treatment. At 8 weeks of age, rats in the DSS and DSS + MHO groups (n = 6 for each group) were sacrificed 2 weeks after DSS treatment. Rats in the CON group (n = 6), not subjected to DSS treatment or exposed to MHO, were sacrificed at 8 weeks of age.
Rats in the DSS + MHO group were placed under normobaric conditions at an atmospheric pressure of 1013 hPa with 20.9% oxygen for 21 hrs (daily from 13:00 to 10:00) and exposed to an atmospheric pressure of 1317 hPa with 40% oxygen for 3 hrs (daily from 10:00 to 13:00) using a MHO chamber. Rats in the CON and DSS groups were placed under normobaric conditions at an atmospheric pressure of 1013 hPa with 20.9% oxygen for 24 hrs a day. All rats were fed a standard diet and water ad libitum and maintained under conditions of 12 hrs light/dark cycle (light period from 08:00 to 20:00) at 24°C ± 2°C with 50% ± 10% relative humidity. The body weight and incidence of diarrhea in each rat were monitored weekly for the clinical assessment of colonic inflammation.6,10 The incidence of diarrhea and fecal blood was scored as follows: grade 0, normal pellets; grade 1, low level of diarrhea; grade 2, high level of diarrhea; and grade 3, bloody diarrhea. Two independent investigators performed clinical assessment in a blinded fashion at two time points weekly and the scores at both time points were averaged. The Institutional Animal Care and Experiment Committee of Kyoto University (Kyoto, Japan) approved the experiment all experiments. All experimental and animal care procedures were performed according to the “Guidelines for the Care and Use of Laboratory Animals” published by the Institutional Animal Care and Experiment Committee of Kyoto University.
Colorectal Preparation
At 8 weeks of age, rats were deeply anesthetized by administering sodium pentobarbital (35 mg/kg of body weight). The colorectum was excised, washed with physiological saline, cut longitudinally along the main axis, and fixed in 10% buffered formalin for histological examination. The fixed colorectum was embedded in paraffin. Furthermore, 4 μm-thick serial sections were cut with a microtome, mounted on slides, and processed for hematoxylin-eosin staining. Colorectal inflammation was scored on the basis of stained hematoxylin-eosin sections according to the morphological criteria described in a previous study:27 grade 0, normal colonic mucosa with no inflammation; grade 1, shortening and loss of the basal one-third of the actual crypts with mild inflammation and edema in the mucosa: grade 2, loss of the basal two-thirds of the crypts with moderate inflammation of the mucosa; grade 3, loss of the entire crypts with severe inflammation in the mucosa, but with retention of the surface epithelium; and grade 4, loss of the entire crypts and surface epithelium with severe inflammation in the mucosa, muscularis propria, and submucosa.
mRNA Levels in The Colonic Mucosa
Total RNA was isolated from the mucosa of the distal colon (3 cm from the anus) and extracted using RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. cDNA was synthesized from 1000 ng of total RNA using PrimeScript RT Master Mix (Takara Bio, Kusatsu, Japan). Gene expression was analyzed using RT-qPCR performed on an Applied Biosystems StepOne platform (Applied Biosystems, Foster City, CA, USA) with TB Green Premix Ex Taq II (Takara Bio). PCR thermal cycling was performed as follows: initial denaturation at 95°C for 30 s followed by 40 cycles at 95°C for 5 s and at 60°C for 30 s. PCR primers for amplification of rat genes were as follows: Interleukin 10 (Il10), 5ʹ-CAGACCCACATGCTCCGAGA-3ʹ (forward) and 5ʹ- CAAGGCTTGGCAACCCAAGTA-3ʹ (reverse); Interleukin 1β (Il1β), 5ʹ-GCTGTGGCAGCTACCTATGTCTTG-3ʹ (forward) and 5ʹ-AGGTCGTCATCATCCCACGAG-3ʹ (reverse); Tumor Necrosis Factor (Tnfa), 5ʹ-AACTCGAGTGACAAGCCCGTAG-3ʹ (forward) and 5ʹ-GTACCACCAGTTGGTTGTCTTTGA- 3ʹ (reverse); and Wingless-type inhibitory factor 1 (Wif1), 5ʹ-AGCCATTCCCGTCAATATCCA-3ʹ (forward) and 5ʹ-TCTGCCATGATGCCTTTATCCA-3ʹ (reverse). β-actin was used as an endogenous housekeeping control; β-actin, 5ʹ-ACGTTGACATCCGTAAAGAC-3ʹ (forward) and 5ʹ-GAAGGTGGACAGTGAGGC-3ʹ (reverse). The mRNA levels were normalized to those of the age-matched control group. A comparative CT method was used for comparative quantification of mRNA species.
Statistical Analysis
Data were expressed as mean and standard deviation of values from six rats. One-way analysis of variance was used to evaluate the overall differences in body weight and mRNA levels among the study groups. When differences were found to be significant, individual group comparisons were made by Scheffé’s post hoc test. Student’s t-test was used to evaluate differences in the incidence of diarrhea and colonic inflammation score between the DSS and DSS + MHO groups. Statistical significance was set at p < 0.05.
Results
The body weight of rats in the DSS and DSS + MHO groups at 7 weeks of age was significantly lower than that in the age-matched CON group (p < 0.05, Figure 1). In contrast, no difference was observed in the body weight at 5, 6, and 8 weeks of age among the CON, DSS, and DSS + MHO groups.
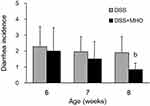
At 6, 7, and 8 weeks of age, the CON group did not show any incidence of diarrhea. No differences were observed in the incidence of diarrhea between the DSS and DSS + MHO groups at 6 and 7 weeks of age (Figure 2). In contrast, the incidence of diarrhea in the DSS + MHO group at 8 weeks of age was significantly lower than that in the age-matched DSS group (p < 0.05).
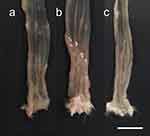
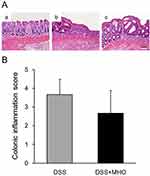
Macroscopically, edematous, reddish, and erosive colorectal mucosa were observed in DSS-treated rats (Figure 3). The colonic inflammation score was 0 for the distal colon of the CON group at 8 weeks of age (Figure 4A). The DSS and DSS + MHO groups showed colonic inflammation at 8 weeks of age (Figure 4B); however, no differences were observed in the inflammation score in these groups (p = 0.12).
No differences were observed in the mRNA levels (fold-change) of Il10 (Figure 5A), Il1β (Figure 5B), Tnfα (Figure 5C), and Wif1 (Figure 5D) among the CON, DSS, and DSS + MHO groups at 8 weeks of age.
Discussion
In this study, we examined the effects of exposure to MHO on DSS-induced diarrhea and colonic inflammation in KAD rats. Exposure to MHO improved (ie, reduced) the incidence of diarrhea (Figure 2). However, exposure to MHO did not improve colonic inflammation, ie, mucosal damage (Figure 4B).
In the rat models of colonic inflammation used in this study, 67% of rats exhibited bloody diarrhea at 6 weeks of age immediately after 1-week DSS treatment (Figure 2). Furthermore, mucosal damage was observed after 1-week DSS treatment (Figure 4B). Mucosal damage, weakened epithelial barrier function, and acute inflammation were observed in the DSS-induced colonic inflammation model due to macrophage activation, altered microbiota, and increased intestinal permeability.8,10,28 At the molecular level, the inflammatory cytokines TNFα and IL1β and the anti-inflammatory cytokine IL10 play key roles in immune response during innate and adaptive periods of colonic inflammation.29,30 The up-regulation of inflammatory cytokines such as TNFα and IL1β accelerates colon carcinogenesis and tumor formation.28,31 The Wnt signaling pathway plays a central role in the development of colorectal cancer.32,33 The Wnt inhibitory factor 1 is secreted as an antagonist that directly binds to Wnt proteins and inhibits the Wnt signaling pathway.34 UC is known to increase the risk of the development of colorectal cancer.35 Previous studies28,31–35 have also suggested that the simultaneous elevation of Il1β and reduction of Wif1 mRNA levels is accompanied by a high incidence of colorectal cancer in rats with colonic inflammation. We measured mRNA levels of these cytokines to understand the underlying mechanisms of the improvement of DSS-induced diarrhea after exposure to MHO. In the present study, contrast with histological results determined using the colonic inflammation scoring system (Figure 4B), no differences were observed in mRNA levels among the CON, DSS, and DSS + MHO groups. These results were induced possibly due to returned inflammation levels at molecular level during the 2 weeks in this study.
]In mice, DSS-induced colonic inflammation showed a reduction in mitochondrial biogenesis in the intestinal epithelium.16 Exercise protected against DSS-induced colonic inflammation,17,18 possibly by rescuing the decreased mitochondrial biogenesis in the intestinal epithelium. Exposure to MHO enhances and improves mitochondrial oxidative capacity of skeletal muscle fibers.22,26 Based on this finding, we hypothesized that exposure to MHO may improve DSS-induced colonic inflammation via enhanced oxidative capacity of the colonic mucosa. Contrary to our hypothesis, we observed no changes in the colonic inflammation grade between rats with DSS-induced colonic inflammation accompanied with or without exposure to MHO. However, DSS-induced diarrhea was improved by exposure to MHO. Together these results suggested that exposure to MHO for a period of 2 weeks may be too short to enhance the oxidative metabolism of the colonic mucosa and to improve colonic inflammation. However, enhancement of the oxidative capacity and improvement of colonic inflammation may be possible by exposure to MHO for longer durations than 2 weeks, which was used in this study. In summary, our study suggests that exposure to MHO at 1317 hPa with 40% oxygen for 2 weeks improves DSS-induced diarrhea but not colonic inflammation in KAD rats.
Acknowledgment
This work was supported by the Japan Society for the Promotion of Science.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi:10.1053/j.gastro.2004.01.063
2. Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi:10.1111/j.0105-2896.2005.00291.x
3. Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15(3):341–352. doi:10.1002/ibd.20753
4. Antoniou E, Margonis GA, Angelou A, et al. The TNBS-induced colitis animal model: an overview. Ann Med Surg (Lond). 2016;11:9–15. doi:10.1016/j.amsu.2016.07.019
5. Meroni E, Stakenborg N, Gomez-Pinilla PJ, et al. Functional characterization of oxazolone-induced colitis and survival improvement by vagus nerve stimulation. PLoS One. 2018;13(5):e0197487. doi:10.1371/journal.pone.0197487
6. Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1328–G1338. doi:10.1152/ajpgi.00467.2004
7. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi:10.1016/0016-5085(90)90290-H
8. Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med. 2012;29(2):202–208.
9. Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126(2):451–459. doi:10.1053/j.gastro.2003.11.010
10. Suzuki R, Miyamoto S, Yasui Y, Sugie S, Tanaka T. Global gene expression analysis of the mouse colonic mucosa treated with azoxymethane and dextran sodium sulfate. BMC Cancer. 2007;7:84. doi:10.1186/1471-2407-7-84
11. Azuma T, Shigeshiro M, Kodama M, Tanabe S, Suzuki T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J Nutr. 2013;143(6):827–834. doi:10.3945/jn.113.174508
12. Irving AA, Yoshimi K, Hart ML, et al. The utility of Apc-mutant rats in modeling human colon cancer. Dis Model Mech. 2014;7(11):1215–1225. doi:10.1242/dmm.016980
13. Yoshimi K, Tanaka T, Takizawa A, et al. Enhanced colitis-associated colon carcinogenesis in a novel Apc mutant rat. Cancer Sci. 2009;100(11):2022–2027. doi:10.1111/j.1349-7006.2009.01287.x
14. Yoshimi K, Tanaka T, Serikawa T, Kuramoto T. Tumor suppressor APC protein is essential in mucosal repair from colonic inflammation through angiogenesis. Am J Pathol. 2013;182(4):1263–1274. doi:10.1016/j.ajpath.2012.12.005
15. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi:10.1038/nri3041
16. Liu WX, Zhou F, Wang Y, et al. Voluntary exercise protects against ulcerative colitis by up-regulating glucocorticoid-mediated PPAR-γ activity in the colon in mice. Acta Physiol (Oxf). 2015;215(1):24–36. doi:10.1111/apha.12534
17. Cunningham KE, Vincent G, Sodhi CP, et al. Peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) protects against experimental murine colitis. J Biol Chem. 2016;291(19):10184–10200. doi:10.1074/jbc.M115.688812
18. Cook MD, Martin SA, Williams C, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013;33:46–56. doi:10.1016/j.bbi.2013.05.005
19. Leach RM, Rees PJ, Wilmshurst P. Hyperbaric oxygen therapy. Bmj. 1998;317(7166):1140–1143. doi:10.1136/bmj.317.7166.1140
20. Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334(25):1642–1648. doi:10.1056/NEJM199606203342506
21. Ishihara A, Nagatomo F, Fujino H, Kondo H. Exposure to mild hyperbaric oxygen increases blood flow and resting energy expenditure but not oxidative stress. J Sci Res Rep. 2014;3:1886–1896.
22. Yasuda K, Adachi T, Gu N, et al. Effects of hyperbaric exposure with high oxygen concentration on glucose and insulin levels and skeletal muscle-fiber properties in diabetic rats. Muscle Nerve. 2007;35(3):337–343. doi:10.1002/mus.20692
23. Matsumoto A, Nagatomo F, Yasuda K, Tsuda K, Ishihara A. Hyperbaric exposure with high oxygen concentration improves altered fiber types in the plantaris muscle of diabetic Goto-Kakizaki rats. J Physiol Sci. 2007;57(2):133–136. doi:10.2170/physiolsci.SC000707
24. Nagatomo F, Roy RR, Takahashi H, Edgerton VR, Ishihara A. Effect of exposure to hyperbaric oxygen on diabetes-induced cataracts in mice. J Diabetes. 2011;3(4):301–308. doi:10.1111/j.1753-0407.2011.00150.x
25. Nagatomo F, Fujino H, Takeda I, Ishihara A. Effects of hyperbaric oxygenation on blood pressure levels of spontaneously hypertensive rats. Clin Exp Hypertens. 2010;32(3):193–197. doi:10.3109/10641960903254521
26. Takemura A, Ishihara A. Mild hyperbaric oxygen inhibits growth-related decrease in muscle oxidative capacity of rats with metabolic syndrome. J Atheroscler Thromb. 2017;24(1):26–38. doi:10.5551/jat.34686
27. Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95(9):721–727. doi:10.1111/j.1349-7006.2004.tb03252.x
28. Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67(3):267–278. doi:10.1002/jlb.67.3.267
29. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–342. doi:10.1038/nri3661
30. Ma J, Yin G, Lu Z, et al. Casticin prevents DSS induced ulcerative colitis in mice through inhibitions of NF-κB pathway and ROS signaling. Phytother Res. 2018;32:1770–1783. doi:10.1002/ptr.6108
31. Wu X, Song M, Wang M, et al. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol Nutr Food Res. 2015;59(12):2383–2394. doi:10.1002/mnfr.201500378
32. Nojima M, Suzuki H, Toyota M, et al. Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene. 2007;26(32):4699–4713. doi:10.1038/sj.onc.1210259
33. Oving IM, Clevers HC. Molecular causes of colon cancer. Eur J Clin Invest. 2002;32(6):448–457. doi:10.1046/j.1365-2362.2002.01004.x
34. Hsieh JC, Kodjabachian L, Rebbert ML, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398(6726):431–436. doi:10.1038/18899
35. Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G7–G17. doi:10.1152/ajpgi.00079.2004
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.